Summary Branches of field-grown mature loblolly pine (Pinus taeda L.) trees were exposed for 2 years (1992 and 1993) to ambient or elevated CO2 concentrations (ambient + 165 µmol mol−1 or ambient + 330 µmol mol−1 CO2). Exposure to elevated CO2 concentrations enhanced rates of net photosyn-thesis (Pn) by 53--111% compared to Pn of foliage exposed to ambient CO2. At the same CO2 measurement concentration, the ratio of intercellular to atmospheric CO2 concentration (Ci/Ca) and stomatal conductance to water vapor did not differ among foliage grown in an ambient or enriched CO2 concentration. Analysis of the relationship between Pn and Ci indicated no significant change in carboxylation efficiency of ribulose-1,5-bisphosphate carboxylase/oxygenase during growth in ele-vated CO2 concentrations. Based on estimates derived from
Pn/Ci curves, there were no apparent treatment differences in dark respiration, CO2 compensation point or Pn at the mean Ci. In 1992, foliage in the three CO2 treatments yielded similar estimates of CO2-saturated Pn (Pmax), whereas in 1993, esti-mates of Pmax were higher for branches grown in elevated CO2 than in ambient CO2. We conclude that field-grown loblolly pine trees do not exhibit downward acclimation of leaf-level photosynthesis in their long-term response to elevated CO2 concentrations.
Keywords: acclimation, intercellular CO2, net photosynthesis,
Pinus taeda.
Introduction
Global atmospheric CO2 concentration has been increasing since the beginning of the industrial revolution in the mid-18th century (Neftel et al. 1985, Keeling and Whorf 1991) and is predicted to double at some time in the mid- to late 21st century (Keeling et al. 1989). Because this potential increase may have both direct and indirect effects on vegetation, studies of the ecological effects of rising atmospheric CO2 and associ-ated global climate change constitute a major research chal-lenge (Taylor and Grotch 1990).
Forests range across some 30% of the Earth’s land surface (Myers 1993) and are estimated to account for approximately
70% of terrestrial carbon assimilation (Waring and Schlesinger 1985). Model predictions (Kohlmaier et al. 1987, Houghton et al. 1987, Tans et al. 1990) and eddy correlation observations (Wofsy et al. 1993) suggest that forests may constitute a potential sink for the increasing atmospheric CO2 concentra-tion. Because estimates of many of the components in the global carbon budget have not been validated (Houghton et al. 1983, Sundquist 1993), information on the reaction of trees and forests to an increase in atmospheric CO2 concentration is needed to assess the ecological consequences of an increasing atmospheric CO2 concentration. Although there have been many studies on this subject, many questions still remain to be answered (Eamus and Jarvis 1989, Graham et al. 1990). In particular, the responses of older trees, i.e., beyond the seed-ling and sapseed-ling stages, have not been well characterized. Older trees have many distinct morphological, phenological and physiological differences from seedlings and saplings (Cregg et al. 1989). These differences raise concerns about whether information gathered from seedlings or saplings can adequately characterize the responses of mature trees to in-creasing atmospheric CO2 concentrations (Barton et al. 1993). We have assessed the effects of elevated CO2 concentrations on the gas exchange characteristics of foliage of mature trees growing in field conditions. The project was begun in 1992 in a 21-year-old plantation of loblolly pine (Pinus taeda L.) using the branch chamber technique (Teskey et al. 1991). The branch chamber technique has the disadvantage that the response of a single branch may not be the same as that of a whole tree when exposed to elevated CO2 concentrations; however, we believe that it may be of value for comparative studies of the effects of long-term CO2 enrichment on physiological and developmen-tal processes in foliage of large trees. An alternative to this technique would be to enclose whole trees in large open-top chambers, but this approach has the disadvantage that it usu-ally results in a large change in the microclimate around the tree. Open air exposure systems are another option but are expensive to operate, which limits replication. In contrast, branch chambers create less alteration of the microclimate than whole-tree chambers, and treatments can easily be replicated because of the small size and low cost of the chambers.
Responses of foliar gas exchange to long-term elevated CO
2
concentrations in mature loblolly pine trees
SHANYIN LIU
1and ROBERT O. TESKEY
1,21 Warnell School of Forest Resources, The University of Georgia, Athens, GA 30602, USA 2 Author to whom correspondence should be addressed
Received October 13, 1994
Materials and methods
Study site and plant material
The research was conducted in 1992 and 1993. The study site was located in the Whitehall Forest, an experimental forest of the University of Georgia, situated about 3 km south of Athens, Georgia (33°57′ N, 83°19′ W). The site is occupied by a plantation of 21-year-old mature loblolly pine trees, with a spacing of approximately 3 × 3 m. Site index of the study area on a 25-year basis is 16.5 m (Bailey et al. 1985), with a low to medium soil fertility; nitrogen concentration of current-year, first-flush foliage is approximately 1.2% in late August to early October. The soil on the site is in the Pacolet series, approxi-mately 0.9 m deep, well drained, and with a sandy loam tex-ture. The average annual precipitation is 1257 mm, and the mean annual air temperature is 16.5 °C, with mean summer and winter temperatures of 25.4 and 7.2 °C, respectively. Ambient CO2 concentrations at the site, monitored during the past several years, are consistent with worldwide seasonal averages, and range between 330 and 340 µmol mol−1 during the day and between 420 and 440 µmol mol−1 at night.
