Stomatal guard cells are unique as a plant cell model and, because of the depth of knowledge now to hand on ion transport and its regulation, serve as an excellent model for the analysis of stimulus-response coupling in higher plants. Parallel controls – mediated by Ca2+, H+protein kinases and
phosphatases — regulate the gating of the K+and Cl–channels that facilitate solute flux for stomatal movements. A growing body of evidence now indicates that oscillations in the cytosolic free concentration of Ca2+contribute to a ‘signalling cassette’, which is integrated within these events through an unusual coupling with membrane voltage. Additional developments during the past two years point to events in membrane traffic that play complementary roles in stomatal control. Research in these areas, especially, is now adding entirely new dimensions to our understanding of guard cell signalling.
Addresses
Laboratory of Plant Physiology and Biophysics, Imperial College of Science, Technology and Medicine at Wye, Wye, Kent TN25 5AH, UK; e-mail: [email protected]
Current Opinion in Plant Biology2000, 3:196–204 1369-5266/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved. Abbreviations
ABA abscisic acid
ARF ADP-ribosylation factor G-protein [Ca2+]
i concentration of cytosolic free calcium cADPR cyclic ADP–ribose
EK K+equilibrium voltage
GEF guanine-nucleotide exchange factor ICl non-inactivating Cl–current IK,in inward-rectifying K+current IK,out outward-rectifying K+current IP3 inositol-1,4,5-trisphosphate Nt-Syr1 Nicotianasyntaxin-related protein 1 PLD phospholipase D
SNARE soluble N-ethylmaleimide-sensitive factor attachment protein receptor
Introduction
Stomata are pores that form between pairs of specialised cells, called guard cells, and that are found on the epidermis of all aerial parts of most higher plants. Guard cells open and close the stoma to regulate gas exchange between the inter-cellular spaces within the plant tissue and the surrounding environment, thereby influencing two of the processes that are most important to the vegetative plant, photosynthesis and transpiration. Thus, the control of stomatal aperture has long been recognised as directly influencing vegetative yields, as well as water resource and arable land manage-ment. Guard cells have also become a focus of attention in fundamental research as a ‘model’ for higher-plant cells. Their ability to integrate environmental and endogenous signals, and their position within the leaf tissue has enabled
explorations of the signal cascades that link guard-cell membrane transport to stomatal control. Of these, the sig-nalling mechanisms evoked by the water-stress hormone abscisic acid (ABA) have received the greatest attention and, in the past four years, have yielded some of the most excit-ing new findexcit-ings in plant cell signallexcit-ing.
Stomatal movements are achieved through changes in guard-cell volume, which, in turn, are driven by the accu-mulation (during opening) and loss (during closing) of osmotically active solutes (i.e. KCl and/or other K+salts).
Flux of these inorganic solutes must take place across the plasma membrane because mature guard cells lack func-tional plasmodesmata [1,2]. K+transport is dominated by
two classes of K+channels. The first of these permits K+
movement into, but not out of, the cell and thus gives rise to an inward-rectifying current (IK,in); the second facilitates K+flux out of, but not into, the cell and therefore appears
as an outward-rectifying current (IK,out). These two
cur-rents contribute to K+ flux during stomatal opening and
closing, respectively, and can be clearly distinguished by their whole-cell, single-channel and pharmacological prop-erties [2–4]. The movement of Cl–through so-called ‘slow’
or ‘non-inactivating’ Cl– channels (I
Cl) accounts for much
of the charge balancing of the fluxes of K+, especially
dur-ing stomatal closure when it is essential that the voltage across the guard-cell plasma membrane is brought positive of the K+ equilibium voltage (E
K) for the loss of K+
through IK,out[4].
Clearly, effective control of stomatal aperture, and hence of guard-cell volume, requires the coordinated regulation of both K+ and Cl- fluxes, and implies a high degree of
integration between the signalling pathways that deter-mine the activities of IK,in, IK,outand ICl. We know now
(see Figure 1) that ABA first, evokes an inward-directed current, mediated at least in part by ICl[5,6]; second,
inac-tivates IK,in, which normally mediates K+uptake [7]; and
third, activates the current through IK,out, which together
with IClfacilitates a net loss of KCl. At least two parallel
signalling pathways that engender changes in cytosolic-free calcium concentration ([Ca2+]
i) and pH (pHi)
underpin these events [2–4]. Nonetheless, major gaps remain in our understanding of these signalling pathways. Even the function and processing of the [Ca2+]
i signal,
itself, is proving surprisingly complex. Furthermore, key developments during the past two years point to related events in membrane traffic that play equally important roles in stomatal control. Research in these areas, espe-cially, is adding entirely new dimensions to our understanding of guard-cell signalling and of plant cell biology in general.
[Ca
2+]
i
increases and Ca
2+channels
Early debates centred around the origins of the [Ca2+] i
sig-nal, whether it is internal or external to the guard cell. Considerable evidence now indicates that increases in [Ca2+]
iarise from both Ca2+entry across the plasma
mem-brane and release from intracellular stores [8–10]. Furthermore, the vacuole is certainly not the only source of Ca2+within the cell [8]. Some uncertainty remains about
the relative contributions of various pathways, which may reflect an inherent redundancy (and, hence, plasticity) within the Ca2+cascade. Nonetheless, we can expect that
the specialisation, and localisation to selected membranes within the cell, of [Ca2+]
i-signalling elements
is also important for signal encoding [11,12].
Within the guard cell, Ca2+release and Ca2+-mediated
sup-pression of IK,inare mediated by inositol-1,4,5-trisphosphate (IP3) [13,14], which is produced rapidly in response to ABA
[15,16]. This lipid metabolite probably interacts with IP3
-sen-sitive Ca2+ channels within the cell to potentiate a rise in
[Ca2+]
i [17]. Several studies have, however, described a
sec-ond class of endomembrane Ca2+-release channels in higher
plants [17,18]. These channels are sensitive to the alkaloid ryanodine and are activated by binding cyclic ADP–ribose (cADPR), a metabolite of nicotinamide adenine dinucleotide.
