A theoretical approach to the genesis of cell layer arrangements in
undifferentiated tissues
Jens Wegner *
Department of Plant Breeding,Faculty of Agriculture and Horticulture,Humboldt Uni6ersity of Berlin,Wendenschloßstr.254,
12557Berlin,Germany
Received 26 May 1999; received in revised form 6 December 1999; accepted 7 December 1999
Abstract
Cell layer arrangements in undifferentiated tissues result from tissue-internal stress generated by a high accumulation of undifferentiated cells. The development of the tunica corpus structure in higher plants is used as an example to explain this thesis. The conditions on which this structure will develop are described. The crucial mark from which a tunica layer in an idealized apex will develop is calculated. This tunica structuring point of 3.85 cell units is a natural constant. A mathematical formula is deduced to determine the existing number of tunica layers in a shoot apex at any given moment. In recent years, a number of genes seen in causal connection with the meristem development could be identified. However, the assumption that the genesis of the layered structure of the shoot apex is also genetically fixed can be refuted with this theory. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cell layer arrangements; Tunica corpus structure; Tunica numbers; Tunica structuring point
www.elsevier.com/locate/plantsci
1. Introduction
The in vitro culture of tissues and organs has reached an advanced stage and even from single cells tissues have been successfully generated but the processes which determine the plane of cell division, and therefore result in the genesis of particular cell arrangement patterns, are unknown. However, there are numerous investigations giving evidence that physical factors are responsible for (or at least strongly modify) the determination of the plane of cell division [1 – 8]. More recently, a number of identified genes are seen in connection with the development, function and cell fate of apical meristems (for review see [9 – 11]). This pa-per explains the processes responsible for cell ar-rangement patterns in undifferentiated tissues by
using the shoot apex as an example. It will be shown that a genetic fixation of the genesis of these cell layer arrangements can be refuted.
Most of the higher plants are characterized by a multi-layered cell structure in their shoot tips (apex). Schmidt [12] and Buder [13] divided the shoot apex into the tunica and the corpus. While cells of the tunica divide in anticlinal direction (=at right angles to the surface) only, cells of the corpus divide anticlinal as well as periclinal (=
parallel to the surface). Because of this distinctive behavior in cell division, the layered structure of the shoot apex can be seen in median longitudinal sections (Fig. 1). Satina et al. [14] termed these layers L1, L2 and L3 from the outside to the inside. Distinction between these cell layers is espe-cially important if these layers bear different ge-netic information.
The tunica-corpus structure develops in the course of ontogenesis. It is currently assumed that during vegetative growth a specific number of cell layers will develop before this cell layer arrange-* Present address: Laboratory of Plant Genetics, Faculty of
Agri-culture, Tokyo University of Agriculture and Technology, 3-5-8 Saiwai-cho Fuchu-shi, Tokyo 183-8509, Japan. Tel./fax: + 81-42-3675625.
E-mail address:[email protected] (J. Wegner)
Fig. 1. Median longitudinal section through a shoot tip of
Pelargonium zonale ‘Kleiner Liebling’. The hemisphere-like shoot tip shows a multi-layered arrangement of cells on the outside; in the center, however, no such cell layers can be found.
ment disappears with the cessation of vegetative growth. Until now there has been no theory that explains the genesis of this layered arrangement of cells in the shoot tip.
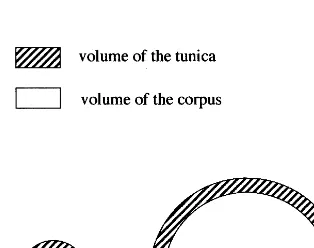
Bergann [15] assumed that pressure and ten-sion in the shoot apex change with the apex’s switch from the embryonic stage to its vegetative and flowering state. His theory is based upon comparison of investigations of different authors. According to Schu¨epp [16] the outer layers of the shoot apex are under tangential pressure. Furthermore, these layers grow faster so that they fold up in what results as in the generation of leaf primordias. Schneider [17] investigated embryonic meristems which were still without leaves and he could not find any sign of tangen-tial pressure. Snow and Snow [18] cut apices and found that the apices gaped immediately. From that the authors concluded that the L1 must be under tangential tension. Finally, Ball [19] cut wedges out of apices which afterwards fit per-fectly into the cut and so he concluded that there is neither tension nor pressure in the apex. Bergann [15,20] undertook a mathematical consideration to bring these apparent contradic-tory findings in line. Bergann employed a hemi-sphere as a model of an idealized apex. When the apex is divided into the tunica and the cor-pus, the tunica is equivalent to the mantle of the entire hemisphere and the corpus corresponds to an inner hemisphere. Bergann pointed out — on condition that the tunica is of constant thickness — that when the corpus radius is small, the tu-nica has a larger volume than the corpus. When the corpus diameter is large, however, the corpus then has a larger volume than the tunica. Fi-nally, there must be one point where both have the same volume (Fig. 2).