Experimental design
The experiment was conducted on upper mid-canopy branches of six healthy trees of average diameter at breast height and canopy size. Each tree was surrounded by four scaffolding towers linked by walkways to hold experimental apparatus and to provide access to branches. On each of the six trees, three main branches were chosen from the middle portion of the crown. Each branch was enclosed in a chamber and randomly assigned to receive one of three CO2 treatments: fumigation with ambient air (about 330 µmol mol−1 CO2, control), fumi-gation with air containing 495 µmol mol−1 CO
2 (ambient air + 165 µmol mol−1 CO2), and fumigation with air containing 660 µmol mol−1 CO2 (ambient air + 330 µmol mol−1 CO2), for a total of 18 branch chambers.
The design of the branch chamber has been described else-where (Teskey et al. 1991). Briefly, each chamber was cylin-drical and consisted of a rigid supporting frame made of aluminum alloy covered with a transparent polyvinylchloride plastic film (Livingstone Coating Corp., Columbia, SC), with dimensions of 1.5 m in length and 0.5 m in diameter. There was a 1.5-m long nylon zipper sewn to the plastic covering to allow easy access to the foliage in the chamber. The chamber was open at the bottom and there was a plenum at the top that had a series of holes to help distribute the air uniformly across the chamber. Each chamber was fastened at the top and bottom to the towers and walkways. Each chamber had one blower (Model AF-8, American Fan Co., Fairfield, OH) mounted on the walkway below it, and air was continuously blown into the chamber. Because of the relatively high rate of air exchange (10 times per min), the temperature and relative humidity within the chambers remained close to ambient. The tempera-ture differed by 0.3 °C and relative humidity by 0.7% inside and outside the chamber, and PAR within the chambers was lowered by approximately 10% (Teskey et al. 1991).
Elevated CO2 was generated from liquid CO2 (Airco,
Mur-ray, NJ), which was vaporized and injected into the air stream as it exited from the blower. Control of CO2 concentrations in the branch chambers was realized by a combination of mass flow controllers and rotometers. To determine CO2 concentra-tions, air samples were measured twice hourly by means of a microcomputer-controlled switching system sequentially routed to an infrared gas analyzer (IRGA) (Model 6252, Li-Cor Inc., Lincoln, NE).
Beginning in March 1992, the branches were exposed to the CO2 treatments for 24 h per day, 7 days per week for the duration of the project. To avoid water stress, the irrigation system was turned on when soil water was at or below −0.05 MPa and turned off at field capacity.
Gas exchange measurements
Gas exchange data were collected with a closed-loop LI-6200 portable photosynthesis system (Li-Cor Inc.) with a 250-ml leaf cuvette. Values of net photosynthesis (Pn), stomatal con-ductance (gs) and intercellular CO2 concentration (Ci), on a total needle surface area basis, were calculated according to von Caemmerer and Farquhar (1981). Measurements were conducted at ambient conditions between 1000 and 1700 h Eastern Standard Time (EST) at saturating sunlight (PAR 1000--2000 µmol m−2 s−1) from late August to mid-October in both 1992 and 1993. Each measurement took approximately 20--50 s. Usually, the change during the measurement period in relative humidity was ≤ 2% and in leaf temperature ≤ 2 °C. All the measurements were made inside the branch chambers on attached, south-facing, current-year foliage from the middle portion of the first flush developed under the experimental conditions. The CO2 concentrations at which measurements were made were 330, 495 and 660 µmol mol−1 CO2 for the ambient CO2 treatment (control), 330 and 495 µmol mol−1 CO2 for the 1.5× ambient CO2 treatment, and 330 and 660 µmol mol−1 CO2 for the 2.0× ambient CO2 treatment. For each branch chamber, four to six measurements were made at each measurement CO2 concentration using different sets of fasci-cles. Measurements of the three treatments on each tree were completed on the same day.
measure-ments. To avoid overestimation of carboxylation efficiency (Pn/Ci curve initial slope), cuvette chamber leaks were mathe-matically corrected using the Li-Cor protocol (Application Note No. 103, 1991). Measurement of each Pn/Ci curve took approximately 20 min. Two to three Pn/Ci curves were meas-ured on each branch using different sets of fascicles.
Data analysis
The initial slopes of the Pn/Ci curves (dPn/dCi) were calculated by linear regression of the first several points (Ci below 200--250 µmol mol−1). The whole Pn/Ci curves were then fitted to the empirical nonlinear model:
Pn= Pmax1 −(1 − Rd /Pmax)(1 −Ci / Γ) , (1)
where Pmax, the asymptotic level, represents the predicted CO2-saturated Pn, Rd is the rate of dark respiration at zero CO2, and Γ is the CO2 compensation point (Taylor and Gunderson 1988). The fitting was carried out by the Marquardt-Levenberg iterative least-square method available in SigmaPlot software (Jandel Scientific, San Rafael, CA).
Relative limitation of stomatal conductance on photosynthe-sis (Ls) was estimated according to the equation:
Ls=(1 − Pi / Pa)100, (2)
where Pi is the estimated Pn at Ci corresponding to the CO2 concentration in a specific ambient air (Ca), and Pa is the estimated Pn when Ci = Ca, the rate at which stomatal resistance to CO2 diffusion is assumed to be zero (Farquhar and Sharkey 1982). Both Pi and Pa were derived using Equation 1.