A statistical analysis of cADPR-injected mesophyll had impli-cated a role for this agonist in r29Aand kin2gene expression evoked by ABA [19]. More recently, Leckie et al. [20••] have
provided direct evidence of cADPR-sensitive, Ca2+
-perme-able channels in guard-cell vacuoles and suggested that they function as a Ca2+-release pathway during ABA stimulation.
In support of this hypothesis, they observed increases in [Ca2+]
ifollowing injection of cADPR into guard cells, and a
slowing of stomatal closure in response to ABA when guard cells were preloaded with the cADPR antagonist 8NH2
-cADPR. Grabov and Blatt [21•] offer parallel evidence that
voltage-evoked increases in [Ca2+]
iare suppressed by
ryan-odine in intact guard cells. The latter study is of particular interest because it offers the first quantitative analysis of the [Ca2+]
idependence of IK,in. Grabov and Blatt [22••] had
pre-viously found evidence of a hyperpolarisation-activated Ca2+
channel at the plasma membranne, implicated by the sensi-tivity of [Ca2+]
i increases to the membrane voltage. In the
more recent study [21•], they made use of changes in clamp
voltage to elicit changes in [Ca2+]
i, monitoring IK,in under
voltage clamp. They found a high (‘switch-like’) sensitivity of the current to [Ca2+]
i, with an apparent K1/2(~330µM) only
marginally above the mean resting [Ca2+]
i. Whether ABA
affects the [Ca2+]
idependence of IK,inis not known;
howev-er, ABA does strongly influence the voltage sensitivity of Figure 1
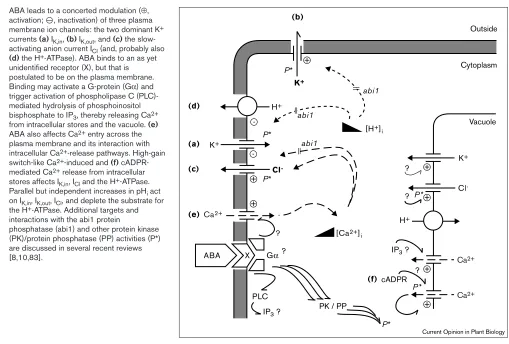
ABA leads to a concerted modulation (⊕, activation; O, inactivation) of three plasma membrane ion channels: the two dominant K+ currents (a) IK,in, (b) IK,out, and (c) the slow-activating anion current ICl(and, probably also (d)the H+-ATPase). ABA binds to an as yet unidentified receptor (X), but that is postulated to be on the plasma membrane. Binding may activate a G-protein (Gα) and trigger activation of phospholipase C (PLC)-mediated hydrolysis of phosphoinositol bisphosphate to IP3, thereby releasing Ca2+ from intracellular stores and the vacuole. (e) ABA also affects Ca2+entry across the plasma membrane and its interaction with intracellular Ca2+-release pathways. High-gain switch-like Ca2+-induced and (f) cADPR-mediated Ca2+release from intracellular stores affects IK,in, ICland the H+-ATPase. Parallel but independent increases in pHiact on IK,in, IK,out, ICl, and deplete the substrate for the H+-ATPase. Additional targets and interactions with the abi1 protein
phosphatase (abi1) and other protein kinase (PK)/protein phosphatase (PP) activities (P*) are discussed in several recent reviews [8,10,83].
rise and its return to resting concentrations [22••]. So, we can
anticipate a compound action of ABA both on Ca2+ entry
across the plasma membrane and on its release from cADPR-dependent or other Ca2+stores.
The spatial distribution of internal Ca2+-release pathways
is also likely to affect the kinetics of [Ca2+]
ichanges and
Ca2+-signal encoding. The vacuole is an obvious source of
Ca2+ and the tonoplast contains slow vacuolar (SV)-type
channels that may be related to animal ryanodine-receptor Ca2+channels [23•], and contribute to Ca2+ release [24].
Nonetheless, Ca2+ channels have also been identified in
the endoplasmic reticulum (ER) of mechanosensitive organs in Bryonia [25] and probably the ER of Lepidium roots [26]. In Brassica florets, Ca2+release appears to be
triggered by IP3in vesicular compartments that are distinct
from the vacuole [27]. Furthermore, in the giant alga Chara, the action potential (which is initiated by Ca2+
-mediated activation of Cl-channels [28,29•,30]) draws on
IP3-dependent Ca2+release from a non-vacuolar
compart-ment close to the plasma membrane. In a particularly elegant study, Plieth et al. [31••] preloaded Chara with
Mn2+, targeting either all intracellular stores (by incubation
in Mn2+medium) or only the central vacuole (by injection
with Mn2+). They found that Mn2+quenches the
fluores-cence of the Ca2+-sensitive dye Fura2 and, because it also
permeates many Ca2+ channels, it provides an assay for
divalent release. When they evoked action potentials, Plieth et al. [31••] observed a quenching of Fura2
fluores-cence in cells that were uniformly loaded with Mn2+, but
not in cells in which Mn2+was loaded only into the
vac-uole. Whether spatial differences in Ca2+ release
underpins [Ca2+]
i signalling in guard cells has yet to be
determined, but there is certainly evidence of local differ-ences in [Ca2+]
idynamics between the peripheral cytosol
and regions of the cell close to the nucleus [22••]. To
sum-marise, both the pharmacological and kinetic characteristics, as well as the spatial distribution of Ca2+
-release pathways within the guard cell, can be expected to play important roles in defining the nature of the [Ca2+] i
response to ABA.
[Ca
2+]
i
oscillations
Ca2+mediates a diversity of responses, even within a
sin-gle cell. The issue of how a sinsin-gle second messenger could give rise to such varied responses was resolved only when it was recognised that the frequency and location of a sequence of repetitive increases in [Ca2+]
iwithin the cell
might determine the nature of the response(s) [12,32,33]. Frequency modulation of [Ca2+]
iincreases or ‘spikes’ has
been shown to give rise to ‘Ca2+ signatures’ that encode
specific responses, including gene expression [34,35] and calmodulin-dependent protein kinase activity [36•]. In
ani-mals, [Ca2+]
i oscillations initiate Ca2+ entry across the
plasma membrane; local [Ca2+]
ielevation then triggers
fur-ther Ca2+ release from intracellular stores (i.e.
