Recent research indicates that several sulfate transporters — exhibiting different tissue specificities and modes of expression — may play distinct roles in sulfate uptake within specific tissues and in long-distance sulfate translocation. The transcription levels of particular genes and feedback inhibition of serine acetyltransferase play major roles in regulating sulfur assimilation and cysteine synthesis. O-acetylserine and glutathione presumably act within the cysteine synthesis pathway as derepressor and repressor, respectively. A unique autoregulatory mechanism that stabilizes mRNA levels has recently been proposed for the regulation of methionine synthesis.
Addresses
Faculty of Pharmaceutical Sciences, Laboratory of Molecular Biology and Biotechnology, Research Center of Medicinal Resources, Chiba University, Yayoi-cho 1-33, Inage-ku, Chiba 263-8522, Japan; e-mail: [email protected]
Current Opinion in Plant Biology 2000, 3:188–195 1369-5266/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved.
Abbreviations
APS adenosine 5′-phosphosulfate
GSH glutathione
Km Michalis–Menten constant
OAS O-acetylserine Sultr sulfate transporter
Introduction
Sulfur, a macronutrient that is essential for plant growth, is found in two sulfur-containing amino acids, cysteine (Cys) and methionine (Met). In addition, sulfur is contained in a variety of cellular components and plays critical biochemi-cal roles in a number of cellular processes, such as redox cycles, detoxification of heavy metals and xenobiotics, and metabolism of secondary products [1••,2••,3].
In the past few years, remarkable progress has been made in understanding the mechanism and regulation of sulfate transport, sulfate assimilation and the synthesis of sulfur-containing amino acids. This progress has been achieved using molecular cloning and transgenic technology [1••,2••,3,4]. In this review, I describe what is known about
the molecular regulation of the sulfur-assimilation system with particular emphasis on recent progress.
Sulfate transport
Sulfur is taken up by plants in its inorganic sulfate form (Figure 1). Sulfate transporters that are localized in the root plasma membrane mediate uptake from external environments (i.e. the soil or apoplasts) into the sym-plastic system [5]. Plasma-membrane transport systems for xylem loading in roots and unloading in leaves are
also necessary. Once inside plant cells, sulfate is trans-ported by organelle-membrane transport systems within the chloroplast envelopes and tonoplast membranes. In each membrane system, transporters or channels that mediate the movement of sulfate in both directions are presumably needed [6].
The cDNA clones encoding plant plasma-membrane sulfate transporters were first isolated from a tropical forage legume, Stylosanthes hamata[7]. Three cDNAs were isolated from this
Regulation of sulfate transport and synthesis of sulfur-containing
amino acids
Kazuki Saito
Figure 1
Biosynthetic pathway for the sulfur-containing amino acids, Cys and Met. The enzymes involved in the pathway are: (a) sulfate transporter;
(b) ATP sulfurylase; (c) APS reductase; (d) sulfite reductase;
(e)OAS(thiol)-lyase [cysteine synthase]; (f)Ser acetyltransferase;
(g)cystathione γ-synthase; (h) cystathione β-lyase; and (i)Met synthase. O-PHS, O-phosphohomoserine. Arrow indicates induction (derepression). Bar indicates inhibition (repression).
APS
S2–
Ser OAS
Cys O-PHS
Aspartate
Threonine Cystathione
Homocysteine
Met Acetyl-CoA
GSH,Cys
Activity inhibition (isoform specific)
mRNA
(isoform specific) SO42– (Extra)
SO32– SO42– (Intra)
Sulfur starvation (a)
(b)
(c)
(d)
(e) (f)
(g)
(h)
(i)
legume each of which encoded a H+/sulfate symporter that
exhibited either high or low affinity for sulfate ions when functionally expressed in yeast. The predicted secondary structures of the sulfate transporter proteins encoded by these cDNAs included 12 membrane-spanning domains, which are typical of cation/solute symporters [7]. Seven cDNAs encod-ing putative sulfate transporters have been isolated and characterized from Arabidopsis thaliana [8–10,11•,12,13••].
Transgenic expression of three of these sulfate transporters (Sultr) cDNAs, Sultr1;1 (AST101), Sultr2;1 (AST68) and Sultr2;2 (AST56), was able to rescue the growth of a yeast mutant that lacked sulfate transporters, suggesting that the proteins encoded by these cDNAs function in the uptake of sulfate. Like the sulfate transporters isolated from S. hamata, these Arabidopsistransporters have variable affinity for sulfate: Sultr1;1 exhibited a high affinity towards the sulfate ion (Km= 3.6µM; where Km is the Michalis–Menten constant,
i.e. the substrate concentration that allows the reaction to pro-ceeed at one-half its maximum rate), whereas Sultr2;1 and Sultr2;2 are low-affinity sulfate transporters (Km= 0.41 mM
and >1.2 mM, respectively) [13••].
The levels of mRNAs that encode the high-affinity sulfate transporters from S. hamata [7], barley [14,15•], A. thaliana
[13••] and maize [16] increased in response to sulfate
starva-tion in roots. The low-affinity transporter mRNA levels were also increased by sulfate deprivation [7,9]. The expression of Sultr1;1 (a high-affinity transporter in A. thaliana) reached its maximum earlier than that of Sultr2;1 (a low-affinity trans-porter in A. thaliana), indicating that the responses of these two transporters to sulfur deficiency are regulated different-ly [13••]. The Sultr1;1gene was expressed in the epidermal
and cortical cells of roots but not in above-ground tissues; whereas the Sultr2;1was found to be expressed in the stele of roots and the vascular tissues of shoots and leaves [9,13••].
These results suggest that Sultr1;1is primarily responsible for the initial uptake of sulfate ions from the rhizosphere in the outermost cell layers, and that its expression is sensitive to the sulfate concentration outside and/or inside of cells. The function of Sultr2;1in roots is presumably to transport apoplastic sulfate into symplasts in the stele, where sulfate leaked from xylem vessels may accumulate. In shoot vascu-lar bundle tissues, Sultr2;1is probably involved in unloading sulfate from xylem vessel into the symplastic system for translocation to the mesophyll cells.
