*Corresponding author. Tel.:#44-118-9-3181-68; fax:#44-118-9-7536-76. E-mail address:[email protected] (C.A. Williams)
1Present address: Cambridge University Botanic Garden, Cory Lodge, Bateman Street, Cambridge CB2 1JF, UK.
Biochemical Systematics and Ecology 28 (2000) 991}1007
Leaf
#
avonoids as systematic characters
in the genera
La
v
andula
and
Sabaudia
Tim M. Upson
!
,1
, Rene
H
e J. Grayer
"
, Jennifer R. Greenham
!
,
Christine A. Williams!
,
*
, Farag Al-Ghamdi!, Fen-Hui Chen!
!Department of Botany, University of Reading, Whiteknights, Reading RG6 6AS, UK
"Jodrell Laboratory, Royal Botanic Gardens, Kew, Richmond, Surrey TW9 3AB, UK Received 28 September 1999; accepted 3 December 1999
Abstract
A comprehensive survey of the leaf #avonoids of the genus Lavandulaand the related
Sabaudia group was carried out using two-dimensional paper chromatography and
high-performance liquid chromatography. The #avonoid patterns obtained were found to be systematically informative at the infrageneric level. Three main groupings were identi"ed: the
"rst containing sectionsLavandula,DentataandStoechascharacterised by the accumulation of
#avone 7-glycosides; the second containing sectionsPterostoechas,SubnudaandChaetostachys
characterised by the accumulation of 8-hydroxylated#avone 7- and 8-glycosides; the third encompassing theSabaudiagroup and accumulating both#avone and 8-hydroxylated#avone 7-glycosides. Such a grouping of taxa is congruent with data from other disciplines, although it is not recognised in any present classi"cations. The taxonomic and evolutionary implications of
the#avonoid data are discussed. ( 2000 Elsevier Science Ltd. All rights reserved.
Keywords: Lavandula;Sabaudia; Lavender; Lamiaceae; Flavonoids; Chemotaxonomy
1. Introduction
The genus Lavandula (lavender, Lamiaceae) is distributed from the Canary and
Cape Verde Islands and Madeira, across the Mediterranean Basin, North Africa,
South}West Asia, the Arabian Peninsula and tropical NE Africa with a disjunction to India. Although the name of the genus is familiar to many people, only a few species are well known, e.g. L. angustifoliaMiller or English lavender,L. latifolia or spike lavender, andL. stoechas or French lavender. The English lavender is widely culti-vated in gardens and is also an important essential oil crop from which many cultivars have been selected.
The last revision covering the whole genus was undertaken more than 60 years ago by Chaytor (1937), but more recently there have been a number of#oristic revisions covering certain geographical areas, e.g. by Miller (1985) on the Arabian and NE tropical African species. Based on Chaytor's work and that of others, six sections can be recognized inLavandula:StoechasGing. (3 spp.),Lavandula(3 spp. ),Dentata(1 sp.),
Pterostoechas(15 spp.),Subnuda(8 spp.) andChaetostachys (2 spp.).
On the basis of its hexacolpate pollen (Erdtman, 1945) and accumulation of essential oils (Hegnauer, 1989), Lavandula clearly belongs to the subfamily
Nepetoideae of the Lamiaceae. According to phylogenetic and molecular studies,
Lavandulais a distinctive clade in the Nepetoideae without close relatives (Cantino
et al., 1992; Kaufmann and Wink, 1994; Wagsta!et al., 1995). The nearest relative appears to be the tribe Ocimeae. However, there is a small genus of two or three species native to the Arabian Peninsula and NE tropical Africa, called Sabaudia, which has sometimes been treated as part ofLavandula(Cufodontis, 1962), although
its tribal position within the Lamiaceae has not yet been fully established.
A vast literature exists on the essential oils in Lavandula, reviewed by Boelens
(1995). For the taxonomist the use of essential oils as characters for the classi"cation is limited by inherent problems of natural variability, although at lower taxonomic levels this can be used to help recognize cultivars (Grayer et al., 1996). The chemical composition and ratios of the individual components making up the oils are also known to change in response to environmental conditions, such as water and nutrient stress or time of year (Ross and Sombrero, 1991). These inherent problems of variation mean that other classes of chemical constituents such as#avonoids are often of greater use and signi"cance to the systematist.