Ca2+-induced Ca2+ release [CICR]) before the Ca2+ is
The result is repetitive [Ca2+]
ispikes, each with a duration
of a few seconds or less.
Interest in [Ca2+]
isignalling in plants has been heightened
by observations that [Ca2+]
i may oscillate in response to
external stimuli [37–40]. In guard cells, however, these oscillations often take place over periods of more than 10 min, with discrete [Ca2+]
imaxima lasting for 2–4 min or
more when evoked by a rise in the extracellular Ca2+
con-centration or CO2[40,41]. A similar oscillation pattern has
now been observed in response to ABA. Staxen et al. [42•]
reported [Ca2+]
i increases from approximately 200 nM at
rest to 400–600 nM within 15–30 min of exposure to 10 nM ABA. Significantly, treatments with higher concentrations of ABA that resulted in a pronounced decrease in stomatal aperture also produced a reduced amplitude in the [Ca2+] i
oscillations and a longer duration of the [Ca2+]
ielevation. A
role for IP3-mediated Ca2+release was suggested because
the ABA-evoked oscillations were partially suppressed by treatment with the phospholipase C antagonist U73122.
This pattern of slow and irregular oscillations in [Ca2+] i
raises questions about the origins and role of this signal in the guard cell. One explanation relates [Ca2+]
ioscillations
to the voltage status of the cell. Guard cells, like many higher-plant cells, have two states of membrane voltage: one state close to EK, and the other largely K+-insensitive
and typified by voltages well negative of EK [43,44].
Transitions between these states take place in response to stimuli, including ABA, that can give rise to oscillations in voltage with periods of between 10 s and many minutes [43,45]. Because membrane hyperpolarisation also evokes a Ca2+influx and a consequent rise in [Ca2+]
i, it is most
likely that the [Ca2+]
i oscillations mirror an underlying
oscillation in plasma membrane voltage [22••]. These
char-acteristics — and the effect of ABA in altering the voltage threshold for the [Ca2+]
i rise [22••] — suggest a role for
[Ca2+]
ielevation in a feedback mechanism that ‘tunes’ the
[Ca2+]
i-dependent currents IK,inand IClto the prevailing
transport status of the guard cell. Thus, [Ca2+]
i feedback
creates a response ‘cassette’ for osmotic balance (Figure 2), switching the membrane between states in which net uptake and net loss of K+and Cl–take place [8,44].
Membrane traffic and cell volume
The activation of Ca2+channels may also play a role in
events one step removed from the regulation of K+and Cl–
of these issues in Charamore than two decades ago. Yet, despite their implied function in stomatal movements, the dynamics of membrane trafficking within the guard cell were largely ignored until recently. Two recent develop-ments have no placed membrane trafficking in the limelight of stomatal physiology and ABA signalling.
In the first of these, Homann [50•] and Kubitscheck et al.
[51••] used in vivo capacitance recording and
membrane-surface labelling with styryl dyes to quantify membrane exocytosis and endocytosis in guard-cell protoplasts during osmotically driven volume changes. Capacitance recording makes use of sine-wave retardation to determine mem-brane capacitance [52–54], which is directly proportional to the membrane surface area. An increase in capacitance represents the sum of all exocytotic events less the sum of all endocytotic events. Endocytotic events can subse-quently be isolated by simultaneously monitoring internalisation of fluorescent styryl dyes, such as FM1-43 [55]. Homann [50•] found that the membrane surface area
of Viciaguard-cell protoplasts increased under hypo-osmot-ic conditions and decreased after hypertonhypo-osmot-ic treatment, and that the rate of change in each case was graded in response to the magnitude of the osmotic potential differ-ence. Kubitscheck et al. [51••] subsequently combined
these techniques with FM1-43 dye measurements. Their results demonstrate a rapid internalisation of membrane during hyperosmotic stimulation that is quantitatively con-sistent with the decrease in plasma membrane surface area (and also capacitance). Intriguingly, the dye also accumu-lated gradually within the cell in a ring 1–2µm below the plasma membrane, even in the absence of an osmotic step. This steady internalisation suggests that constitutive endocytosis continues under constant osmotic pressure, resulting in the accumulation of a pool of sub-plasma membrane vesicles. Whether these vesicles are available for re-incorporation into the plasma membrane remains to be determined. The role of ABA and of Ca2+ in these
events can also be expected to draw attention in the near future. So far, all of the evidence indicates that Ca2+does
not have a direct impact on the membrane trafficking that is evoked by osmotic gradients [50•]. It may be that
osmot-ic potential- and ABA-sensitive traffosmot-icking controls overlap only in part. Whether or not this is the case, experimental results point to a highly dynamic process of membrane cycling that is responsive to membrane tension.