Besides the plasma membrane transporters that mediate the uptake and long-distance transport of sulfate, an intra-cellular transport system for sulfate is also thought to be present. A system that transports sulfate into chloroplasts is necessary because chloroplasts are the almost exclusive sites for the reduction of sulfate into sulfide. The analysis of the transient expression of a fusion protein made up of Sultr4;1 (AST82) and jellyfish green fluorescent protein revealed that Sultr4;1 is a chloroplast-localized protein [11•]. In addition to the H+/sulfate symporter, a
bacterial-type ATP-dependent sulfate permease is also suspected to be present in chloroplasts of higher plants. Although
the vacuole is presumed to be the major compartment for sulfate storage within cells, little information is available on sulfate transporters in tonoplasts [17]. Molecular infor-mation on such intracellular sulfate-transport systems and channels in the plasma membrane [18] is also limited.
Activation and reduction of sulfate
ATP sulfurylase activates sulfate by converting it to adeno-sine 5′-phosphosulfate (APS) (Figure 1b). ATP-sulfurylase activity has been detected in chloroplasts and the cytosol. Indeed, two cDNAs encoding both a chloroplastic and a cytosolic isoform of ATP sulfurylase have been isolated from potato [19]. In A. thaliana, four cDNAs have been iso-lated that all seem to encode chloroplastic forms of ATP sulfurylase [20,21,22••]. The cytosolic isoform of ATP
sul-furylase is presumably produced from one of these four genes by the use of a different translational start codon.
Two reports have described the over-expression of ATP sulfurylase in plants [23•,24•]. Its over-expression in
tobac-co cells had no effect on sulfate influx and sulfate tobac-content, although the ATP-sulfurylase activity in transgenic cells was eight-times higher than that in the cells of wild-type plants [23•]. In contrast, transgenic Brassica juncea that
over-expressed ATP sulfurylase accumulated more glu-tathione (GSH) than normal and exhibited resistance to selenate, which is a toxic analog of sulfate [24•].
The enzymatic step involved in the conversion of APS to sulfite (Figure 1c) has been the subject of debate for a long time. Recent data confirm that this reaction is catalyzed by a single enzyme called APS reductase [25•]. This enzyme is
a GSH-dependent reductase [26•], which used to be called
APS sulfotransferase [27•]. In B. juncea, both ATP
sulfury-lase and APS reductase are known to be induced upon exposure to cadmium [28]. The expression of APS reduc-tase is also regulated by light and diurnal rhythm [29•]. In
maize, ATP-sulfurylase and APS-reductase activities take place almost exclusively in the bundle-sheath cells [30]. Ferredoxin-dependent sulfite reductase catalyzes the six-electron reduction of sulfite to sulfide [31,32] (Figure 1d). Also in maize, distinct electron transfer systems using dif-ferent isoforms of ferredoxin and ferredoxin-NADP+
reductase have been shown to operate in the leaves and roots [33•]. It is interesting to note how the supply of
elec-trons from the photosystems or NADPH to sulfur or nitrogen assimilation is regulated via feredoxin.
Cys synthesis
The final step in cysteine synthesis is the reaction that incorporates a sulfide moiety into the β-position of ala-nine [1••] (Figure 1e). The carbon skeleton is derived
from serine (Ser) via O-acetylserine (OAS) (Figure 1f). Two enzymes, Ser acetyltransferase and OAS(thiol)-lyase [Cys synthase], are involved in this step [1••]. Although
compartments of plant cells, that is the cytosol, chloro-plasts and mitochondria [34–36,37••,38]. In mitochondria,
there is an additional β-cyanoalanine synthase that exhibits similar catalytic activity to that of OAS(thiol)-lyase [39,40••], although cytosolic β-cyanoalanine
synthase activity is subscribed to side activity of OAS(thiol)-lyase (Cys synthase) [41•]. Mitochondrial
β-cyanoalanine synthase was previously identified as the mitochondrial form of OAS(thiol)-lyase [35,40••,42].
It has been shown that cytosolic Ser acetyltransferase is feedback inhibited by Cys at a physiological concentration (2–10µM), but the plastidic and mitochondrial forms are not subject to this regulation [37••]. These results suggest
that feedback inhibition is an important regulatory mecha-nism for OAS levels in the cytosol. Because OAS is presumably a positive regulatory factor that derepresses the genes that encode enzymes involved in sulfur assimilation (see below), the feedback regulation of cytosolic Ser acetyl-transferase by Cys is especially important. The specific residues of Ser acetyltransferase that are responsible for feedback regulation have been identified recently [43•].
Ser acetyltransferase and OAS(thiol)-lyase [44,45] form a bienzyme complex in the chloroplast. OAS(thiol)-lyase con-centrations in the chloroplast are, however, far in excess of Ser-acetyltransferase concentrations indicating that only a fraction of OAS(thiol)-lyase associates with Ser acetyltrans-ferase [46••]. A large amount of OAS(thiol)-lyase is therefore
present in a free form. The bound form of OAS(thiol)-lyase, which has dramatically reduced catalytic activity, seems to modulate the activity of Ser acetyltransferase in the enzyme complex. The free form of OAS(thiol)-lyase is presumably responsible for the actual catalytic function of this enzyme. This is a special mechanism that is responsible for main-taining Cys biosynthesis at full capacity [46••]. In
A. thaliana, the expression of cytosolic OAS(thiol)-lyase is induced by exposure to salt and heavy-metal stress, via a mechanism that probably involves mediation by abscisic acid [47•]. Under these stress conditions, cytosolic
OAS(thiol)-lyase is expressed in the leaf trichomes, suggest-ing a possible connection between this enzyme and detoxification of heavy metals in trichomes [48].
Overexpression of OAS(thiol)-lyase or Ser acetyltrans-ferase in transgenic tobacco conferred increased tolerance of oxidative stress caused by sulfite [49], paraquat (M Noji et al., personal communication) or hydrogen peroxide [50•]. These results suggest that it
may become possible to engineer stress-resistant plants by manipulating Cys synthesis.