The utility of#avonoids within the Lamiaceae has been demonstrated at various taxonomic levels, including the assessment of generic relationships within subfamilies, generic a$nities of species, and even in verifying the parents of hybrids (reviewed by TomaHs-BarberaHn and Gil, 1992). With regard to the genusLavandula, no#avonoid
survey of the genus has yet been undertaken, although some species have been investigated as part of wider taxonomic surveys of the family. The following #avonoids were reported fromL. dentata(Ferreres et al., 1986): genkwanin (apigenin 7-methyl ether), luteolin, apigenin, luteolin 7-glucoside, apigenin 7-glucoside, luteolin 7-rutinoside, vitexin and vicenin-2. Several species ofLavandulawere investigated as
species both belonging to section Pterostoechas. These compounds were the 7-glucoside and 8-glucuronide of hypolaetin, and the 8-glucuronides of hypolaetin 4@-methyl ether and isoscutellarein (El Garf et al., 1999). Glycosides of 8-hydroxyf-lavones are uncommon plant constituents, especially the 8-glycosides, and in several cases have provided useful characters at the sectional level within genera of the Lamiaceae (TomaHs-BarberaHn et al., 1988; TomaHs-BarberaHn and Gil, 1992). To see whether that is also the case in the genus Lavandula, we have surveyed a large
proportion of the species of the genus for the presence or absence of these and other #avonoids, and also studied representatives ofSabaudiain the hope that #avonoid pro"les would help in clarifying relationships between this genus and Lavandula.
2. Materials and methods
2.1. Plant material
Specimens and voucher details for the taxa surveyed are presented in Table 1. The fresh material used in the investigations was "rst grown in a greenhouse and then moved outside to grow under natural daylight for six weeks before extraction.
2.2. Extraction
A few chopped leaves of each sample were placed in a test tube, covered with, ca. 5 ml of 100% MeOH for fresh material or 70% aqueous MeOH for dry herbarium material, and placed in a heating block at 703C for about 5 min. This procedure kills the plant tissue preventing enzymic oxidation or hydrolysis. The samples were left to extract for at least 8 h or overnight. Excess chlorophyll was removed using petroleum ether (BP 40}603) before the extracts were evaporated to dryness on watchglasses.
2.3. Two-dimensional (2-D) -paper chromatography
The dried extracts were reconstituted using 80% MeOH and run two-dimen-sionally on quarter sheets of Whatman no. 1 chromatography paper. The chromato-grams were run by descent in BAW (n-BuOH, HOAc, H
2O"4 : 1 : 5; v/v; upper layer) "rst direction, and in 15% aqueous HOAc or H
2O in the second direction. After drying, the chromatograms were viewed in UV light (366 nm)"rst without and later in the presence of ammonia vapour. The colours of the spots before and after NH
3 were recorded andR&-values calculated.
2.4. Paper electrophoresis
Table 1
Specimen and voucher details for the taxa surveyed. Accession numbers are given for those living specimens held in the collection at the University of Reading; herbarium acronyms refer to vouchers and follow Holmgren, Keuken and Scho"eld (1981). Authority abbreviations follow Brummitt and Powell (1992). L"living material used, H"herbarium material used
Taxa Specimen and voucher details
SectionLavandula
L. angustifoliaMill. subsp.angustifolia L Cultivatedex.Kiev Botanic Garden, Russia (RNG); Cultivatedex.Tsukuba Medicinal Plant Research Station, Japan (RNG); Cultivated variant (RNG)
L. angustifoliaMill. subsp.pyrenaica(DC.) Masclans L Cultivatedex. Botanischer Garten Kiel(RNG); Spain, Prov. of Huesca, Pyrenaen Plan-Benasqueex. Botanischer Garten Berlin Dahlem(RNG); France, Frankreich Pyrenees Orientalisex. Botanischer Garten Berlin Dahlem(RNG)
L. angustifoliaMill. subsp.delphinensis(Jord.) O. Bolo`s & Vigo
L Cultivatedex. Bundesgarten Wien}Alpengarten Im Belvedere(RNG)
L. latifoliaMedic. L France, Prov. of Aude, Montagne de Tauchex. Jardin Botanique Leige(RNG); France, Aude Fore(t de Rives-Hautesex. Leige(RNG); Spain, Prov. Gerona Angles,ex. Botanis-cher Garten Berlin Dahlem(RNG); Italy, Siena, Pomona in Chiantiex. Jardin Botanico Siena(RNG); France Angersex. Instituto Botanico Siena(RNG)
L. lanataBoiss. L Spain, Prov. of Granada, Sierra Nevadaex. Botanischer Garten Berlin Dahlem(RNG); Cultivatedex. Jardin Botanique Ronen(RNG); Spain, Prov. of Malaga,S.L. Jury 13138
(RNG)
SectionStoechas
L. stoechasL. subsp.stoechas L Corsica, Mt. Tomboni,L. Springate 93.289(RNG) Crete,J.D. Ross s.n. (RNG); Turkey, Mugla province, SW of Marmaris, L.C. Jury 334 (RNG); France, Angersex. Jardin Botanique de Dijon(RNG); Spain, Prov. of Granada, Sierra Nevadaex. Jardin Botanico Cordoba(RNG); Morocco, SE of Tetouan, 1 km N of Oued Laou,S.L. Jury 12408 et al.