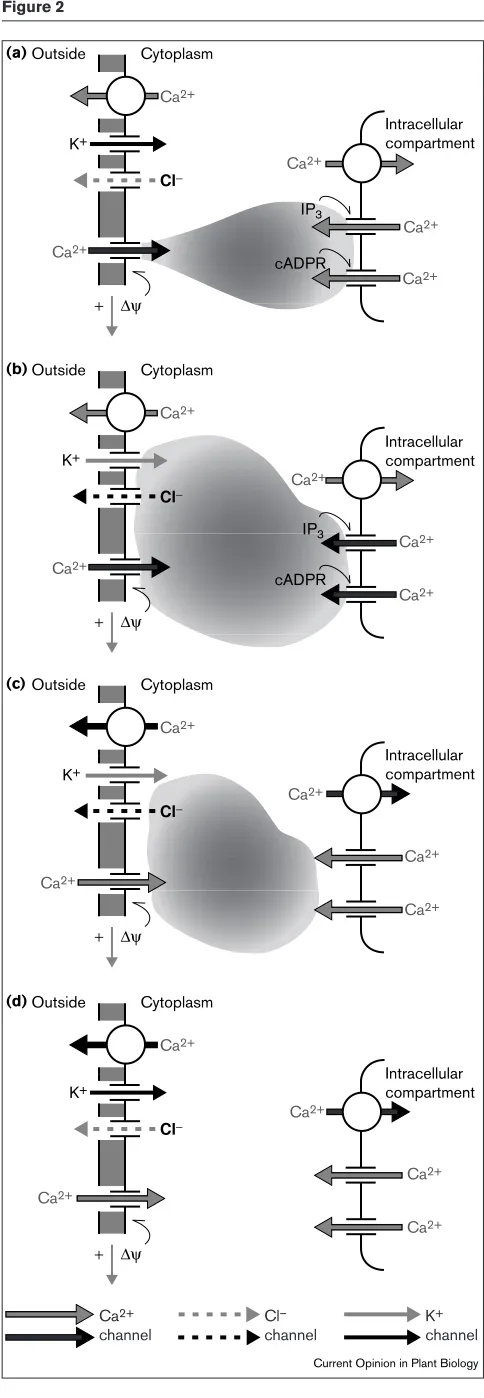
Figure 2
Cytoplasm Outside
Ca2+
Ca2+ Ca2+ Ca2+
Ca2+
K+
Cl−
+ ∆ψ
cADPR IP3
Intracellular compartment
(a)
Cytoplasm Outside
Ca2+
Ca2+ Ca2+ Ca2+
Ca2+
K+
Cl−
+ ∆ψ
cADPR IP3
Intracellular compartment
(b)
Cytoplasm Outside
Ca2+
Ca2+ Ca2+ Ca2+
Ca2+
K+
Cl−
+ ∆ψ
Intracellular compartment
(c)
Cytoplasm Outside
Ca2+
Ca2+ Ca2+ Ca2+
Ca2+
K+
Cl−
+ ∆ψ
Intracellular compartment
(d)
Current Opinion in Plant Biology
Ca2+ channel
Cl– channel
K+ channel
Figure 2 Legend
The voltage–[Ca2+]
iresponse ‘cassette’ cycle. (a) Negative membrane voltage (downward arrow indicates amplitude) triggers Ca2+influx across the plasma membrane that (b) stimulates intracellular Ca2+ release, activates Cl–channels for Cl–efflux and inactivates I
K,in. (c) The rise in [Ca2+]
i, bias to Cl–efflux and additional changes in membrane conductance (not shown) lead to membrane depolarisation, inactivate the Ca2+influx and engage Ca2+extrusion processes. (d) Finally, with [Ca2+]
The second development has come from the identifica-tion of the Nicotiana syntaxin homologue Nt-Syr1. Syntaxins belong to a group of integral membrane pro-teins that form the core of the molecular machinery for vesicle trafficking and membrane fusion (see below). Surprisingly, Leyman et al. [56••] isolated the Nt-Syr1
gene by expression cloning in a heterologous screen designed to identify an ABA receptor. Their studies demonstrate not only that ABA enhances Nt-Syr1 tran-script and Nt-Syr1 protein levels in the plant but also that disrupting Nt-Syr1 function, with either the Clostridial neurotoxin BotN/C (an endopeptidase that specifically cleaves the syntaxin) or by loading guard cells with the soluble (carboxy-truncated) portion of Nt-Syr1, prevents both K+and Cl–channel responses to
ABA in vivo. These results, and observations of a high molecular weight band recognised by Nt-Syr1 antibod-ies, suggest that Nt-Syr1 functions in a complex that is competitively blocked through substitution with the car-boxy-truncated protein. They also indicate that Nt-Syr1 functions within or very close to the early steps of the ABA signal cascade, either as a scaffolding protein, a sec-ond messenger or a modulator of the activity/availability of one or more signalling elements.
Further evidence of membrane trafficking in
stomatal control?
Two additional lines of evidence hint at roles for mem-brane trafficking in guard-cell volume control and ABA signalling. First, in screening for ABA-hypersensitive mutants of Arabidopsis, Cutler et al. [57] identified Era1, the gene that encodes a protein farnesyl-transferase. Farnesyl- and geranylgeranyl-transferases covalently add lipid moieties to small GTPases, anchoring the latter at the membrane surface [58] to target GTPase activity in a vari-ety of cellular functions. Although the substrate(s) for Era1 is not known, Rac- and Rho-type GTPases, which are important for cytoskeletal function [59], are commonly far-nesylated. In fact, a Rho-like GTPase, Rop1, that is localised to the apex of Pisumpollen tubes [60] has been implicated in microfilament-based intracellular transport of vesicles. Intriguingly, actin organisation has also been suggested to regulate guard-cell ion channels and stomatal movements, although the function of actin in this case has been interpreted in the context of control by mechanical stress rather than membrane trafficking [61–63].
The Era1 farnesyl-transferase also contributes to ABA sig-nalling. Pei et al. [64•] have reported an enhanced
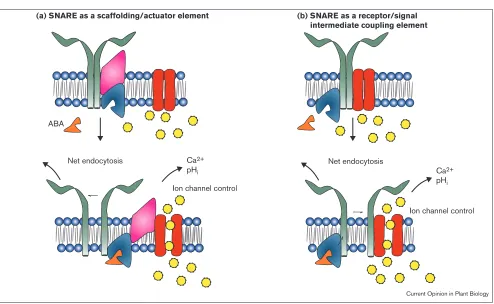
(b) SNARE as a receptor/signal intermediate coupling element
ABA
Net endocytosis Net endocytosis
Ion channel control
Ion channel control Ca2+
pHi Ca2+
pHi (a) SNARE as a scaffolding/actuator element
Current Opinion in Plant Biology
Two models for the dual functioning of Nt-Syr1 (and other SNARE proteins) in controlling ion channels and vesicle trafficking. Soluble ABA (orange) binding to its receptor (blue) alters both net endocytosis and the permeation of ions (yellow) through ion channels (red).
sensitivity of guard-cell Cl– channels to ABA in the era1
mutant and in wild-type Arabidopsis plants after treatment with broad-range farnesyl-transferase antagonists. The era1 phenotype is dominant and persists throughout the life cycle of the plant. So, although these results do not dis-tinguish between possible roles for Era1 as an ABA-signalling element per se and as a secondary element necessary to maintain the signalling machinery, they do implicate a role for small GTPases and potentially for membrane trafficking in guard-cell function.