Regulatory circuit for sulfate transport,
assimilation and Cys synthesis
It is well known that sulfate uptake and assimilation activ-ities are derepressed under sulfur-deficient conditions. In Arabidopsis, this derepression is correlated with the inducible expression of a particular set of genes that
encode sulfate transporter isoforms, Sultr1;1and Sultr2;1, and APS reductase [9,13••]. As yet, we do not know which
signals are involved in the induction of these genes and how they operate.
OAS is thought to be a positive regulator (i.e. derepressor or inducer) of gene expression in sulfate-starved plants, as it is in bacteria [51]. In fact, OAS has also been shown to induce the expression of genes encoding a high-affinity sulfate transporter in barley, thereby overriding the repressive effect of sulfur-rich nutritional conditions [14]. Similar results have been obtained using Arabidopsis, in which only Sultr1;1and Sultr2;1of seven sulfate-transporter genes were induced by the addition of OAS (Y Hatzfeld et al., personal communica-tion). The expression of mRNA encoding APS-reductase was also induced by OAS in Arabidopsis[52]. In the cotyle-dons of Arabidopsisand soybean, cellular OAS concentration increases under sulfur-starved conditions. The application of OAS to immature soybean cotyledons resulted in the elevat-ed expression of mRNA encoding a sulfur-poor seelevat-ed storage protein; this response is similar to that caused by sulfur star-vation [53•]. In maize cells, addition of OAS resulted in
increased sulfate uptake and ATP-sulfurylase activity [54]. In contrast to the role of OAS as a positive regulator, GSH and Cys are thought to be negative regulators (i.e. repressors) of genes whose expression is regulated by sulfur status [16,55]. Using ‘split-root’ experiments, it was shown that GSH is a phloem-translocated signal molecule that represses the expression of the genes that encode the sulfate trans-porter Sultr2;1and ATP sulfurylase [56,57••].
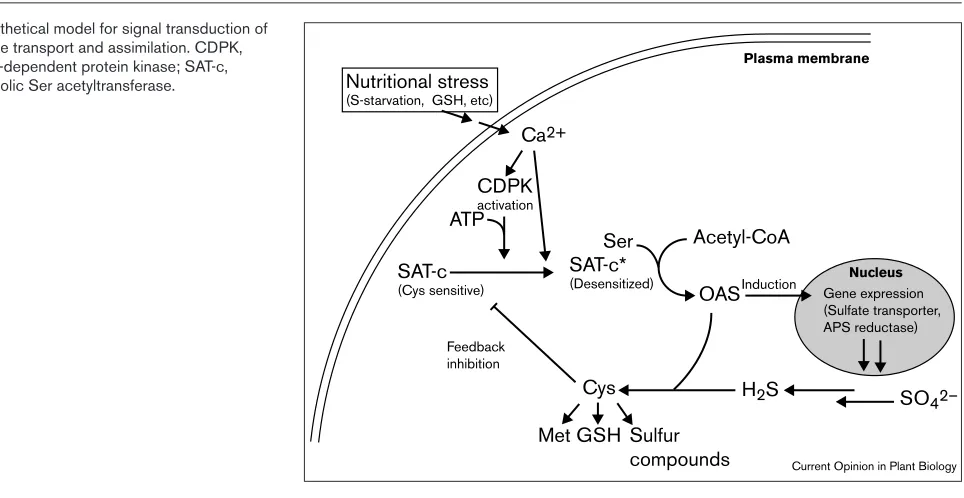
The role of OAS in regulating the entire sulfur assimilation pathway takes us back to the its role in the control of Ser acetyltransferase activity. Given the fact that cytosolic Ser acetyltransferase is regulated by Cys feedback inhibition, this feedback inhibition may be controlled further by cytosolic signal(s) that are induced by a change of sulfur sta-tus. Recent investigations have clearly demonstrated that transient increases in cytosolic Ca2+ concentration are
caused by sulfate-deprivation in Arabidopsis(K Inoue et al., personal communication). In addition, Ca2+ can partially
increase the activity of cytosolic Ser acetyltransferase even in the presence of inhibitory cysteine concentrations. These results suggest a previously unknown role for Ca2+in
desensitizing Ser acetyltransferase to cysteine inhibition. They suggest that cytosolic Ca2+finely modulates the
feed-back regulation of Ser acetyltransferase and ultimately controls cytosolic OAS levels. In soybean, Ca2+-dependent
protein kinase phosphorylates a putative cytosolic Ser acetyltransferase, which is a feedback-inhibited isoform [58]. The resulting phosphorylated enzyme is still active but no longer subject to feedback inhibition by Cys.
levels are kept low, resulting in the repression of the expres-sion of the genes that encode sulfate transporters and APS reducatse. External or internal stimuli such as sulfate defi-ciency or decreasing GSH levels trigger Ca2+mobilization
into the cytosol. Ca2+ partially desensitizes cytosolic Ser
acetyltransferase to Cys inhibition, and Ca2+-dependent
protein kinase phosphorylates this isoform; both of these changes result in increased Ser-acetyltransferase activity and subsequently OAS production. The elevated OAS concen-tration activates the expression of the repressed genes that encode proteins involved in sulfate transport and assmila-tion. This hypothetical mechanism explains the fine modulation of sulfate transport and assimilation that ensures that Cys levels remain constant even prior to a decrease in Cys level caused by sulfate starvation. The molecules that are known to be involved in early signal perception and transduction have been isolated from the unicellualar green algae Chlamydomonas reinhardtiiby mutant analysis [59,60•];
these molecules might also be involved in sulfate sensing in higher plants.