(RNG)
L. stoechasL. subsp.atlanticaBraun-Blanq L Morocco, Rif Central, 17 km S. Ketama,S.L. Jury 11566 et al.(RNG); Morocco, Haut Ouerrha, 47 km S. of Ketama,S.L. Jury 11575 et al.(RNG)
L. stoechasL. subsp.cariensis(Boiss.) Rozeira L NW Turkey, Balikesir,Haziren s.n. (RNG)
L. stoechasL. subsp.luisieri(Rozeira) Rozeira L Portugalex. Jardim Botanico Porto(RNG); Portugal, Algarve, Serra do Caldeira8oex. Jardim Botanico Lisboa(RNG) Spain, Sa de CoHrdobaex. Jardin Botanico Cordoba(RNG); Portugal, Coimbraex.Jardim Botanico Coimbra (RNG)
L. stoechasL. subsp.lusitanica(Chaytor) Rozeira L Portugal, Prov. Baixo Alentejo, Reguengos de Monsarazex. Jardim Botanico de Lisboa
(RNG)
L. stoechasL. subsp.pedunculata(Mill.) Samp. ex. Rozeira
L Spain, Prov. of Leo`n, La Magdalenaex. Jardin Botanico Bilbao(RNG); Spain, Prov. of Avila, Sierra de Gredosex. Botanisher GartenOsnabruKck (RNG)
L. stoechasL. subsp.sampaianaRozeira L Portugal, Prov. Alto Alentejo, Marva8oex. Jardim Botanico Lisboa(RNG) Portugal, nr. Coimbraex. Jardim Botanico Coimbra(RNG)
L.viridisL@HeHr. L Portugal, outside Barradaex Joan Head 91.10(RNG); Portugal, Barranco Velhoex. Joan Head 91.08(RNG); Cultivatedex Jardin Botanico Nice(RNG)
SectionDentata
L. dentataL. var.dentata L Balearic Islands, Mallorca (RNG); Morocco, S. of Tetouan on rd. to Oued Laou, Cap Mazari,S.L. Jury 12383 et al.(RNG); Morocco, N. of Agadir, rd. to Immouzzer,S.L. Jury 11984 et al.(RNG); Cultivatedex. Jardin Botanico Barcelona(RNG)
L. dentataL. var.candicansBatt. L Morocco, NW of Agadir, Cap Rhir, S.L. Jury 12000 et al.(RNG); Morocco, nr. Al Hoceima,S.L. Jury 13533(RNG)
SectionPterostoechas
L. antineaeMaire H Algeria, Hoggar Mts,Miller 4144 et al.(E); Algeria, Hoggar Massif, Oued Ideles,D. Podlech 36932(M)
L. buchiiWebb & Berthel. var.buchii H Canary Islands, Tenerife: Anaga Peninsula, near Las Carboneras,T.M. Upson 299(RNG); Anaga Peninsula, NW of Taganana, Playa de Benijo,T.M. Upson 307(RNG)
L. buchiiWebb & Berthel. var.gracileLeoHn L Canary Islands, Tenerife, Teno Regionex. Joan Head(RNG); Teno Region, c. 6 km SW of Tamaimo, T.M. Upson 301 (RNG); Teno Region, Mirador de Don Pompeyo, nr. Buenavista,T.M. Upson 297(RNG)
L. canariensisMill. L Cultivatedex. Norfolk Lavender (RNG); Canary Islands, Gran Canaria, Arucas,Joan Head 93.21(RNG)
H Canary Islands, Tenerife, above San Juan,T.M. Upson 298(RNG)