Second, Jacob et al. [65•] have recently reported a
tran-sient rise in the phospholipase D (PLD) activity of guard-cell protoplasts following stimulation with ABA. They also observed a reduction in the activity of IK,in
that complemented the action of the cADPR antagonist nicotinamide when the protoplasts were treated with the PLD by-product phosphatidic acid. It is worth noting that both PLD and phosphatidic acid have been impli-cated in secretion by the barley aleurone [66]. Phosphatidic acid contributes to the regulation of vesicle trafficking at the Golgi apparatus [67], and PLD activity has been associated with the functioning of ARF- and Rho-GTPases in the secretory processes of mammals and yeast [68,69]. Once again, the data suggest a link between membrane trafficking and ABA.
Coordinating membrane traffic and ion
channel control
How might membrane trafficking contribute to ion-chan-nel control and ABA signalling? Membrane cycling and fusion between the membranes of eukaryotic cells are facilitated by a family of SNARE (i.e. soluble N -ethyl-maleimide-sensitive factor attachment protein receptor) proteins that are associated with each membrane [70–72]. During synaptic transmission, which depends on vesicle fusion and release of a neurotransmitter, the target (t-SNARE) proteins syntaxin and SNAP-25 form a stable ternary complex with the vesicle (v-SNARE) protein synaptobrevin that serves to bring the two membrane bilayers into close proximity for fusion [73]. In yeast and plants, SNARE proteins are thought to be essential for the related functions of interorganelle transport, growth and cell division [72,74]. Thus, SNAREs have been recognised as contributing to response mechanisms and not as compo-nents of signal cascades per se.
Even so, SNAREs are probably important for signal transduction, its plasticity and adaptation. SNAREs may contribute to ion-transport regulation simply by mediat-ing the selective addition and removal of transport proteins from the membrane. These events can take place with remarkable rapidity. A recent study of the
Arabidopsis GNOM gene, which encodes a GTP–GDP
exchange factor (GEF) for an ADP-ribosylation factor G-protein (ARF) GTPase, is a case in point. Steinmann et al. [75••] report that treatments with the ARF–GEF
antagonist brefeldin A result in dramatic alterations in
protein localisation within 15–30 min, including a loss of the polar distribution of the putative auxin efflux carrier protein Pin1. Auxin efflux, itself, is altered within 10 min of brefeldin A treatments [76], suggesting that substan-tial changes to the make-up of the membrane transport proteins take place within the same time period. The data certainly highlight the dynamic flux of membrane and protein within the plant cell.
The role(s) carried out by SNAREs may prove to be much more subtle still. In neurons, SNAREs, including syntax-ins, bind to and regulate Ca2+channels [77,78] as well as
CFTR Cl-channels [79]. Syntaxins interact with proteins
that may not be related to secretion processes directly, including the orphan G-protein-coupled receptor CIRL [80] and tomosyn, a microfilament-associated protein [81]. Furthermore, SNARE proteins have been associated with responses to membrane tension and osmotic stress [82]. So, one possibility (Figure 3) is that SNARE proteins such as Nt-Syr1 [56••] contribute to the scaffolding of an
ABA-receptor complex and, therefore, serve dual roles by marrying the functions of a signalling element and of the machinery for membrane trafficking.
Conclusions
Research into the cellular and molecular mechanisms of stomatal control has begun to take on new dimensions in relating signalling pathways with response ‘cassettes’ that comprise several ion transporters and their control mecha-nisms. Understanding how these cassettes are assembled will present a major challenge in the coming years. Defining the interactions within these cassettes is already providing a clearer picture of the functions of Ca2+as a
sec-ond messenger. At the same time, attention to the related processes of membrane dynamics and trafficking promises to add to our understanding of both cellular transport and volume control, as well as to the guard-cell system as a higher-plant cell model.
Update
Following up the evidence of a hyperpolarisation-activated rise in [Ca2+]
i, Hamilton et al. [84••] have now identified a
small-conductance Ca2+channel at the plasma membrane
by patch-clamp of Viciaguard-cell protoplasts. The chan-nel shows characteristics that are entirely consistent with those expected from the previous studies, including a dependence on negative voltage and block by Gd3+and
La3+[21•,22••]. Furthermore, ABA evokes a large increase
in channel opening, even in isolated membrane patches. These results suggest that the ABA receptor is physically close to the Ca2+channel and localised to the inner surface
of the plasma membrane.
Acknowledgements
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Wille A, Lucas W: Ultrastructural and histochemical studies on guard cells.Planta1984, 160:129-142.
2. Willmer C, Fricker MD: Stomata. London: Chapman and Hall; 1996.
3. Thiel G, Wolf AH: Operation of K+channels in stomatal
move-ment.Trends Plant Sci1997, 2:339-345.
4. Blatt MR, Leyman B, Grabov A: Cellular responses to water stress.
In Plant Responses to Environmental Stress. Edited by Shinozaki K. Houston: Landes; 1999:90-126.
5. Pei ZM, Kuchitsu K, Ward JM, Schwarz M, Schroeder JI: Differential abscisic acid regulation of guard cell slow anion channels in
Arabidopsiswild-type and abi1and abi2mutants.Plant Cell1997,
9:409-423.
6. Grabov A, Leung J, Giraudat J, Blatt MR: Alteration of anion chan-nel kinetics in wild-type and abi1-1transgenic Nicotiana ben-thamianaguard cells by abscisic acid.Plant J1997, 12:203-213.
7. Blatt MR: Potassium channel currents in intact stomatal guard cells: rapid enhancement by abscisic acid.Planta1990,
180:445-455.
8. Blatt MR: Reassessing roles for Ca2+in guard cell signalling.
J Exp Botany1999, 50:989-999.