Met synthesis
As shown in Figure 1, Met is synthesized in three steps from Cys and O-phosphohomoserine (which is derived from aspartic acid) [61,62•]. Cystathione γ-synthase catalyzes the
first step (Figure 1g) that involves the β-replacement of the phosphate group of O-phosphohomoserine with Cys to form cystathione. The second enzyme in the pathway is cys-tathione β-lyase (Figure 1h), which catalyzes the cleavage of a β-C-S bond to produce homocysteine. The final step (Figure 1i) is catalyzed by Met synthase, which transfers the methyl group from methyltetrahydrofolate to homocysteine. This reaction is important not only in de novoMet synthesis but also in the recycling of S-adenosylmethionine, which is
a key compound serving as a methyl donor in a variety of methy-transfer reactions.
Plastids contain all of the enzymes involved in the conver-sion of aspartate to homocysteine. Nevertheless, the steps from homocysteine to Met and the recycling of S-adenosyl-methionine are localized in the cytosol. Cystathione γ-synthase has been shown to be localized in the plastids of spinach leaves [63] and A. thaliana[64•]. The predicted
cys-tathione γ-synthase structures deduced from its cDNAs from A. thaliana[65] and potato [66•] also suggest that this
enzyme is localized in the plastids. In spinach, there are two distinct forms of cystathione β-lyase, one localized in the plastids and one in the cytosol [67]. The cytosolic isoform catalyzes the β-cleavage of cystine more efficiently than that of cystathione, indicating that the proper substrate for the cytosolic cystathione β-lyase is cystine rather than cys-tathione [68••]. In contrast, the plastidic isoform efficiently
catalyzes the β-cleavage of cystathione. These findings were confirmed in A. thaliana[69] andEchinochloa colonum [70], in which cystathione β-lyase is only detected in the plastids. A cDNA encoding cystathione β-lyase was cloned from A. thaliana[69]. The predicted polypeptide encoded by this cDNA contained a putative plastid-transit peptide. The plant Met synthase is localized in the cytosol, and its cDNAs have been cloned from several plant species [68••,71,72].
Multiple regulatory mechanisms control the synthesis of amino acids from the aspartate family (i.e. Met, lysine and threonine). The control of cystathione γ-synthase activity primarily regulates the Met-biosynthetic enzymes. The cellular activity of cystathione γ-synthase is regulated nega-tively by the presence of Met, and posinega-tively by lysine and threonine [62•]. A recent analysis of a Met over-producing Figure 2
Hypothetical model for signal transduction of sulfate transport and assimilation. CDPK, Ca2+-dependent protein kinase; SAT-c,
cytosolic Ser acetyltransferase. Nutritional stress (S-starvation, GSH, etc)
SAT-c
(Cys sensitive)
SAT-c*
(Desensitized)
Cys
Feedback inhibition
Acetyl-CoA Ser
OASInduction
Met GSH Sulfur compounds
Plasma membrane
Nucleus
ATP
Ca2+
Gene expression (Sulfate transporter, APS reductase)
H2S SO42– CDPK
activation
A. thalianamutant revealed that the stability of the mRNA that encodes cystathione γ-synthase is negatively auto-reg-ulated by an amino-acid stretch in its own translational product (i.e. cystathione γ-synthase) that takes place when Met or its metabolites are present [73••]. This amino-acid
stretch is highly conserved amongst several cystathione γ -synthases from different species, and hence, this unique regulatory mechanism is likely to be functionally conserved in plant cells. The antisense suppression of the cystathione γ-synthase encoding gene resulted in severe growth reduc-tion, which is restored by the exogenous addition of Met [68••,74]. This finding suggests an essential role for
cys-tathione γ-synthase in controlling plant growth.
Conclusions
Although events in the sulfur assimilation pathway in plants have been confirmed only in the past few years, progress in understanding the mechanism and molecular regulation of this pathway has been remarkably rapid. Nevertheless, the available information on sulfur allocation [30,75•] and signal
transduction is still limited. We can expect that further mol-ecular analysis of sulfur transport and assimilation will provide significant insights into the pathways involved in the biosynthesis of sulfur-containing amino acids and will ultimatley lead to the molecular engineering of these path-ways. The biosynthesis pathways for sulfur-containing amino acids are of particular interest because of a variety of cellular functions (e.g. redox cycling, detoxification etc.) are ascribed to sulfur-containing compounds.
Note added in proof
Detailed functional characterisation and tissue localization of three distinct sulfate transporters from A. thaliana has recently been provided [13••]. A fourth ATP sulfurylase
has been isolated from A. thaliana[22••]. Evidence is
pro-vided for the identity of β-cyanoalanine synthase with a mitochondrial OAS(thiol)-lyase (cysteine synthase) in spinach and A. thaliana[40••].
Acknowledgements
I would like to thank all of those colleagues in plant sulfur research, both inside and outside of my laboratory, who provided unpublished results and preprints of papers. The research in my group was supported in part by grants-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture, Japan, by the Research for the Future Program (96I00302) of the Japan Society for the Promotion of Science, and by the Asahi Glass Foundation.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest ••of outstanding interest
1. Saito K: Biosynthesis of cysteine.In Plant Amino Acids •• Biochemistry and Biotechnology. Edited by Singh BK. New York:
Marcel Dekker, Inc.; 1999:267-291.
One of the most recent reviews describing the mechanism and regulation of Cys biosynthesis.
2. Leustek T, Saito K: Sulfate transport and assimilation in plants.
•• Plant Physiol1999, 120:637-643.
One of the most recent reviews on sulfate transport and assimilation in plants.
3. Hell R: Molecular physiology of plant sulfur metabolism.Planta
1997, 202:138-148.
4. Saito K, Takahashi H, Noji M, Inoue K, Hatzfeld Y: Molecular regulation of sulfur assimilation and cysteine synthesis.In Sulfur Nutrition and Assimilation in Higher Plants.Edited by Brunold C, Rennenberg H, De Kok LJ, Stulen I, Davidian J-C. Berne: Paul Haupt; 2000: in press.
5. Clarkson DT, Hawkesford MJ, Davidian J-C: Membrane and long-distance transport of sulfate.In Sulfur Nutrition and Assimilation in Higher Plants — Regulatory Agricultural and Environmental Aspects. Edited by De Kok LJ, Stulen I, Rennenberg H, Brunold C, Rauser WE. The Hague: SPB Academic Publishing; 1993:3-20.