Assuming that all apical cells are of the same size and frequency of division, a big corpus will put the tunica under strong pressure resulting in tangential tension in the tunica (Fig. 3). There-fore, periclinal divisions in tunica cells are sup-pressed. From that Bergann drew conclusions on the formation of leaves which cannot be agreed with. Attempting a different theoretical ap-proach, Bergann’s corpus pressure theory shall be taken up again, leading to the idea of the nature of cell layer arrangement patterns in tis-sues.
Fig. 2. Comparison of two theoretical apices. While in both apices the tunica is of the same thickness the radius of the corpus is different. This results in an opposite volume ratio of tunica and corpus in these apeces.
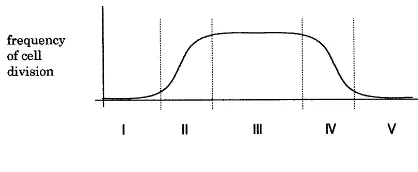
Fig. 4. The development of the tunica corpus structure in the course of ontogenesis on the basis of the general growth curve. Phase I: activity of cell division is low (the plant is a young seedling); no development of a tunica corpus structure. Phase II: high increase in activity of cell division; between cell division and expansion a certain amount of time passes which results in a disproportional accumulation of meristematic cells; genesis of the tunica corpus structure. As long as the pace of cell division is higher than the pace of cell differentia-tion the number of tunica layers will increase. Phase III: rate of cell division is equal to the rate of cell differentiation; the number of tunica layers is constant. Phase IV: decrease in the activity of cell division; the pace of division is lower than the pace of cell differentiation; tunica layers decrease in number and the tunica corpus structure eventually disappears. Phase V: cell divisions come to an end; the remaining meristematic cells differentiate and so the meristem will be used up.
sionally which means as a unit of space and time. Fig. 2 explains the spatial relation between tunica and corpus and Fig. 4 explains the timewise devel-opment in the course of ontogenesis on the basis the general growth curve.
On condition that there are no external effects and differentiation does not start in meristematic cells, the shoot apex will naturally form a hemisphere.
Excursus: When looking over different plant species, apices of various kinds can be found. Beside the hemispherical ones conical or flattened apices can be found as well. Among others, Steeve and Sussex [21] give a survey of a variety of known apex forms. Then, what justifies the as-sumption that a theoretical apex will naturally form a hemisphere?
The understanding of what the apex comprises varies greatly and stretches from the meristematic cell group to the shoot tip including the leaf primordias. Here, the apex is reduced to an accu-mulation of meristematic cells. In principle, undif-ferentiated cells can divide in any direction. If cell divisions continue over a certain period of time (and neither cell enlargement nor differentiation sets in) this accumulation will take on the shape of a sphere. At the shoot apical meristem, however, cells are differentiated into the shoot axis and therefore, the meristem can form a hemisphere, only. A deviation of the apical meristem from the hemisphere is caused by inner or outer sources of interference. Inner sources of interference are en-largement and differentiation of meristematic cells and an outer source of interference is, e.g. neigh-boring tissue. A conical apex will be formed by the enlargement of central apical cells or by pressure from outside generated by leaf primordias sessile to the shoot tip. A flat apex develops if leaf primordias are differentiated at a very early stage so that these leaf primordias grow above the meristematic cell group. (At this point a compari-son to the meristem of the root tip suggests that at the very tip of the root no secondary roots are differentiated whereas cell enlargement sets in soon. Because of that, the conical shape of the root tip is formed.) For the purpose of clarity, all sources of interference and possible special cases must be left aside. Therefore, the assumption that the shoot apical meristem will naturally form a hemisphere is justified.
2. The theory
Theoretical assumptions concerning the shoot apex:
Following Bergann:
1. All apical cells are of the same size In contrast to Bergann:
2. The apex is looked at purely histologically with no respect to further development of apical cells
3. The layers of the tunica are independent from one another which means that the layers of a multi-layered tunica must not be compared to the corpus as a unit but each layer separately (of course, a tunica far outside is always of larger volume than the corpus. However, the several other tunica layers between the outer tunica and the corpus exert pressure as well. That means, in this case the volume of the inner tunica layers must be added to the corpus).