9. McAinsh MR, Brownlee C, Hetherington AM: Calcium ions as sec-ond messengers in guard cell signal transduction.Physiol Plant
1997, 100:16-29.
10. MacRobbie EAC: Signalling in guard cells and regulation of ion channel activity.J Exp Botany1997, 48:515-528.
11. Bootman MD, Berridge MJ, Lipp P: Cooking with calcium: the recipes for composing global signals from elementary events. Cell1997, 91:367-373.
12. Berridge MJ: Neuronal calcium signaling.Neuron1998, 21:13-26.
13. Gilroy S, Read ND, Trewavas AJ: Elevation of cytoplasmic calcium by caged calcium or caged inositol trisphosphate initiates stom-atal closure.Nature1990, 346:769-771.
14. Blatt MR, Thiel G, Trentham DR: Reversible inactivation of K+
chan-nels of Viciastomatal guard cells following the photolysis of caged inositol 1,4,5- trisphosphate.Nature1990, 346:766-769.
15. Parmar PN, Brearley CA: Metabolism of 3-phosphorylated and 4-phosphorylated phosphatidylinositols in stomatal guard cells of Commelina communisl..Plant J1995, 8:425-433.
16. Lee YS, Choi YB, Suh S, Lee J, Assmann SM, Joe CO, Kelleher JF, Crain RC: Abscisic acid-induced phosphoinositide turnover in guard-cell protoplasts of Vicia faba.Plant Physiol1996,
110:987-996.
17. Allen GJ, Muir SR, Sanders D: Release of Ca2+from individual
plant vacuoles by both InsP(3) and cyclic ADP–ribose.Science
1995, 268:735-737.
18. Muir SR, Sanders D: Pharmacology of Ca2+release from red beet
microsomes suggests the presence of ryanodine receptor homologs in higher-plants.FEBS Lett1996, 395:39-42.
19. Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, Chua N-H:
Abscisic acid signaling through cyclic ADP–ribose in plants. Science1997, 278:2126-2130.
20. Leckie CP, McAinsh MR, Allen GJ, Sanders D, Hetherington AM:
•• Abscisic acid-induced stomatal closure mediated by cyclic ADP–ribose.Proc Natl Acad Sci USA1998, 95:15837-15842. This paper represents an important milestone, demonstrating the action of cADPR in promoting Ca2+flux across the tonoplast of guard cells and its
possible link to ABA-mediated increases in [Ca2+] i.
21. Grabov A, Blatt MR: A steep dependence of inward-rectifying
• potassium channels on cytosolic free calcium concentration increase evoked by hyperpolarization in guard cells.Plant Physiol
1999, 119:277-287.
The authors elegantly quantified the dependence of IK,inon voltage-evoked
[Ca2+]iincreases in vivo. Mn2+loading and Fura2-fluorescence quench
were used to demonstrate the role of intracellular Ca2+release. The
phar-22. Grabov A, Blatt MR: Membrane voltage initiates Ca2+waves and
•• potentiates Ca2+increases with abscisic acid in stomatal guard
cells.Proc Natl Acad Sci USA1998, 95:4778-4783.
This is a seminal work that links plasma-membrane voltage to oscillations of [Ca2+]iin guard cells. The data implicate the function of a plasma-membrane
Ca2+-channel that is activated at negative voltages and demonstrate an action
of ABA in altering the kinetics and voltage-dependence of these evoked [Ca2+]iincreases. A report of an ABA-sensitive, hyperpolarisation-activated
Ca2+channel at the guard-cell plasma membrane will follow shortly.
23. Pottosin II, Dobrovinskaya OR, Muniz J: Cooperative block of the
• plant endomembrane ion channel by ruthenium red.Biophys J
1999, 77:1973-1979.
This is an excellent, quantitative analysis of ruthenium-red block of the ‘slow-vacuolar’ channel of Beta vulgaris. The characteristics of this blockage are surprisingly similar to those of many ruthenium red- and ryanodine-sensitive Ca2+-release channels in mammalian tissues. The data thus raise a question
about targeting of ryanodine action and, possibly, cADPR at the tonoplast. 24. Bewell MA, Maathuis FJM, Allen GJ, Sanders D: Calcium-induced
calcium release mediated by a voltage-activated cation channel in vacuolar vesicles from red beet.FEBS Lett1999, 458:41-44.
25. Klusener B, Boheim G, Liss H, Engelberth J, Weiler EW:
Gadolinium-sensitive, voltage-dependent calcium release chan-nels in the endoplasmic reticulum of a higher-plant mechanore-ceptor organ.EMBO J1995, 14:2708-2714.
26. Klusener B, Weiler EW: A calcium-selective channel from root-tip endomembranes of garden cress.Plant Physiol1999,
119:1399-1405.
27. Muir SR, Sanders D: Inositol 1,4,5-trisphosphate-sensitive Ca2+
release across nonvacuolar membranes in cauliflower.Plant Physiol1997, 114:1511-1521.
28. Thiel G, Homann U, Plieth C: Ion channel activity during the action potential in Chara: new insights with new techniques.J Exp Botany 1997, 48:609-622.
29. Thiel G, Dityatev AE: Transient activity of excitatory Cl-channels in
• Chara: evidence for quantal release of a gating factor.J Membr Biol1998, 163:183-191.
The authors use data from concurrent whole-cell voltage clamp and patch clamp recordings to demonstrate that Cl-channels are activated in discrete
subpopulations, each with a fixed amplitude. The results suggest a localised, quantal action of a control factor and its release in close proximity to the plasma membrane.
30. Biskup B, Gradmann D, Thiel G: Calcium release from InsP(3)-sen-sitive internal stores initiates action potential in Chara.FEBS Lett
1999, 453:72-76.
31. Plieth C, Sattelmacher B, Hansen UP, Thiel G: The action potential
•• in Chara: Ca2+release from internal stores visualized by Mn2+
-induced quenching of fura-dextran.Plant J1998, 13:167-175. An elegant demonstration showing that the rise in [Ca2+]ithat creates the
action potential across the Charaplasma membrane draws on Ca2+release
from non-vacuolar stores. The authors make use of Mn2+ fluorescence
quench of cytosolic Fura2-dextran and Mn2+microinjections to discriminate
between vacuolar and non-vacuolar pools. The results also raise some intriguing questions about the coupling of membrane voltage to intracellular Ca2+release. Compare these results with those of Grabov and Blatt [22••].