Treatment differences in foliar gas exchange data, Pn/Ci curve initial slopes and estimates of other parameters were determined by analysis of variance (ANOVA) with SAS soft-ware (SAS Institute Inc., Cary, NC). The experiment was treated as a randomized complete block design, with individ-ual trees as blocks and individindivid-ual branches receiving specific treatments as experimental units. The basic linear additive model was:
yijk=µ+τi+βj+εij+δijk, (3)
where yijk is the value of the kth measurement for the ith branch on the jth tree, µ is the overall mean, τi is the branch (i.e., CO2 treatment) effect where i = 1, 2 or 3, βj is the tree (i.e., block) effect where j = 1, 2, 3, 4, 5 or 6, εij is the experimental error (= (τβ)ij, treatments × trees), and δijk is the sampling error.
Least significant difference (LSD) pairwise comparisons were made to separate the means of the parameters when ANOVAs indicated significant treatment effects.
Results
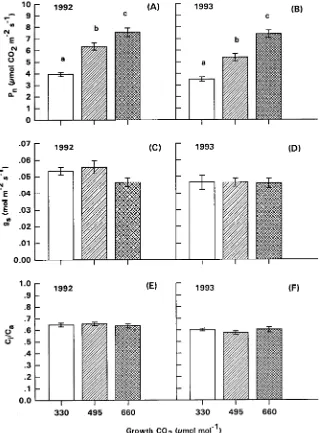
Net photosynthetic rate (Pn) of current-year, first-flush foliage of mature loblolly pine trees consistently responded positively to elevated growth CO2 concentrations in both 1992 and 1993 (P < 0.0002 for 1992, Figure 1A; P < 0.0001 for 1993, Figure
1B). Doubling of the ambient CO2 concentration (660 µmol mol−1) increased Pn by 92% in 1992 and by 111% in 1993, whereas enhancement of Pn for foliage grown at 495 µmol mol−1 CO2 was 61and 53% in 1992 and 1993, respectively. A comparison of the results for 1992 and 1993 indicated that there was no reduction in the enhancement of Pn with exposure time for branches grown at high concentrations of CO2.
Stomatal conductance, gs, was not significantly affected by elevated CO2. In 1993, foliage of the branches in the three CO2 treatments had almost identical gs (P < 0.6594, Figure 1D). Similarly, in 1992, the treatment difference was not statisti-cally significant (P < 0.3692), although gs was somewhat higher for foliage of branches grown at 495 µmol mol−1 CO2 and lower for foliage grown at 660 µmol mol−1 CO2 compared with foliage of branches grown under ambient conditions (Fig-ure 1C). The ratio of intercellular CO2 concentration to atmos-pheric CO2 concentration (Ci/Ca) was unaffected by the CO2 enrichment treatments in both years (P < 0.4229 for 1992, Figure 1E; P < 0.3363 for 1993, Figure 1F).
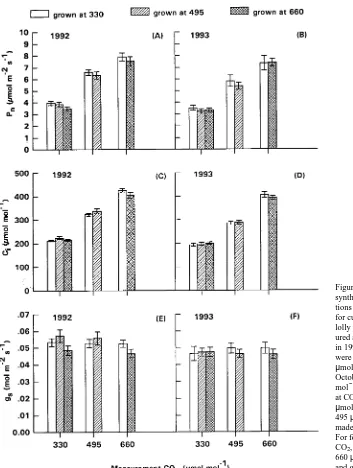
Net photosynthesis tended to be lower in foliage of branches grown at elevated CO2 concentrations than in foliage of branches grown at 330 µmol mol−1 CO
2 when measured at the same atmospheric CO2 concentration (Figure 2). Stomatal conductance and Ci also tended to be slightly less in branches grown at 660 µmol mol−1 CO2 than in branches grown at 330 µmol mol−1 CO2. Nevertheless, results of ANOVAs for the two years of measurements indicated that there were no statisti-cally significant differences in Pn, gs or Ci among the three treatments when measured at the same CO2 concentration (Figure 2A--F, P-values ranged from 0.2391 to 0.9609).
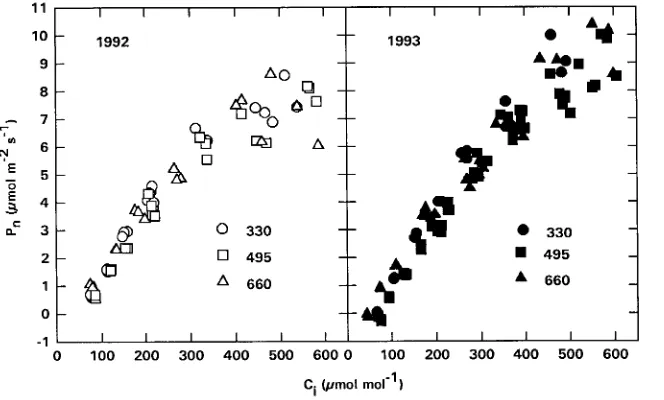
Elevated CO2 concentrations during growth had little effect on the relationship between Ci and Pn (Figure 3). Foliage in the three treatments had similar Pn/Ci function curves in both 1992 and 1993. Analysis of initial slopes of the Pn/Ci curves showed that, although the initial slope tended to be slightly lower for branches grown at elevated CO2 concentrations, the treatment differences were not significant (Table 1).