6. Davidian J-C, Hatzfeld Y, Cathala N, Tagmount A, Vidmar JJ: Sulfate uptake and transport in plants.In Sulfur Nutrition and Assimilation in Higher Plants.Edited by Brunold C, Rennenberg H, De Kok LJ, Stulen I, Davidian J-C. Berne: Paul Haupt; 2000: in press.
7. Smith FW, Ealing PM, Hawkesford MJ, Clarkson DT: Plant members of a family of sulfate transporters reveal functional subtypes.Proc Natl Acad Sci USA1995, 92:9373-9377.
8. Takahashi H, Sasakura N, Noji M, Saito K: Isolation and chracterization of a cDNA encoding a sulfate transporter from
Arabidopsis thaliana.FEBS Lett1996, 392:95-99.
9. Takahashi H, Yamazaki M, Sasakura N, Watanabe A, Leustek T, de Almeida Engler J, Engler G, Van Montagu M, Saito K: Regulation of cysteine biosynthesis in higher plants: a sulfate transporter induced in sulfate-starved roots plays a central role in Arabidopsis thaliana.Proc Natl Acad Sci USA1997, 94:11102-11107.
10. Takahashi H, Sasakura N, Kimura A, Watanabe A, Saito K: Identification of two leaf-specific sulfate transporters in
Arabidopsis thaliana(Accession No. AB012048 and AB004060).
Plant Physiol1999, 121:686.
11. Takahashi H, Asanuma W, Saito K: Cloning of an ArabidopsiscDNA
• encoding a chloroplast localizing sulphate transporter isoform.
J Exp Botany1999, 50:1713-1714.
This paper provides evidence of the in vivo translocation of a putative
Arabidopsissulfate transporter into chloroplasts. The investigation that is described used a fusion protein in which a sulfate transporter isoform is bound to a green fluorescent protein.
12. Yamaguchi Y, Nakamura T, Harada E, Koizumi N, Sano H: Isolation and characterization of a cDNA encoding a sulfate transporter from Arabidopsis thaliana(Accession No. D89631).Plant Physiol
1997, 113:1463.
13 Takahashi H, Watanabe-Takahashi A, Smith FW, Blake-Kalff M,
•• Hawkesford MJ, Saito K: The roles of three functional sulfate transporters involved in uptake and translocation of sulfate in
Arabidopsis thaliana.Plant J 2000, in press.
This study provides the first detailed characterization of three functional sul-fate transporters. The different tissue localizations of these three trans-porters is emphasized and suggests that these transtrans-porters have distinct roles as high- and low-affinity transporters.
14. Smith FK, Hawkesford MJ, Ealing PM, Clarkson DT, Berg PJV, Belcher AR, Warrilow AGS: Regulation of exprression of a cDNA from barley roots encoding a high affinity sulphate transporter.
Plant J1997, 12:875-884.
15. Vidmar JJ, Schjoerring JK, Touraine B, Glass ADM: Regulation of the
• hvst1 gene encoding a high-affinity sulfate transporter from
Hordeum vulgare.Plant Mol Biol1999, 40:883-892.
The authors of this paper and [14] describe the cloning of a high-affinity sulfate transporter from barley. This transporter is induced by sulfate depletion. GSH down-regulated the concentration of mRNA encoding this sulfate transporter even in sulfate-starved plants in which the transporter is usually derepressed. 16. Bolchi A, Petrucco S, Tenca PL, Foroni C, Ottonello S: Coordinate
modulation of maize sulfate permease and ATP sulfurylase mRNAs in response to variations in sulfur nutritional status: stereospecific down-regulation by L-cysteine.Plant Mol Biol1999 39:527-537.
17. Mornet C, Tommasini R, Hörtensteiner S, Martinoia E: Transport of sulphate and reduced sulphur compounds at the tonoplast membrane.In Sulphur Metabolism in Higher Plants Molecular, Ecophysiological and Nutritional Aspects. Edited by Cram WJ, De Kok LJ, Stulen I, Brunold C, Rennenberg H. Leiden: Backhuys Publishers; 1997:1-11.
voltage-dependent anion channel of Arabidopsishypocotyl cells.Plant Physiol1999, 121:253-261.
19. Klonus D, Höfgen R, Willmitzer L, Riesmeier JW: Isolation and characterization of two cDNA clones encoding ATP-sulfurylase from potato by complementation of a yeast mutant.Plant J1994, 6:105-112.
20. Leustek T, Murillo M, Cervantes M: Cloning of a cDNA encoding ATP sulfurylase from Arabidopsis thalianaby functional expression in
Saccharomyces cerevisiae.Plant Physiol1994, 105:897-902.
21. Logan HM, Cathala N, Grignon C, Davidian J-C: Cloning of a cDNA encoded by a member of the Arabidopsis thalianaATP sulfurylase multigene family.J Biol Chem1996, 271:12227-12233.
22. Hatzfeld Y, Lee S, Lee M, Leustek T, Saito K: Functional
•• characterization of a gene encoding a fourth ATP sulfurylase isoform from Arabidopsis thaliana. Gene2000, in press.
In this study, the gene encoding fourth chloroplastic ATP sulfurylase was iso-lated. The authors discuss the possibility that the cytosolic ATP sulfurylase is produced from one of the genes encoding chloroplastic isoforms by the use of a different initiation Met codon.
23. Hatzfeld Y, Cathala N, Grignon C, Davidian J-C: Effect of ATP
• sulfurylase overexpression in Bright Yellow 2 tobacco cells.Plant Physiol1998, 116:1307-1313.
This is the first report to describe the effects of overexpression of ATP sul-furylase. Although the ATP sulfurylase activity was increased 8-fold in the BY-2 cells of transgenics tobacco, no effects on cell growth and sensitivity to selenate were observed.