L. coronopifoliaPoir. L Morocco: SW Anti-Atlas, c.27 W of Tata,S.L. Jury 14464 et al.(RNG); SW Anti-Atlas, c. 20 km W of Tata,S.L. Jury 14448 et al.(RNG)
L. maireiHumbert var.mairei L Morocco: 24 km N. of Er Rachidia,S.L. Jury 14612(RNG); c. 26 km ESE of Tinerhir,S.L. Jury 14589(RNG)
H Morocco, Gorges du Ziz,S.L. Jury 14614(RNG)
L. maireiHumbert var.antiatlanticaMaire L Morocco, SW Anti-Atlas: W of Tata, S.L. Jury 14463a et al. (RNG); 27 km NE of Tafraoute,S.L. Jury 14384 et al.(RNG); SE of AmKt-Baha,S.L. Jury 14385 et al.(RNG)
Table*continued
Taxa Specimen and voucher details
L. maroccanaMurb. L Morocco: Gorges du Ziz,S.L. Jury 14615(RNG); Morocco: Immouzzer Valley, 10 km N of Agadir,S.L. Jury 14245(RNG); Morocco: High Atlas, c. 12 km NNE of Ijoukak,S.L. Jury 14200(RNG)
L. minutoliiBolle var.minutolii L Canary Islands, Gran Canaria,Joan Head s.n. (RNG); Canary Islands, Gran Canaria, Roque Bentrigaex. Botanischer Garten Berlin Dahlem(RNG)
L. minutoliiBolle var.tenuipinnaSvent. H Canary Islands, Tenerife, Teno Region, near Masca,T.M. Upson 295(RNG)
L. multixdaL. Culivatedex. Jardin Botanique De Dijon, France (RNG)
L. pinnataL. ff. L Cultivatedex. Orto Botanico Torino(RNG); Cultivatedex. Botanischer Garten Innsbruck
(RNG)
L. rotundifoliaBenth. L Cape Verde Islandex. Botanischer Garten Bonn(RNG)
H Cape Verde Islands, mountains SW of the village Ribeira Grande, below Faja8 Bedonda, 26.1.1982,C. Brochmann 533/82(O)
L. tenuisectaCoss. ex. Ball H High Atlas, Toubkal range, side of gorge from refuge Neltner to Imlil village, 14.7.1966,
T.K. Thorpno.95(BM)
SectionSubnuda
L. aristibracteataA.G. Mill. L Somalia, Surundi Hills,M. Thulin 9051(RNG)
L. dhofarensisA.G. Mill. subsp.dhofarensis L Oman, Dhofar, Wadi Sahalnawt, nr. Salalah,A.G. Miller 6158(RNG)
L. nimmoiBenth. H Socotra, base of escarpment c. 6 km SE of Qalansiyah, 23.2.1989,A.G. Milleret al.8395
(E)
L. subnudaBenth. L N. Oman, Wadi Mistral,S. Gbazdrfai 2482(RNG); Oman, Musandam by road to Khawr Niad,H.D.V. Prendergast(RNG)
SectionChaetostachys
L. bipinnataKuntze L India, Prov. of Maharashtra, Poona District, Vede-Bowdhanex. Botanical Survey of India
(BSI, RNG)
Sabaudiagroup
S. atriplicifolia(Benth.) Chiov. H Yemen Arab Republic, Kaukaban,J.R.I. Wood Y/75/311(BM)
S. erythreaeChiov. H Ethiopia (Eritrea), Monti Lesa,A. Pappi(C)
996
T.M.
Upson
et
al.