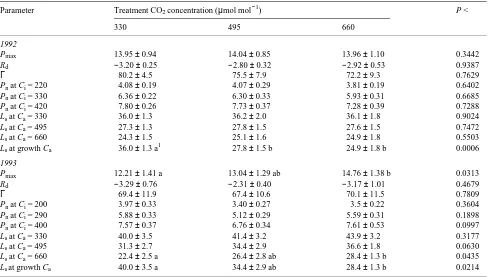
Carbon dioxide-saturated Pn (Pmax), foliar dark respiration at zero CO2 (Rd), CO2 compensation point (Γ) and Pn at Ci values corresponding to atmospheric CO2 concentrations of 330, 495 and 660 µmol mol−1 were estimated from the individual Pn/Ci curves using Equation 1 (Table 2). There were no effects of the treatments on Pmax of current-year, first-flush foliage of branches in 1992. In contrast, a significant difference in Pmax was detected among treatments in 1993. Compared with the branches grown at ambient CO2 concentration, Pmax increased by 7 and 21% in branches grown at 495 and 660 µmol mol−1 CO2, respectively. There were no differences in Rd and Γ among the three CO2 treatments. The estimated average Pn at a given Ci had a tendency to decrease with increasing treatment CO2 concentration, but the differences among treatments were consistently nonsignificant for both 1992 and 1993.
grown at 330 µmol mol−1 CO2 in 1993 (28.4% versus 22.4%,
P < 0.0435). When treatments were compared for Ls at their respective growth Ca, however, the differences were consis-tently significant in 1992 and 1993. Relative limitation of stomatal conductance on photosynthesis was lower in foliage grown at the elevated CO2 concentrations compared with Ls in foliage grown at the ambient CO2 concentration. Compared with Ls of the control (330 µmol mol−1 CO2) treatment, Ls was 23 and 31% lower in the 495 and 660 µmol mol−1 CO2 treatments, respectively, in 1992, and in 1993, the decline in Ls was 14 and 29% in the 495 and 660 µmol mol−1 CO2 treat-ments, respectively.
Discussion
Short-term CO2 enrichment typically enhances leaf net photo-synthesis in trees (Eamus and Jarvis 1989); however, this
short-term leaf-level photosynthetic enhancement may not be maintained in trees during long-term exposure to elevated CO2 concentrations (Gunderson and Wullschleger 1994). We found that branches of mature loblolly pine trees exhibited increased
Pn in current-year, first-flush foliage in response to long-term elevated exposures to 495 or 660 µmol mol−1 CO2 (Figures 1A and 1B). We conclude that there was no reduction in the enhancement of photosynthetic capacity of the foliage grown at elevated CO2 over the 2-year study, because there was no difference in Pn between CO2 treatments when measured at the same CO2 concentrations (Figures 2A and 2B). These results suggest that there was no downward photosynthetic acclima-tion of Pn during two growing seasons. This result is consistent with other CO2-enrichment studies conducted in the field or in large pots (Sage 1994).
acclimation and to identify the mechanisms contributing to acclimation (Harley et al. 1992, Gunderson and Wullschleger 1994, Sage 1994). In the Pn/Ci curve, Pn is theoretically limited by carboxylation efficiency (amount, activity and kinetic prop-erties) of Rubisco at low Ci, by ribulose-1,5-bisphosphate (RuBP) regeneration at intermediate Ci, and by inorganic phos-phate (Pi) regeneration capacity at high Ci (von Caemmerer and Farquhar 1981, Sharkey 1985, Harley and Sharkey 1991). We observed no significant change in Rubisco carboxylation efficiency in foliage grown at elevated CO2 (Table 1). Also, there was no reduction in CO2-saturated Pn (Pmax) or Pn at average Ci values, further demonstrating that long-term ele-vated CO2 fumigation of branches does not induce down-regu-lation of photosynthetic capacity. In 1993, estimates of Pmax were higher for branches grown at elevated CO2 than for
branches grown at ambient CO2, perhaps reflecting a gradual increase in Pi regeneration capacity for growth during long-term exposure to elevated CO2 (Makino 1994, Sage 1994). Positive acclimation of photosynthesis has been observed in mature Picea sitchensis (Bong.) Carr. (Barton et al. 1993) and nonwoody plants (Campbell et al. 1988, Arp and Drake 1991) grown in the field.
The absence of downward photosynthetic acclimation is similar to the findings of the other few field studies on woody species (Idso and Kimball 1991, Barton et al. 1993, Dufrêne et al. 1993, Gunderson et al. 1993), but contrasts with responses in many experiments with potted plants (see reviews by Eamus and Jarvis 1989 and Sage 1994). Field-grown trees have a large, if not unlimited, rooting volume, which represents a large sink for photosynthates. The fact that only a portion of Figure 2. Comparative rates of net photo-synthesis (Pn), intercellular CO2 concentra-tions (Ci) and stomatal conductance (gs) for current-year, first-flush foliage of lob-lolly pine tree branches grown and meas-ured at 330, 495 or 660 µmol mol−1 CO2 in 1992 and 1993. The measurements were made at saturating PAR (≥ 1000
µmol m−2 s−1) during late August to mid-October. For foliage grown at 330 µmol mol−1 CO2, gas exchange was measured at CO2 concentrations of 330, 495 and 660
the tree crown was exposed to elevated CO2 concentrations in this study may be another reason for the large sink strength in our trees. In contrast, root growth is restricted in many pot experiments, and this may reduce sink demand for photosyn-thates thereby causing down-regulation of Pn in the potted plants (Ehret and Joliffe 1985, Arp 1991, Stitt 1991, Thomas and Strain 1991). The pot-size effect could also be attributed to limited nutrient supply (Conroy et al. 1986, Fetcher et al. 1988, Norby and O’Neill 1991, Silvola and Ahlholm 1992, Tissue et al. 1993, El Kohen and Mousseau 1994, Nobel et al. 1994, Thomas et al. 1994) and the release of inhibitory signals (e.g., ABA) in response to pot-associated physical stresses (Sage 1994).