24. Pilon-Smit EAH, Hwang S, Lytle CM, Zhu Y, Tai JC, Bravo RC,
• Chen Y, Leustek T, Terry N: Overexpression of ATP sulfurylase in Indian mustard leads to increased selenate uptake, reduction, and tolerance.Plant Physiol1999, 119:123-132.
This paper describes similar work to that reported in [23•]. ATP sulfurylase
was overexpressed in transgenic Indian mustard (Brassica juncea). The transformants showed increased selenate uptake, reduction and tolerance, suggesting that ATP sulfurylase mediates the reduction of selenate and lim-its the rate of selenate assimilation.
25. Bick JA, Leustek T: Plant sulfur metabolism — the reduction of
• sulfate to sulfite.Curr Opin Plant Biol 1998, 1:240-244.
An up-to-date short review on the reduction of sulfate to sulfite and the enzymes involved in this reaction.
26. Bick J-A, Åslund F, Chen Y, Leustek T: Glutaredoxin function for the
• carboxyl-terminal domain of the plant-type 5¢-adenylylsulfate reductase.Proc Natl Acad Sci USA1998, 95:8404-8409. The authors show that APS reductase has two domains. They present evidence showing that the carboxyl-terminal domain has a glutaredoxin-like function and that APS reductase requires GSH for its catalytic activity. 27. Suter M, von Ballmoos P, Kopriva S, Op den Camp R, Schaller J,
• Kuhlemeier C, Schürmann P, Brunold C: Adenosine 5′′-phosphosulfate sulfotransferase and adenosine
5′′-phosphosulfate reducatse are identical enzymes.J Biol Chem
2000, 75:930-936.
This is a conclusive report that shows that APS reductase and APS sulfo-transferase, which are thought to be involved in the carrier-bound pathway of APS reduction, are identical enzymes. APS sulfotransferase was purified, cloned and characterized using a recombinant form of the enzyme from
Lemna minor.
28. Heiss S, Schäfer HJ, Haag-Kerwer A, Rausch T: Cloning sulfur assimilation genes of Brassica junceaL.: cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase.Plant Mol Biol 1999, 39:847-857.
29. Kopriva S, Muheim R, Koprivova A, Trachsel N, Catalano C, Suter M,
• Brunold C: Light regulation of assimilatory sulphate reduction in
Arabidopsis thaliana.Plant J1999, 20:37-44.
This paper describes the evidence for the existence of a diurnal rhythm in the expression of the mRNA that encodes APS reductase and for the activity of this enzyme. This evidence suggests that APS reductase is regulated by a similar mechanism to that controlling nitrate reductase. Sucrose is shown to be a positive effector of the expression of mRNA encoding APS reductase and of the activity of this enzyme.
30. Burgener M, Suter M, Jones S, Brunold C: Cyst(e)ine is the transport metabolite of assimilated sulfur from bundle-sheath to mesophyll cells in maize leaves.Plant Phsyiol 1998, 116:1315-1322.
31. Bork C, Schwenn JD, Hell R: Isolation and characterization of a gene for assimilatory sulfite reductase from Arabidopsis thaliana.
Gene1998, 212:147-153.
32. Yonekura-Sakakibara K, Ashikari T, Tanaka Y, Kusumi T, Hase T: Molecular characterization of tobacco sulfite reductase: enzyme purification, gene cloning, and gene expression analysis.
J Biochem1998, 124:615-621.
33. Yonekura-Sakakibara K, Onda Y, Ashikari T, Tanaka Y, Kusumi T,
• Hase T: Analysis of reductant supply systems for ferredoxin-dependent sulfite reductase in photosynthetic and
nonphotosynthetic organs of maize.Plant Physiol2000, 122:887-894. In this paper, the authors reconstituted the sulfite-reduction system, which involves ferredoxin, ferredoxin-NADP+reductase and sulfite reductase, using an appropriate combination of tissue-specific enzyme isoforms. This allowed the authors to investigate the efficiency of electron transfer by different enzyme isoforms in different tissues, and to examine the complementation of an E. coli
sulfite reductase-deficient mutant by genes encoding the various isoforms. 34. Lunn JE, Droux M, Martin J, Douce R: Localization of ATP sulfurylase
and O-acetylserine(thiol)lyase in spinach leaves.Plant Physiol
1990, 94:1345-1352.
35. Takahashi H, Saito K: Subcellular localization of spinach cysteine synthase isoforms and regulation of their gene expression by nitrogen and sulfur.Plant Physiol1996, 112:273-280.
36. Ruffet M-L, Lebrun M, Droux M, Douce R: Subcellular distribution of serine acetyltransferase from Pisum sativumand characterization of an Arabidopsis thalianaputative cytosolic isoform.Eur J Biochem1995, 227:500-509.
37. Noji M, Inoue K, Kimura N, Gouda A, Saito K: Isoform-dependent
•• differences in feedback regulation and subcellular localization of serine acetyltransferase involved in cysteine biosynthesis from
Arabidopsis thaliana.J Biol Chem1998, 273:32739-32745. The subcellular localization of three Ser acetyltransferases from A. thaliana
was determined by transient expression of fusion proteins in which each of these three enzymes were fused with green fluorescence protein. Furthermore, it is shown that only the cytosolic isoform of Ser acetyltrans-ferase (i.e. not the chloroplastic and mitochondrial isoforms) is regulated by feedback inhibition by Cys. This work provides evidence of the important regulatory role of cytosoic Ser acetyltransferase in Cys biosynthesis. 38. Hesse H, Lipke J, Altmann T, Höfgen R: Molecular cloning and
expression analyses of mitochondrial and plastidic isoforms of cysteine synthase (O-acetylserine (thiol)lyase) from Arabidopsis thaliana.Amino Acids1999, 16:113-131.
39. Warrilow AGS, Hawkesford MJ: Separation, subcellular location
• and influence of sulphur nutrition on isoforms of cysteine synthase in spinach.J Exp Botany1998, 49:1625-1636.
This paper and [41•] deal with the relation between β-cyanoalanine synthase
and OAS(thiol)-lyase [Cys synthase]. The authors of this paper present defin-itive evidence, which was obtained using purification techniques, to show that
β-cyanoalanine synthase is predominantly located in mitochondria of spinach.