4. Reproduction of the shoot apex structure in axillary buds is not a criteria to discriminate the tunica and the corpus (as it was essential to Bergann)
dimen-First, the point of equal volume of the tunica and the corpus shall be calculated. The volume of a hemisphere is calculated according to the formula:
V=2
3p×r
3
To calculate the mantle volume of a hemisphere, the formula:
is used. These formulas will be transcribed for the shoot apex:
radius of the corpus.
VT=
the shoot apex; rC=radius of the corpus.
Both formulas will be equated to find the condi-tion on which the tunica and the corpus are of the same volume:
The radius of the shoot apex represents the sum of the corpus radius plus the thickness of the tunica. This can be described by the formula:
rA=rC+dT
where dT=thickness of the tunica.
For a single layered tunica, the radius of the corpus will be calculated as follows:
rA=rC+dT
That means a single layered tunica is of equal volume to its corpus if the corpus has a radius of 3.85 cell units. The mark of 3.85 cell units repre-sents the equilibrium of the tunica and the corpus, which means that every complete cell layer beyond this mark will behave like a tunica. Therefore the mark of 3.85 cell units can be termed the tunica structuring point. From that, the formula for cal-culating the number of tunica layers existing in a specific apex can be deduced:
nT=
rA−3.85dCell dCell
wherenT=number of tunica layers;rA=radius of
the shoot apex; dCell=diameter of the cell (in
direction of the radius).
Since the tunica structuring point is a crucial mark, the calculated number of tunica layers must always be rounded down. It follows that:
rAB(3.85+1)dCell=no tunica
(3.85+1)dCellrAB(3.85+2)dCell=one tunica
(3.85+2)dCellrAB(3.85+3)dCell=two tunicas
(3.85+3)dCellrAB(3.85+4)dCell
=three tunicas, etc.
3. Discussion
To calculate the equilibrium mark of the tunica and the corpus, the radius of the apex (rA) and the
corpus (rC), as well as the thickness of the tunica
(dT), have been calculated as cell units. Of course, all terms may also be measured in mm. That means, however, that the values are specific for each apex, and therefore the naturally constant value (3.85dCell) of the tunica structuring point is
not recognizable (in fact, the derivation of the tunica structuring point is analogous to the deriva-tion of the number p). The formula to calculate the existing number of tunica layers could also be:
nT=
In empirical investigations rA and dCell can be
measured, rC cannot. Therefore it may be
sug-gested that rC is replaced by 3.85 dCell.
Soon after the tunica corpus concept was estab-lished by Schmidt [12] attempts have been made to determine a fixed number of tunica layer for each species. When it comes to interpreting microscopic pictures, however, it is especially difficult to sepa-rate the tunica from the corpus since cell division can not be observed directly, but is retrospectively concluded from the pattern of cell arrangement. Another way of determining the number of apical layers is the analysis of periclinal chimeras. Using this method, Bergann [22,23], Bergann and Bergann [24,25], Pohlheim [26 – 28] and Tilney-Bassett [29] describe the apex of Pelargonium
zonale as three-layered. However, Stewart et al.
[30] could show the existence of five apical layers by employing the same analytical method. Today it is well-known that the number of tunica layers may vary within one species as well as during ontogenesis. The presented theory describes the genesis of the shoot apex as a continuum by which it becomes possible to bring seemingly contradic-tory findings in line.
The proportion of apical cell size to the size of the belonging apex not only plays a decisive role in the development of the tunica-corpus structure as such, but also in the number of tunica layers at any given moment. Since the layered apex struc-ture is not the cause but the result of inner apical conditions of stress, it becomes clear that a gene for the development of this structure cannot exist. From the simple fact that periclinal division can be found in the tunica and that these periclinal divisions occur in inner tunica layers more often than in outer tunicas it may be concluded that the genesis of layered structures in undifferentiated tissues is not a genetic but a mechanical question. The apparent genetic fixation of this structure is illogical because its development is dependent on the accumulation of meristematic cells, which itself may be genetically fixed. In fact, a number of genes have been seen in causal connection with the development of the shoot apical meristem (for review see [9 – 11]). All these investigations, how-ever, show only that meristematic cells are accu-mulated to a different degree or that the kind or the pace of differentiation, respectively, is encoded genetically. In the most extreme case, cell divisions cease as early as prior to apical meristem
forma-tion [31,32]. Mayer et al. [33] found gurke gene mutations occurring to different degrees which found expression accordingly in altered meristem development. However, the opposite case has also been documentated where due to overprolifera-tion, the apical meristem is abnormally large [34]. Finally, e.g. Mandel et al. [35] and Mandel and Yanowsky [36] identified a genetic encoding for meristem fate with regard to the timing of the switch from the vegetative to the floral meristem. All these findings, however, cannot directly be regarded as causal connected with the develop-ment of a layered arrangedevelop-ment of apical cells in the shoot meristem.