/
Biochemical
Systematics
and
Ecology
28
(2000)
991
}
control. The paper was subjected to electrophoresis at pH 4.4, using a sodium acetate/acetic acid bu!er at 400 V for about 2 h.
2.5. Preparative paper chromatgraphy (PPC)
Flavonoids were separated from rosmarinic acid and other ca!eic acid derivatives which interfered with the HPLC analysis of crude extracts by means of PPC. Each crude extract was applied as a streak onto one quarter sheet of Whatman no. 3 chromatography paper. This was run by descent in 15% HOAc. The bottom half of the paper which showed dark#avonoid bands in UV light (roughly from the origin to, ca.R
&0.50) was cut out, cut into 2 cm squares, and these were eluted in 80% MeOH
for 3}4 h. The top half of the paper (R
&-value above 0.50) which showed#uorescent
blue bands of ca!eic acid derivatives such as rosmarinic acid (R
& 0.58 in 15% HOAc)
was not eluted. The#avonoid eluate was evaporated and resuspended in ca. 0.8 ml of 80% MeOH. This was"ltered using a 13 mm Gelman Acrodisc, pore size 5lm, ready
for HPLC analysis. A few extracts were further separated by PPC to isolate #avonoids, using the solvent BAW (see above) as a second puri"cation step.
2.6. HPLC
The HPLC system consisted of a Waters 600E systems controller with a Waters 996 photodiode array detector. Injection of the extracts (40ll) took place with a Waters
717 autosampler. Millennium software was used to record and analyse the results. Two di!erent elution programmes were used; a LiChrospher 100RP-18 column was employed for programme 1 and a Bondapak Phenyl RP-18 column for programme 2. Both columns measured 4.0 mm (i.d.)]250 mm with a 5lm particle size. Elution
took place by means of two solvents, solvent A consisting of 2% of aqueous HOAc and solvent B of MeOH, HOAc, H
2O, 18: 1: 1, and elution started with 25% of B at
t"0. For programme 1 there was a linear gradient reaching 100% B att"20 min,
but for programme 2 it reached 65% B att"23 min. In both programmes this was
followed by an isocratic elution with 100% B for 5 or 1 min, respectively, before going back to the initial conditions. The#ow rate was 1.0 ml/min, and the temperature of the column was 253C.
2.7. Identixcation ofyavonoids
The#avonoids were identi"ed by comparingR
&-values on paper chromatograms,
colours of spots, HPLCR
5sand UV spectra of the compounds in the extracts with
hypolaetin 7-glucoside (#avonoid collection at Kew); xanthomicrol and salvigenin (kindly provided to R.J.G. by Prof. E. Wollenweber).
3. Results
The extracts of 23 species ofLavandulaand two of Sabaudiawere examined by
means of 2-D paper chromatography. In addition, seven di!erent subspecies of L.
stoechas were studied together with three subspecies of L. angustifolia and two
varieties ofL. dentata, L. buchii, L. maireiandL. minutolii. Besides, for many of the taxa more than one accession was examined (see Table 1). Extracts of 13 species, represent-ing all sections, were subjected to paper electrophoresis to check for the presence of #avonoid glucuronides which are characteristic constituents of the family Lamiaceae (TomaHs BarberaHn et al., 1988). The crude extracts used for the 2-D paper chromato-grams were also analysed by HPLC. However, the HPLC traces did not give clear results, because of the presence of large amounts of rosmarinic acid and related substances in the extracts, which masked many of the#avonoid peaks and deformed their UV spectra. For this reason about half of the extracts, representing all six sections ofLavandulaand all species ofSabaudia, were puri"ed by means of PPC to
separate the #avonoids from rosmarinic acid. HPLC of these puri"ed #avonoid fractions gave much better results.