40. Hatzfeld Y, Maruyama A, Schmidt A, Noji M, Ishizawa K, Saito K:
•• ββ-Cyanoalanine synthase is a mitochondrial cysteine synthase-like protein in spinach and Arabidopsis thaliana.Plant Physiol
2000, in press.
Definitive evidence is provided for the identity of β-cyanoalanine synthase with a mitochondrial OAS(thiol)-lyase [cysteine synthase], of which cDNA was previously isolated from spinach [42].
41. Maruyama A, Ishizawa K, Takagi T, Esashi Y: Cytosolic ββ
• cyanoalanine synthase activity attributed to cysteine synthases in Cocklebur seeds. Purification and characterization of cytosolic cysteine synthases.Plant Cell Physiol1998, 39:671-680. The authors used purification and a partial amino-acid sequence analysis of the purified β-cyanoalanine synthase to show that the cytosolic activity of β -cyanoalanine synthase is controlled by OAS(thiol)-lyase [Cys synthase]. 42. Saito K, Tatsuguchi K, Takagi Y, Murakoshi I: Isolation and
characterization of cDNA that encodes a putative mitochondrion-localizing isoform of cysteine synthase (O-acetylserine(thiol)-lyase) from Spinacia oleracea.J Biol Chem1994, 269:28187-28192.
43. Inoue K, Noji M, Saito K: Determination of the sites required for the
• allosteric inhibition of serine acetyltransferase by L-cysteine in plants.Eur J Biochem1999, 266:220-227.
This study uses a series of recombinant mutant proteins to determine the residues and domains of Ser acetyltransferase that are involved in the feed-back inhibition of this enzyme by Cys.
45. Bogdanova N, Hell R: Cysteine synthesis in plants: protein–protein interactions of serine acetyltransferase from Arabidopsis thaliana. Plant J1997, 11:251-262.
46. Droux M, Ruffet M-L, Douce R, Job D: Interactions between serine
•• acetyltransferase and O-acetylserine (thiol) lyase in higher plants. Structural and kinetic properties of the free and bound enzymes.
Eur J Biochem1998, 255:235-245.
The significance of the bienzyme complex involving OAS(thiol)-lyase and Ser acetyltransferase, and the importance of greater cellular concentrations of uncomplexed OAS(thiol)-lyase Ser acetyltransferase relative to those of the uncomplexed enzyme, is explained. An interesting regulatory mechanism is proposed that involves both free and bound forms of the two enzymes. Within the complex, the bound form of OAS(thiol)-lyase may act as a structural and/or regulatory subunit of Ser acetyltransferase. Actual OAS(thiol)-lyase activity is attributed to the free form, because the activity of bound form of OAS(thiol)-lyase is dramatically decreased.
47. Barroso C, Romero LC, Cejudo FJ, Vega JM, Gotor C: Salt-specific
• regulation of the cytosolic O-acetylserine (thiol)lyase gene from
Arabidopsis thalianais dependent on abscisic acid.Plant Mol Biol
1999, 40:729-736.
Salt and heavy metal stresses induced OAS(thiol)-lyase activity suggesting a connection between these stresses and trichome-specific gene expres-sion [48]. The induction of gene expresexpres-sion is presumably mediated by abscisic acid.
48. Gotor C, Cejudo FJ, Barroso C: Tissue-specific expression of ATCYS-3A, a gene encoding the cytosolic isoform of
O-acetylserine (thiol)lyase in Arabidopsis.Plant J1997 11:347-352.
49. Saito K, Kurosawa M, Tatsuguchi K, Takagi Y, Murakoshi I: Modulation of cysteine biosynthesis in chloroplasts of transgenic tobacco overexpressing cysteine synthase [O-acetylserine(thiol)lyase].
Plant Physiol1994, 106:887-895.
50. Blaszczyk A, Brodzik R, Sirko A: Increased resistance to oxidative
• stress in transgenic tobacco plants overexpressing bacterial serine acetyltransferase.Plant J1999, 20:237-243.
This paper describes the first study of overexpression of Ser acetyltrans-ferase in plants. Although overexpression of this enzyme resulted in only two or three times higher concentrations of Cys and GSH, much greater resis-tance to hydrogen peroxide was generated.
51. Kredich NM: Biosynthesis of cysteine.In Escherichia coli and Salmonella typhimurium: Cellular and molecular biology,vol 1, edn 2. Edited by Neidhardt FC, Curtis RC III, Ingraham JL, Low KB, Magasanik B, Reznikoff RZ, Riley M, Schaechter M, Umbarger HE. Washington DC: American Society for Microbiology; 1996:514-527. 52. Koprivova A, Suter M, Op den Camp R, Brunold C, Kopriva S:
Regulation of sulfate assimilation by nitrogen in Arabidopsis thaliana.Plant Physiol 2000, 122:737-746.
53. Kim H, Hirai MY, Hayashi H, Chino M, Naito S, Fujiwara T: Role of
• O-acetyl-L-serine in the coordinated regulation of the expression of a soybean seed storage-protein gene by sulfur and nitrogen nutrition.Planta1999, 209:282-289.
In conditions in which sulfur is deficient (or a low ratio of sulfur to nitrogen availability exists), the β-subunit of β-conglycinin (i.e. the sulfur-poor soybean seed storage protein) is accumulated. This paper provides evidence that OAS accumulates to higher concentrations in A. thaliana and soybean cotyledons under sulfate-deficient conditions. The addition OAS results in an elevated accumulation of the mRNA encoding the β-subunit and of the pro-tein itself. These findings suggest that OAS is a positive signal molecule for gene expression in sulfur-starved plants.
54. Clarkson D, Diogo E, Amâncio S: Uptake and assimilation of sulphate by sulphur deficient Zea mays cells: the role of O -acetyl-L-serine in the interaction between nitrogen and sulphur assimilatory pathways.Plant Physiol Biochem 1999, 37:283-290.