Recognizing that the tunica formation depends on pressure and tension, it logically follows that a tunica layer can never be of a larger volume than its belonging corpus: the moment the tunica vol-ume exceeds the volvol-ume of the corpus, the tangen-tial tension inside the tunica will change into tangential pressure and radial tension will come from the corpus instead. Subsequently, tunica cells divide periclinal and thus tunica cells behave like corpus cells. Since a change of outer and inner conditions causes a cell to change from corpus to tunica behavior and vice versa, it seems adequate to refer to the tunica-corpus structure as primarily unstable. Stability is a merely secondary result.
divi-sion, the outermost tunica layers would be lacer-ated; tunica perforations, however, occur rather seldom. Besides that apices are often squeezed into leaf primordias which may be seen as an outer source of interference. It can be concluded that the inner-apical forces do not spread out equally. Hence, the so far assumed layered structure must be dissected into single cells, and if their periclinal divisions are prevented they must be defined as belonging to the tunica. The termination of these apical layers with Ln, as introduced by Satina et al. [14], may be upheld if ‘L’ (=layer) is not strictly regarded as a complete and stable layer, but rather a cell clone which may temporarily appear as a layer.
The tunica corpus structure has served as an example to explain the theory of the genesis of cell layer arrangements. This multi-layered arrange-ment of meristematic cells has especially attracted attention because of its relatively high stability. If these layers bear different genetic information (=
chimeras), plants with a fairly stable coexistence of genetically different tissues can develop. Beside the shoot apex a number of further examples — like calli and tumors — can be found, which also can be described as an accumulation of undifferenti-ated cells. Comparing them to different geometri-cal models and considering specific developmental conditions, the same basic course of development may be expected.
Acknowledgements
I thank F. Pohlheim and E. Thomas of the Humboldt University of Berlin, K. Klopfer of the University of Potsdam and Y. Hirata of the Tokyo University of Agriculture and Technology for discussions. I received financial support from the state of Berlin and the FAZIT-Stiftung Frankfurt.
References
[1] L. Errera, Ueber Zellformen und Seifenblasen, Bot. Zbl. 34 (1888) 395 – 398.
[2] L. Kny, Ueber den Einfluss von Zug und Druck auf die Richtung der Scheidewa¨nde in sich theilenden Pflanzen-zellen (Zweite Mittheilung), Jahrb. Wiss. Bot. 37 (1902) 55 – 98.
[3] M.M. Yeoman, R. Brown, Effects of mechanical stress on the plane of cell division in developing callus cultures, Ann. Bot. 35 (1971) 1101 – 1112.
[4] P.M. Lintilhac, Differentiation, organogenesis and the tectonics of cell wall orientation. III. Theoretical consid-erations of the cell mechanics, Am. J. Bot. 3 (1974) 230 – 237.
[5] B.A. Palevitz, P.K. Hepler, The control of the plane of division during stomatal differentiation in Allium. I. Spindle reorientation, Chromosoma (Berl.) 46 (1974) 297 – 326.
[6] B.F. Wilson, R.R. Archer, Reaction wood: induction and mechanical action, Ann. Rev. Plant Physiol. 28 (1977) 23 – 43.
[7] J.H. Miller, Orientation of the plane of cell division in fern gametophytes: the role of cell shape and stress, Am. J. Bot. 67 (1980) 534 – 542.
[8] T.J. Cooke, D.J. Paolillo Jr, The control of the orienta-tion of cell division in fern gametophytes, Am. J. Bot. 67 (1980) 1320 – 1333.
[9] I.M. Sussex, Developmental programming of the shoot meristem, Cell 56 (1989) 225 – 229.
[10] J.I. Medford, Vegetative apical meristems, Plant Cell 4 (1992) 1029 – 1039.
[11] E.M. Meyerowitz, Genetic control of cell division pat-terns in developing plants, Cell 88 (1997) 299 – 308. [12] A. Schmidt, Histologische Studien an phanerogamen
Vegetationspunkten, Bot. Arch. 8 (1924) 345 – 404. [13] J. Buder, Der Bau des phanerogamen
Sproßvegetationspunktes und seine Bedeutung fu¨r die Chima¨rentheorie, Ber. Dtsch. Bot. Ges. 46 (1928) 20 – 21. [14] S. Satina, A.F. Blakeslee, A.G. Avery, Demonstrations of the three germ layers in the shoot apex ofDatura by means of induced polyploidy in periclinal chimeras, Am. J. Bot. 30 (1940) 895 – 905.