Stomatal response to CO2 is a common phenomenon (Mori-son 1987, Mott 1990) and stomatal conductance (gs) in many plants decreases in response to increasing atmospheric CO2 concentrations (Oberbauer et al. 1985, Tolley and Strain 1985, Fetcher et al. 1988, Eamus et al. 1993, Thomas et al. 1994). In our study, however, elevated CO2 treatments did not alter foliar
gs in mature loblolly pine trees (Figures 1C and 1D). In loblolly pine seedlings, Tolley and Strain (1985) also found that there
was no difference in gs between CO2 treatments when plants were grown at high irradiances (1000 µmol m−2 s−1). Similar results have been reported in Pinus contorta Dougl. ex Loud. (Higginbotham et al. 1985), Pinus radiata D. Don (Conroy et al. 1986, 1988), Malus domestica Borkh, Quercus prinus L. and Quercus robur L. (Bunce 1992), Fagus sylvatica L. (Du-frêne et al. 1993), and Liriodendron tulipifera L. and Quercus
alba L. (Gunderson et al. 1993). However, using the same
branch bag technique, Barton et al. (1993) found that, in mature P. sitchensis, gs was higher in shoots grown in an elevated CO2 concentration than in shoots grown in ambient CO2. Although the mechanisms by which CO2 affects stomatal aperture and gs are not known (Morison 1987, Mott 1990), there may be interspecific variation in CO2 sensitivity of gs (Eamus and Jarvis 1989). In our study, lack of stomatal sensi-tivity to CO2 might be associated with environmental condi-tions during growth. The branches receiving the CO2 treatments were grown at full PAR with an adequate supply of soil water, and stomatal sensitivity to CO2 may be reduced at high PAR in well-watered plants (Eamus and Jarvis 1989).
The ratio of intercellular to atmospheric CO2 (Ci/Ca) is directly linked to changes in the relationship between gs and the biochemical characteristics of Pn (Ball and Berry 1982) and may be a useful parameter for evaluating possible stomatal acclimation to long-term CO2 exposure (Sage 1994), although no clear mechanistic basis for this relationship has been dem-onstrated (Mott 1990). The Ci/Ca ratio was constant during the 2 years of elevated CO2 exposure (Figures 1E and 1F), indicat-ing that there was no stomatal acclimation to long-term ele-vated CO2 in mature loblolly pine trees. Results of the treatment comparison of gs and Ci supported this conclusion, i.e., when measured at the same Ca concentrations, no differ-ences in gs and Ci between CO2 treatments were observed (Figures 2C--F). However, elevated CO2 concentrations during growth decreased the stomatal limitation to photosynthesis (Ls) (Table 2), reflecting unchanged rates of gas phase diffusion of CO2 and an increase in biochemical limitations with increasing
Ci. This result is similar to those observed in cotton (Thomas Figure 3. Representative response curves of net photosynthesis (Pn) to intercellular CO2 concentration (Ci) for current-year, first-flush foliage of loblolly pine branches grown at 330, 495 or 660 µmol mol−1 CO2 in 1992 and 1993. The meas-urements were made at ambient tempera-ture and saturating PAR (≥ 1000 µmol m−2 s−1) in early October in one tree.
Table 1. Slopes of the initial linear portion of the Pn/Ci curves for current-year, first-flush foliage of loblolly pine branches grown at 330, 495 or 660 µmol mol−1 CO2 , derived from the linear regression of the first several points with Ci below 200--250 µmol mol−1. Measure-ments were taken from late August to mid October for both years (1992 and 1993). Values presented are means ± SE (n = 9--13). The unit for the initial slopes is mol m−2 s−1. The level of significance (P) is given in the last row.
Treatment [CO2] Initial slope
1992 1993
330 0.0276 ± 0.0014 0.0283 ± 0.0017
495 0.0258 ± 0.0016 0.0253 ± 0.0010
660 0.0250 ± 0.0023 0.0249 ± 0.0021
and Strain 1991) and P. radiata (Whitehead et al. 1992). To conclude, current-year, first-flush foliage of branches exposed to ambient + 165 or ambient + 330 µmol mol−1 CO2 concentrations in branch chambers for 2 years had greatly enhanced Pn compared with Pn of foliage exposed to ambient CO2 concentration. Various foliar gas exchange measurements and the modeling results of Pn/Ci curves indicated that there was no reduction in photosynthetic capacity in response to elevated CO2 in the field-grown mature loblolly pine trees during the 2-year study. We also demonstrated that field-grown trees do not experience the downward photosynthetic acclima-tion typically observed in long-term elevated CO2 experiments with potted plants. Although there is need for caution when extrapolating data derived from the branch chamber technique to whole trees, we note that our findings are consistent with other long-term whole-plant field studies (Idso and Kimball 1991, Gunderson et al. 1993, Drake et al. 1994).