55. Herschbach C, Rennenberg H: Influence of glutathione (GSH) on net uptake of sulphate and sulphate transport to tobacco plants.
J Exp Botany1994, 45:1069-1076.
56. Lappartient AG, Touraine B: Demand-driven control of root ATP sulfurylase activity and SO42–uptake in intact canola.Plant Physiol1996, 111:147-157.
57. Lappartient AG, Vidmar JJ, Leustek T, Glass ADM, Touraine B: Inter
•• organ signaling in plants: regulation of ATP sulfurylase and sulfate transporter genes expression in roots mediated by phloem-translocated compound. Plant J1999, 18:89-95. An impressive report that suggests a role for GSH as a phloem-translocat-ed signal molecule that represses the expression of genes encoding ATP sulfurylase and a sulfate transporter. These conclusions are based on the results of ‘split root’ experiments using A. thalianaand B. napus.
58. Yoo B-C, Harmon AC: Regulation of recombinant soybean serine acetyltransferase by CDPK.Plant Physiol Suppl1997, 114:267.
59. Davies JP, Yildiz F, Grossman A: Sac1, a putative regulator that is critical for survival of Chlamydomonas reinhardtii during sulfur deprivation.EMBO J1996, 15:2150-2159.
60. Davies JP, Yildiz FH, Grossman AR: Sac3, an Snf1-like
• serine/threonine kinase that positively and negatively regulates the responses of Chlamydomonasto sulfur limitation.Plant Cell
1999, 11:1179-1190.
This paper and [59] demonstrate the benefits of using the unicellular green algae Chlamydomonasas a model organism in which to study the effects of nutritional stress on signal transduction. Sac3cDNA was iden-tified by its complementation of a Chlamydomonas Sac3mutant, which lacked the proper responses to sulfate deprivation. The Sac3product is a Snf1-related serine/threonine kinase. The involvement of the same product in responses to sulfur deprivation in higher plants is an intrigu-ing possibility.
61. Azevedo RA, Arruda P, Turner WL, Lea PJ: The biosynthesis and metabolism of the aspartate derived amino acids in higher plants.
Phytochemistry1997, 46:395-419.
62. Matthews BF: Lysine, threonine, and methionine biosynthesis.In
• Plant Amino Acids Biochemistry and Biotechnology. Edited by Singh BJ. New York: Marcel Dekker, Inc.; 1999:205-225.
An excellent review describing the up-to-date information on biosynthesis of amino acids in the aspartate family.
63. Ravanel S, Droux M, Douce R: Methionine biosynthesis in higher plants. I. Purification and characterization of cystathione
γγ-synthase from spinach chloroplasts.Arch Biochem Biophys
1995, 316:572-584.
64. Ravanel S, Gakiere B, Job D, Douce R: Cystathione γγ-synthase
• from Arabidopsis thaliana: purification and biochemical chracterization of the recombinant enzyme overexpressed in
Escherichia coli.Biochem J1998, 331:639-648.
The authors describe the first biochemical characterization of recombinant cystathione γ-synthase from plants.
65. Kim J, Leustek T: Cloning and analysis of the gene for cystathione
γγ-synthase from Arabidopsis thaliana.Plant Mol Biol1996, 32:1117-1124.
66. Riedel K, Mangelsdorf C, Streber W, Willmitzer L, Höfgen R, Hesse H:
• Cloning and characterization of cystathione γγ-synthase from
Solanum tuberosumL.Plant Biol1999, 1:638-644.
The cloning of cystathione γ-synthase from potato is reported. The expres-sion pattern of the gene encoding this enzyme exhibits a diurnal rhythm and is induced by light.
67. Droux M, Ravanel S, Douce R: Methionine biosynthesis in higher plants. II. Purification and characterization of cystathione ββ-lyase from spinach chloroplasts.Arch Biochem Biophys1995, 316:585-595.
68. Ravanel S, Gakiere B, Job D, Douce R: The specific features of
•• methionine biosynthesis and metabolism in plants.Proc Natl Acad Sci USA1998, 95:7805-7812.
An excellent article describing the current status of our knowledge of Met biosynthesis in plants.
69. Ravanel S, Ruffet M-L, Douce R: Cloning of an Arabidopsis thaliana
cDNA encoding cystathione ββ-lyase by functional complementation in Escherichia coli.Plant Mol Biol 1995, 29:875-882.
70. Turner WL, Pallett KE, Lea PJ: Cystathione ββ-lyase from Echinochloa colonum tissue culture.Phytochemistry1998, 47:189-196.
71. Eichel J, Gonzalez JC, Hotze M, Matthews RG, Schröder J: Vitamin-B12-independent methionine synthase from higher plant (Catharanthus roseus). Molecular characterization, regulation, heterologous expression, and enzyme properties.Eur J Biochem
1995, 230:1053-1058.
72. Petersen M, Van Der Straeten D, Bauw G: Full-length cDNA clone from Coleus blumei(GenBank Z49150) with high similarity to cobalamine-independent methionine synthase.Plant Physiol
1995, 109:338.
73. Chiba Y, Ishikawa M, Kijima F, Tyson RH, Kim J, Yamamoto A,
•• Nambara E, Leustek T, Wallsgrove RM, Naito S: Evidence for authoregulation of cystathione γγ-synthase mRNA stability in
Arabidospsis.Science1999, 286:1371-1374.
repression of this enzyme by its own translational product (i.e. cystathione γ -synthase) in the presence of Met (or its metabolites).
74. Kim J, Leustek T: Repression of cystathione γγ-synthase in
Arabidopsis thalianaproduces partial methonine auxotrophy and developmental abnormalities.Plant Sci2000, 151:9-18.
75. Bourgis F, Roje S, Nuccio ML, Fisher DB, Tarczynski MC, Li C,
• Herschbach C, Rennenberg H, Pimenta MJ, Shen T-L et al.: S-Methylmethionine plays a major role in phloem sulfur transport
and is synthesized by a novel type of methyltransferase.Plant Cell1999, 11:1485-1497.