32. Meyer T, Stryer L: Calcium spiking.Annu Rev Biophys Biophys Chem1991, 20:153-174.
33. Neher E: Vesicle pools and Ca2+microdomains: new tools for
understanding their roles in neurotransmitter release.Neuron
1998, 20:389-399.
34. Dolmetsch RE, Lewis RS, Goodnow CC, Healy JI: Differential acti-vation of transcription factors induced by Ca2+response
ampli-tude and duration.Nature1997, 386:855-858.
35. Dolmetsch RE, Xu KL, Lewis RS: Calcium oscillations increase the efficiency and specificity of gene expression.Nature1998,
392:933-936.
36. DeKoninck P, Schulman H: Sensitivity of CaM kinase II to the
• frequency of Ca2+oscillations.Science1998, 279:227-230. The authors present a remarkable synthesis of molecular, biochemical and biophysical techniques to demonstrate the modulation of protein kinase activity by an oscillating [Ca2+]ienvironment. The activity of immobilised
37. Bauer CS, Plieth C, Hansen UP, Sattelmacher B, Simonis W, Schonknecht G: Repetitive Ca2+spikes in a unicellular green alga.
FEBS Lett1997, 405:390-393.
38. Bauer CS, Plieth C, Bethmann B, Popescu O, Hansen UP, Simonis W, Schonknecht G: Strontium-induced repetitive calci-um spikes in a unicellular green alga.Plant Physiol1998,
117:545-557.
39. Ehrhardt DW, Wais R, Long SR: Calcium spiking in plant-root hairs responding to Rhizobium nodulation signals.Cell1996,
85:673-681.
40. McAinsh MR, Webb AAR, Taylor JE, Hetherington AM: Stimulus-induced oscillations in guard cell cytosolic-free calcium.Plant Cell1995, 7:1207-1219.
41. Webb AAR, McAinsh MR, Mansfield TA, Hetherington AM: Carbon dioxide induces increases in guard cell cytosolic free calcium. Plant J1996, 9:297-304.
42. Staxen I, Pical C, Montgomery LT, Gray JE, Hetherington AM,
• McAinsh MR: Abscisic acid induces oscillations in guard-cell cytosolic free calcium that involve phosphoinositide-specific phospholipase C.Proc Natl Acad Sci USA1999, 96:1779-1784. This is a notable addition to the literature on [Ca2+]
iand ABA action in
guard cells. The authors demonstrate ABA-evoked oscillations in [Ca2+] i
and link their occurrence to Ca2+ release from
inositol-1,4,5-trisphos-phate-sensitive stores.
43. Thiel G, MacRobbie EAC, Blatt MR: Membrane transport in stom-atal guard cells: the importance of voltage control.J Membr Biol
1992, 126:1-18.
44. Gradmann D, Blatt MR, Thiel G: Electrocoupling of ion transporters in plants.J Membr Biol1993, 136:327-332.
45. Blatt MR, Thiel G: K+channels of stomatal guard cells: bimodal
control of the K+inward-rectifier evoked by auxin.Plant J1994,
5:55-68.
46. Louguet P, Coudret A, Couotgastelier J, Lasceve G: Structure and ultrastructure of stomata.Biochemie Physiologie Pflanzen1990,
186:273-287.
47. Wolfe J, Steponkus PL: Mechanical-properties of the plasma-membrane of isolated plant-protoplasts — mechanism of hyper-osmotic and extracellular freezing-injury.Plant Physiol1983,
71:276-285.
48. Wolfe J, Steponkus PL: The stress-strain relation of the plasma-membrane of isolated plant-protoplasts.Biochim Biophys Acta
1981, 643:663-668.
49. MacRobbie EAC: Vesicle trafficking: a role in trans-tonoplast ion movements?J Exp Botany 1999, 50:925-934.
50. Homann U: Fusion and fission of plasma membrane material
• accommodates for osmotically induced changes in the surface area of guard cell protoplasts.Planta1998, 206:329-333. The authors provide the first direct evidence of homeostatic endo/exocytot-ic vesendo/exocytot-icle traffendo/exocytot-ic at the guard-cell plasma membrane in response to osmotendo/exocytot-ic gradients. Intriguingly, the data do not support a role for [Ca2+]iin
control-ling these events.
51. Kubitscheck U, Homann U, Thiel G: Osmotically-evoked shrinking
•• of guard cell protoplasts causes retrieval of plasma membrane into the cytoplasm.Planta2000, 210:423-431.
This study represents a significant technical advance in the analysis of mem-brane trafficking in guard cells in vivo. The authors combined membrane capacitance and styryl-dye fluorescence recordings to examine the dynam-ics of exocytotic and endocytotic events at the plasma membrane. They found a rapid internalisation of membrane in response to hyperosmotic steps, which raises some interesting questions about membrane recycling during stomatal movements.
52. Angleson JK, Betz WJ: Monitoring secretion in real time: capaci-tance, amperometry and fluorescence compared.Trends Neurosci
1997, 20:281-287.
53. Penner R, Neher E: The patch-clamp technique in the study of secretion.Trends Neurosci1989, 12:159-163.
54. Thiel G, Kreft M, Zorec R: Unitary exocytotic and endocytotic events in Zea maysL. coleoptile protoplasts.Plant J1998,
13:117-120.
55. Smith CB, Betz WJ: Simultaneous independent measurement of endocytosis and exocytosis.Nature1996, 380:531-534.
56. Leyman B, Geelen D, Quintero FJ, Blatt MR: A tobacco syntaxin with
•• a role in hormonal control of guard cell ion channels.Science
1999, 283:537-540.