Acknowledgments
This research was supported by the USDA Forest Service Southern Global Change Program.
References
Arp, W.J. 1991. Effects of source--sink relations on photosynthetic acclimation to elevated CO2. Plant Cell Environ. 14:869--875.
Arp, W.J. and B.G. Drake. 1991. Increased photosynthetic capacity of
Scirpus olneyi after 4 years of exposure to elevated CO2. Plant Cell
Environ. 14:1003--1006.
Bailey, R.L., G.E. Grider, J.W. Rheney and L.V. Pienaar. 1985. Stand structure and yields for site-prepared loblolly plantations in the piedmont and upper coastal plain of Alabama, Georgia, and South Carolina. The University of Georgia, Agricultural Experimental Station Bulletin 328, 118 p.
Ball, J.T. and J.A. Berry. 1982. The Ci/Cs ratio: a basis for predicting stomatal control of photosynthesis. Carnegie Inst. Washington Year-book 81:88--92.
Barton, C.V.M., H.S.J. Lee and P.G. Jarvis. 1993. A branch bag and CO2 control system for long-term CO2 enrichment of mature Sitka spruce (Picea sitchensis (Bong.) Carr.). Plant Cell Environ. 16:1139--1148.
Bunce, J.A. 1992. Stomatal conductance, photosynthesis and respira-tion of temperate deciduous tree seedlings grown outdoors at an elevated concentration of carbon dioxide. Plant Cell Environ. 15:541--549.
Campbell, W.J., L.H. Allen and G. Bowes. 1988. Effects of CO2 concentration on Rubisco activity, amount, and photosynthesis in soybean leaves. Plant Physiol. 88:1310--1316.
Conroy, J.P., M. Kuppers, B. Kuppers, J. Virgona and E.W.R. Barlow. 1988. The influence of CO2 enrichment, phosphorus deficiency and water stress on growth, conductance and water use of Pinus radiata D. Don. Plant Cell Environ. 11:91--98.
Table 2. Foliar gas exchange parameters for branches of mature loblolly pine grown at 330, 495 or 660 µmol mol−1 CO2 in branch chambers, estimated from response curves of net photosynthesis (Pn) to intercellular CO2 concentrations (Ci) measured in late August to mid-October of 1992 and 1993. Values are means ± SE for each treatment (n = 9--13). The fitting model used was Equation 1. The Ls value was calculated using Equation 2. Parameters are defined in the text. The level of significance (P) is given in the last column. Units are µmol m−2 s−1 for Pmax , Rd and
Pn, µmol mol−1 for Γ, and Ls is a percentage.
Parameter Treatment CO2 concentration (µmol mol−1) P <
330 495 660
1992
Pmax 13.95 ± 0.94 14.04 ± 0.85 13.96 ± 1.10 0.3442
Rd −3.20 ± 0.25 −2.80 ± 0.32 −2.92 ± 0.53 0.9387
Γ 80.2 ± 4.5 75.5 ± 7.9 72.2 ± 9.3 0.7629
Pn at Ci = 220 4.08 ± 0.19 4.07 ± 0.29 3.81 ± 0.19 0.6402
Pn at Ci = 330 6.36 ± 0.22 6.30 ± 0.33 5.93 ± 0.31 0.6685
Pn at Ci = 420 7.80 ± 0.26 7.73 ± 0.37 7.28 ± 0.39 0.7288
Ls at Ca = 330 36.0 ± 1.3 36.2 ± 2.0 36.1 ± 1.8 0.9024
Ls at Ca = 495 27.3 ± 1.3 27.8 ± 1.5 27.6 ± 1.5 0.7472
Ls at Ca = 660 24.3 ± 1.5 25.1 ± 1.6 24.9 ± 1.8 0.5503
Ls at growth Ca 36.0 ± 1.3 a1 27.8 ± 1.5 b 24.9 ± 1.8 b 0.0006
1993
Pmax 12.21 ± 1.41 a 13.04 ± 1.29 ab 14.76 ± 1.38 b 0.0313
Rd −3.29 ± 0.76 −2.31 ± 0.40 −3.17 ± 1.01 0.4679
Γ 69.4 ± 11.9 67.4 ± 10.6 70.1 ± 11.5 0.7809
Pn at Ci = 200 3.97 ± 0.33 3.40 ± 0.27 3.5 ± 0.22 0.3604
Pn at Ci = 290 5.88 ± 0.33 5.12 ± 0.29 5.59 ± 0.31 0.1898
Pn at Ci = 400 7.57 ± 0.37 6.76 ± 0.34 7.61 ± 0.53 0.0997
Ls at Ca = 330 40.0 ± 3.5 41.4 ± 3.2 43.9 ± 3.2 0.3177
Ls at Ca = 495 31.3 ± 2.7 34.4 ± 2.9 36.6 ± 1.8 0.0630
Ls at Ca = 660 22.4 ± 2.5 a 26.4 ± 2.8 ab 28.4 ± 1.3 b 0.0435
Ls at growth Ca 40.0 ± 3.5 a 34.4 ± 2.9 ab 28.4 ± 1.3 b 0.0214
Conroy, J.P., R.M. Smillie, M. Kuppers, D.I. Bevege and E.W. Barlow. 1986. Chlorophyll a fluorescence and photosynthetic and growth responses of Pinus radiata to phosphorus deficiency, drought stress, and high CO2. Plant Physiol. 81:423--429.