[15] F. Bergann, Die Sproßvariation als anatomisches, ent-wicklungsphysiologisches und zu¨chterisches Problem. Habilitationsschrift, Karl-Marx-Universita¨t Leipzig, Landwirtschaftlich-Ga¨rtnerische Fakulta¨t, 1955. [16] O. Schu¨epp, U8 ber den Nachweis von
Gewebespannun-gen in der Sproßspitze, Ber. Dtsch. Bot. Ges. 35 (1917) 703 – 706.
[17] E. Schneider, U8 ber die Gewebespannung der Vegetation-spunkte, Ber. Dtsch. Bot. Ges. 44 (1926) 326 – 328. [18] M. Snow, R. Snow, On the determination of leaves, New
Phytologist 46 (1947) 5 – 19.
[19] E. Ball, Isolation, removal and attempted transplants of the central portion of the shoot apex ofLupinus albusL., Am. J. Bot. 37 (1950) 117 – 136.
[20] F. Bergann, Zur Theorie des angiospermen Vegetations-punktes und zur Peridermbildung, Ber. Dtsch. Bot. Ges. 68 (1955) 29 – 30.
[21] T.A. Steeves, I.A. Sussex, Patterns in Plant Develop-ment, second edition, Cambridge University Press, Cam-bridge, 1989.
[22] F. Bergann, Praktische Konsequenzen der Chima¨ren-forschung fu¨r die Pflanzenzu¨chtung, Wiss. Z. d. Karl-Marx-Universita¨t Leipzig 4 (1954) 281 – 291.
[24] F. Bergann, L. Bergann, U8ber experimentell ausgelo¨ste vegetative Spaltungen und Umlagerungen an chi-ma¨rischen Klonen, zugleich ein Beispiel erfolgreicher Staudenauslese. I. Pelargonium zonale Ait. ‘Madame Salleron’, Zu¨chter 29 (1959) 361 – 374.
[25] F. Bergann, L. Bergann, U8 ber Umschichtungen (Translokationen) an den Sproßscheiteln periklinaler Chima¨ren, Zu¨chter 32 (1962) 110 – 119.
[26] F. Pohlheim, Untersuchungen zur periklinalchima¨rischen Konstitution vonPelargonium zonale‘Freak of Nature’, Flora 162 (1973) 284 – 294.
[27] F. Pohlheim, Untersuchungen an der Trichima¨re
Pelargonium zonale ‘Freak of Nature’ ein Beitrag zur Herstellung von Plastommutanten, Wiss. Z. d. Pa¨d. Hochschule Potsdam 21 (1977) 115 – 127.
[28] F. Pohlheim, Vergleichende Untersuchungen zur A8nderung der Richtung von Zellteilungen in Blattepider-men, Biol. Zentralbl. 3 (1983) 323 – 336.
[29] R.A.E. Tilney-Bassett, Plant Chimaras, Edward Arnold, London, 1986.
[30] R.N. Stewart, P. Semeniuk, H. Dermen, Competition and accommodation between apical layers and their derivatives in the ontogeny of chimerical shoots of
Pelargonium x hortorum, Am. J. Bot. 61 (1974) 54 – 67.
[31] J.A. Long, E.I. Moan, J.I. Medford, M.K. Barton, A member of the KNOTTED class of homeodomain proteins encoded by the STM gene ofArabidopsis, Na-ture 379 (1996) 66 – 69.
[32] E. Souer, A.v. Houwelingen, D. Kloos, J. Mol, R. Koes, The No apical meristem gene of petunia is re-quired for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries, Cell 85 (1996) 159 – 170.
[33] U. Mayer, R.A. Torres Ruiz, T. Berleth, S. Misera, G. Ju¨rgens, Mutations affecting body organization in the
Arabidopsisembryo, Nature 353 (1991) 402 – 407. [34] S.T. Clark, S.E. Jacobsen, J.Z. Levin, E.M.
Meyero-witz, The CLAVITA and SHOOT MERISTEMLESS
loci competitively regulate meristem activity in Ara
-bidopsis, Development 122 (1996) 1567 – 1575.
[35] M.A. Mandel, C. Gustafson-Brown, B. Savidge, M.F. Yanowsky, Molecular characterization of theArabidop
-sis floral homeotic gene APETALA1, Nature 360 (1992) 273 – 277.
[36] M.A. Mandel, M.F. Yanowsky, A gene triggering flower formation in Arabidopsis, Nature 377 (1995) 522 – 524.