This paper describes the first application of expression-cloning in Xenopus
oocytes to screen for a plant hormone receptor, with a secondary screen yielding the syntaxin Nt-Syr1 from tobacco. The molecular, biochemical and electrophysiological evidence presented suggests that Nt-Syr1 possibly functions as part of a signalling protein complex coupling the ABA stimulus to control of guard-cell K+and Cl-channels. Structural homologies suggest
a parallel function for Nt-Syr1 in vesicle trafficking, although this role remains to be established.
57. Cutler S, Ghassemian M, Bonetta D, Cooney S, McCourt P: A pro-tein farnesyl transferase involved in abscisic acid signal trans-duction in Arabidopsis.Science1996, 273:1239-1241.
58. Zerial M, Huber LA: Small GTPases. Oxford: Oxford University Press; 1995.
59. Kaibuchi K, Kuroda S, Amano M: Regulation of the cytoskeleton and cell adhesion by the Rho family GTPases in mammalian cells.Annu Rev Biochem1999, 68:459-486.
60. Lin YK, Wang YL, Zhu JK, Yang ZB: Localization of a Rho GTPase implies a role in tip growth and movement of the generative cell in pollen tubes.Plant Cell1996, 8:293-303.
61. Kim M, Hepler PK, Fun SO, Ha KS, Lee Y: Actin filaments in mature guard cells are radially distributed and involved in stomatal movement.Plant Physiol1995, 109:1077-1084.
62. Hwang JU, Suh S, Yi HJ, Kim J, Lee Y: Actin filaments modulate both stomatal opening and inward K+-channel activities in guard
cells of Vicia faba L.Plant Physiol1997, 115:335-342.
63. Liu K, Luan S: Voltage-dependent K+channels as targets of
osmosensing in guard cells.Plant Cell1998, 10:1957-1970.
64. Pei ZM, Ghassemian M, Kwak CM, McCourt P, Schroeder JI: Role of
• farnesyltransferase in ABA regulation of guard cell anion channels and plant water loss.Science1998, 282:287-290. The authors combined genetics, pharmacology and electrophysiology to gather evidence of Era1 farnesyl-transferase’s role in ABA-related sig-nalling of guard cells. The substrate that is farnesylated has not yet been identified.
65. Jacob T, Ritchie S, Assmann SM, Gilroy S: Abscisic acid signal
• transduction in guard cells is mediated by phospholipase D activity.Proc Natl Acad Sci USA1999, 96:12192-12197.
The data presented in this paper suggest a role for phospholipase D and its metabolite phosphatidic acid in the regulation of guard-cell K+channels and
stomatal movements by ABA.
66. Ritchie S, Gilroy S: Abscisic acid signal transduction in the barley aleurone is mediated by phospholipase D activity.Proc Natl Acad Sci USA1998, 95:2697-2702.
67. Siddhanta A, Shields D: Secretory vesicle budding from the trans-Golgi network is mediated by phosphatidic acid levels.J Biol Chem 1998, 273:17995-17998.
68. Martin TFJ: Phosphoinositides as spatial regulators of membrane traffic.Curr Opin Neurobiol1997, 7:331-338.
69. Williger BT, Ostermann J, Exton JH: Arfaptin 1, an ARF-binding pro-tein, inhibits phospholipase D and endoplasmic reticulum Golgi protein transport.FEBS Lett1999, 443:197-200.
70. Martin TFJ: Stages of regulated exocytosis.Trends Cell Biol1997,
7:271-276.
71. Hanson PI, Heuser JE, Jahn R: Neurotransmitter release — four years of SNARE complexes.Curr Opin Neurobiol1997,
7:310-315.
72. Blatt MR, Leyman B, Geelen D: Molecular events of vesicle traf-ficking and control by SNARE proteins in plants.New Phytol
2000, 44:389-418.
73. Jahn R, Sudhof TC: Membrane fusion and exocytosis.Annu Rev Biochem1999, 68:863-911.
auxin efflux carrier PIN1 by GNOM ARF GEF.Science1999,
286:316-318.
This is a remarkable study of the function of the Arabidopsis GNOM gene product, a putative ARF–GTPase GTP–GDP exchange factor involved in Pin1 protein distribution at the plasma membrane. Disrupting GNOM function by mutation of the GNOMgene or by treatments with the ARF–GEF antagonist brefeldin A causes a rapid loss of Pin1 locali-sation to the basipetal region of the plasma membrane. The results high-light both the spatial localisation of a specific membrane protein (i.e. Pin1) within the cell and the dynamic nature of the processes that underpin the localisation.
76. Morris DA, Robinson JS: Targeting of auxin carriers to the plasma membrane: differential effects of brefeldin A on the traffic of auxin uptake and efflux carriers.Planta1998, 205:606-612.
77. Sheng ZH, Rettig J, Takahashi M, Catterall WA: Identification of a syntaxin-binding site on N-type calcium channels.Neuron1994,
13:1303-1313.
78. Stanley EF, Mirotznik RR: Cleavage of syntaxin prevents G-protein regulation of presynaptic calcium channels.Nature1997,
385:340-343.
protein–protein interactions.Proc Natl Acad Sci USA1998,
95:10972-10977.
80. Lelianova VG, Davletov BA, Sterling A, Rahman MA, Grishin EV, Totty NF, Ushkaryov YA: alpha-Latrotoxin receptor, latrophilin, is a novel member of the secretin family of G protein-coupled recep-tors.J Biol Chem1997, 272:21504-21508.
81. Fujita Y, Shirataki H, Sakisaka T, Asakura T, Ohya T, Kotani H, Yokoyama S, Nishioka H, Matsuura Y, Mizoguchi A et al.: Tomosyn: a syntaxin-1-binding protein that forms a novel complex in the neu-rotransmitter release process.Neuron1998, 20:905-915.
82. Dai JW, Sheetz MP, Wan XD, Morris CE: Membrane tension in swelling and shrinking molluscan neurons.J Neurosci1998,
18:6681-6692.
83. Leung J, Giraudat J: Abscisic acid signal transduction.Annu Rev Plant Physiol Mol Biol1998, 49:199-222.
84. Hamilton D, Hills A, Köhler B, Blatt M: Ca2+channels at the plasma