Cregg, B.M., J.E. Halpin, P.M. Dougherty and R.O. Teskey. 1989. Comparative physiology and morphology of seedling and mature forest trees. In Air Pollution Effects on Forest Vegetation. Eds. R.D. Noble, J.L. Martin and K.F. Jensen. USDA Forest Service, North-eastern Forest Experiment Station, Broomall, PA, pp 111--118. Davis, J.E., T.J. Arkebauer, J.M. Norman and J.R. Brandle. 1987.
Rapid field measurement of the assimilation rate versus internal CO2 concentration relationship in green ash (Fraxinus
pennsyl-vanica Marsh.): the influence of light intensity. Tree Physiol.
3:387--392.
Drake, B.G., G.J. Peresta and E. Beugeling. 1994. No decline in effect of elevated atmospheric CO2 on net ecosystem CO2 exchange (NCE), biomass production and tissue nitrogen concentration [N] of a C3 plant community in a Chesapeake Bay wetland after 7 years. Suppl. Bull. Ecol. Soc. Am. 75(abstr.):57.
Dufrêne, E., J.-Y. Pontailler and B. Saugier. 1993. A branch bag technique for simultaneous CO2 enrichment and assimilation meas-urements on beech (Fagus sylvatica L.). Plant Cell Environ. 16:1131--1138.
Eamus, D., C.A. Berryman and G.A. Duff. 1993. Assimilation, sto-matal conductance, specific leaf area and chlorophyll responses to elevated CO2 of Maranthes corymbosa, a tropical monsoon rain forest species. Aust. J. Plant Physiol. 20:741--755.
Eamus, D. and P.G. Jarvis. 1989. The direct effects of increase in the global atmospheric CO2 concentration on natural and commercial temperate trees and forests. Adv. Ecol. Res. 19:1--55.
Ehret, D.L. and P.A. Joliffe. 1985. Photosynthetic carbon dioxide exchange of bean plants grown at elevated carbon dioxide concen-trations. Can. J. Bot. 63:2026-- 2030.
El Kohen, A. and M. Mousseau. 1994. Interactive effects of elevated CO2 and mineral nutrition on growth and CO2 exchange of sweet chestnut seedlings (Castanea sativa). Tree Physiol. 14:679--690. Farquhar, G.D. and T.D. Sharkey. 1982. Stomatal conductance and
photosynthesis. Annu. Rev. Plant Physiol. 33:317--345.
Fetcher, N., C.H. Jaeger, B.R. Strain and N. Sionit. 1988. Long-term elevation of atmospheric CO2 concentration and the carbon ex-change rates of saplings of Pinus taeda L. and Liquidamber
styraci-flua L. Tree Physiol. 4:255--262.
Graham. R.L., M.G. Turner and V.H. Dale. 1990. How increasing CO2 and climate change affect forests. BioScience 40:575--587. Gunderson, C.A., R.J. Norby and S.D. Wullschleger. 1993. Foliar gas
exchange responses of two deciduous hardwoods during 3 years of growth in elevated CO2: no loss of photosynthetic enhancement. Plant Cell Environ. 16:797--807.
Gunderson, C.A. and S.D. Wullschleger. 1994. Photosynthetic accli-mation in trees to rising atmospheric CO2: a broader perspective. Photosynth. Res. 39:369--388.
Harley, P.C. and T.D. Sharkey. 1991. An improved model of C3 photosynthesis at high CO2: reversed O2 sensitivity explained by lack of glycerate reentry into the chloroplast. Photosynth. Res. 27:169--178.
Harley, P.C., R.B. Thomas, J.F. Reynolds and B.R. Strain. 1992. Modelling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ. 15:271--282.
Higginbotham, K.O., J.M. Mayo, S. L’Hirondelle and D.K. Krystofiak. 1985. Physiological ecology of lodgepole pine (Pinus
contorta) in an enriched CO2 environment. Can. J. For. Res.
15:417--421.
Houghton, R.A., R.D. Boone, J.R. Fruci, J.E. Hobbie, J.M. Melillo, C.A. Palm, B.J. Peterson, G.R. Shaver and G.M. Woodwell. 1987. The flux of carbon from terrestrial ecosystems to the atmosphere in 1980 due to changes in land use: geographic distribution of the global flux. Tellus 39B:122--139.
Houghton, R.A., J.E. Hobbie, J.M. Melillo, B. Moore, B.J. Peterson, G.R. Shaver and G.M. Woodwell. 1983. Changes in the carbon content of terrestrial biota and soils between 1860 and 1980: a net release of CO2 to the atmosphere. Ecol. Monogr. 53:235--262. Idso, S.B. and B.A. Kimball. 1991. Downward regulation of
photosyn-thesis and growth at high CO2 levels. Plant Physiol. 96:990--992. Keeling, C.D., R.B. Bacastow, A.F. Carter, S.C. Piper, T.P. Whorf, M.
Heimann, W.G. Mook and H. Roeloffzen. 1989. A three-dimen-sional model of atmospheric CO2 transport based on observed winds. I. Analysis of observational data. In Aspects of Climate Variability in Pacific and the Western Americas. Ed. D.H. Peterson. Geophys. Monogr. 55:165--235.
Keeling, C.D. and T.P. Whorf. 1991. Atmospheric CO2----modern record: Mauna Loa. In Trends ’91: A Compendium of Data on Global Change. Eds. T.A. Boden, R.J. Sepanski and F.W. Stoss. Carbon Dioxide Information Analysis Center, Oak Ridge National Laboratory, Oak Ridge, TN, pp 12--15.
Kohlmaier, G.H., H. Bröhl, E.O. Siré and M. Plöchl. 1987. Modelling simulation of plants and ecosystem response to present levels of excess atmospheric CO2. Tellus 39B:155--170.
Makino, A. 1994. Biochemistry of C3-photosynthesis in high CO2. J. Plant Res. 107:79--84.
McDermitt, D.K., J.M. Norman, J.T. Davis, T.M. Ball, T.J. Arkebauer, J.M. Welles and S.R. Roemer. 1989. CO2 response curves can be measured with a field-portable closed-loop photosynthesis system. Ann. Sci. For. 46(suppl.):416-- 420.
Morison, J.I.L. 1987. Intercellular CO2 concentration and stomatal response to CO2. In Stomatal Function. Eds. E. Zeigler, G.D. Farquhar and I.R. Cowan. Stanford University Press, Stanford, CA, pp 229--252.
Mott, K.A. 1990. Sensing of atmospheric CO2 by plants. Plant Cell Environ. 13:731--737.
Myers, N. 1993. Gaia: an atlas of planet management. Anchor Books, Doubleday, New York, NY, 272 p.
Neftel, A., E. Moor, H. Oeschger and B. Stauffer. 1985. Evidence from polar ice cores for the increase in atmospheric CO2 in the past two centuries. Nature 315:45--47.
Nobel, P.S., M. Cui, P.M. Miller and Y. Luo. 1994. Influences of soil volume and an elevated CO2 level on growth and CO2 exchange for the Crassulacean acid metabolism plant Opuntia ficus-indica. Physiol. Plant. 90:173--180.
Norby, R.J. and E.G. O’Neill. 1991. Leaf area compensation and nutrient interactions in CO2-enriched seedlings of yellow-poplar (Liriodendron tulipifera L.). New Phytol. 117:515--528.
Oberbauer, S.F., B.R. Strain and N. Fetcher. 1985. Effects of CO2 -en-richment on seedling physiology and growth of two tropical tree species. Physiol. Plant. 65:352--356.
Sage, R.F. 1994. Acclimation of photosynthesis to increasing atmos-pheric CO2: the gas exchange perspective. Photosynth. Res. 39:351--368.
Sharkey, T.D. 1985. Photosynthesis in intact leaves of C3 plants: physics, physiology and rate limitations. Bot. Rev. 51:53--106. Silvola, J. and U. Ahlholm. 1992. Photosynthesis in willows (Salix ×
dasyclados) grown at different CO2 concentrations and fertilization
levels. Oecologia 91:208--213.
Sundquist, E.T. 1993. The global carbon dioxide budget. Science 259:934--941.
Tans, P.P., I.Y. Ting and T. Takahashi. 1990. Observational constraints on the global atmospheric CO2 budget. Science 247:1431--1438. Taylor, G.E., Jr. and C.A. Gunderson. 1988. Physiological site of
ethylene effects in carbon dioxide assimilation in Glycine max L. Merr. Plant Physiol. 86:85--92.
Taylor, K.E. and S.L. Grotch. 1990. Observational and theoretical studies of greenhouse climate effects. In Environmental Problems and Solutions: Greenhouse Effect, Acid Rain, Pollution. Ed. T.N. Veziroglu. Hemisphere Publishing Corporation, New York, NY, pp 3--16.
Teskey, R.O., P.M. Dougherty and A.E. Wiselogel. 1991. Design and performance of branch chambers suitable for long-term ozone fu-migation of foliage in large trees. J. Environ. Qual. 20:591--596. Thomas, R.B., J.D. Lewis and B.R. Strain. 1994. Effects of leaf
nutrient status on photosynthetic capacity in loblolly pine (Pinus
taeda L.) seedlings grown in elevated atmospheric CO2. Tree
Physiol. 14:947--960.
Thomas, R.B. and B.R. Strain. 1991. Root restriction as a factor in photosynthetic acclimation of cotton seedlings grown in elevated carbon dioxide. Plant Physiol. 96:627--634.
Tissue, D.T., R.B. Thomas and B.R. Strain. 1993. Long-term effects of elevated CO2 and nutrients on photosynthesis and Rubisco in loblolly pine seedlings. Plant Cell Environ. 16:859--865.
Tolley, L.C. and B.R. Strain. 1985. Effects of CO2 enrichment and water stress on gas exchange of Liquidambar styraciflua and Pinus
taeda seedlings grown under different irradiance levels. Oecologia
65:166--172.
von Caemmerer, S. and G.D. Farquhar. 1981. Some relationships between the biochemistry of photosynthesis and the gas exchange of leaves. Planta 153:376--387.
Waring, R.H. and W.H. Schlesinger. 1985. Forest ecosystems: con-cepts and management. Academic Press Inc., Orlando, FL, 340 p. Whitehead, D., R.O. Teskey and J.F.F. Hobbs. 1992. The response of
large Pinus radiata trees to enhanced carbon dioxide concentration: what can we learn with a Li-Cor porometer? In Proc. Ann. Meeting N.Z. Soc. Plant Physiologists. Lincoln University, New Zealand, pp 14--16.