To identify some of the compounds, an extract ofL. angustifoliassp.pyrenaicawas used to isolate #avonoids by means of preparative paper chromatography and preparative paper electrophoresis. Ten #avonoids were obtained, 1+8, 18 and 19. Their colours on the chromatograms before and after fuming with ammonia, R
&
-values in two or three solvents, HPLC retention times in two di!erent solvent systems and diode array UV spectra were determined (see Table 2). In addition,1,2,4,5,7and
8were studied by UV spectroscopy using shift reagents (Mabry et al., 1970) and by means of acid hydrolysis of the compounds and subsequent analysis of the aglycones and sugars (Harborne, 1998). In this way,1and2were identi"ed as the 7-glucoside and 7-glucuronide of luteolin, respectively, and4and5as apigenin 7-glucoside and 7-glucuronide. These identi"cations were con"rmed by means of co-chromatography with standard markers on paper and by HPLC. After partial and total hydrolysis of
7and8and analysis of the hydrolysis products, these compounds were tentatively identi"ed as luteolin 7,4@-di-glucuronide and luteolin 7-glucoside-4@-glucuronide. No standards were available for comparison. Not enough pure compound for3and6was obtained for complete identi"cation. HPLC of these compounds revealed that3had a similar UV spectrum to1and 2, but a longer retention time. The colour of3on chromatograms in UV light after fuming with ammonia (yellow}green) and the UV spectrum suggested that it may be a chrysoeriol 7-glycoside. In the same way,6may be a glycoside of a methylated apigenin derivative. Compounds 18 and 19 were identi"ed by means of co-chromatography with standards as the#avone aglycones, apigenin and genkwanin.Table 2 shows another 11 #avonoids, 9}17, 20 and 21, frequently encountered in extracts ofLavandulaspecies, which were completely or
Table 2
Chromatographic and UV spectral data of#avonoids fromLavandulaandSabaudiaspecies!
Compound Identi"cation(Htentative) Colour in UV
1 Luteolin 7-O-glucoside D Y 0.40 0.12 0.02 14.2 17.4 256, 267sh, 350
2 Luteolin 7-O-glucuronide D Y 0.32 0.15 0.09 14.1 17.4 256, 267sh, 350
3 Chrysoeriol 7-O-glycosideHD GnY 0.42 n.d n.d. 15.9 21.0 252, 267sh, 348
4 Apigenin 7-O-glucoside D dYGn 0.58 0.25 0.04 15.7 20.3 268, 338
5 Apigenin 7-O-glucuronide D dYGn 0.50 0.28 0.15 15.7 20.3 268, 338
6 Derivative of4or5 n.d. n.d. n.d. n.d. n.d. 17.2 23.7 268, 338
10 Scutellarein 7-O-glycosideHD D 0.35 0.13 n.d. 14.1 n.d. 285, 338
11 Vitexin D dY 0.40 0.30 n.d 13.2 n.d. 269, 338
12 Hypolaetin 8-O-glu-curonide
D D 0.40 0.12 n.d. 15.9 n.d. 256sh, 271, 354
13 Derivative of12 D D 0.45 0.25 n.d. 16.7 n.d. 256sh, 271, 354
14 Hypolaetin 4@-methyl ether 8-O-glucuronide
D D 0.48 0.14 n.d. 17.3 n.d. 257, 271, 352
15 Isoscutellarein 8-O-glucur. D D 0.55 0.20 n.d. 17.4 n.d. 271, 343
16 Hypolaetin 7-O-glucoside D D 0.20 0.08 n.d. 13.9 n.d. 276, 300sh, 343
17 Isoscutellarein 7-O-glycoside
D D 0.35 0.15 n.d. 15.4 n.d. 276, 306, 328sh
18 Apigenin D Ygn 0.85 0.08 n.d. 20.5 26.8 268, 338
19 Genkwanin D GnY 0.90 0.08 n.d. 24.2 32.5 268, 338
20 Xanthomicrol D D 0.90 0.15 n.d. 23.0 n.d. 281, 290sh, 333
21 Salvigenin D D 0.90 0.15 n.d. 24.9 n.d. 276, 333
!Solv. 1 and Solv. 2: HPLC solvent programs 1 and 2, respectively. For composition of the solvents used for HPLC and PC, see Experimental. Abbreviations: D"dark; Y"yellow; L"light; Gn"green; d"dull; Br"brown; n.d."not determined.
PC. The 8-hydroxylated #avonoids 12, 14}16 were previously isolated from two species of Lavandula, L. coronopifoliaand L. pubescens, and identi"ed by means of
NMR, MS and UV spectroscopy as the 8-glucuronides of hypolaetin, hypolaetin 4@-methyl ether and isoscutellarein and the 7-glucoside of hypolaetin, respectively (El Garf et al., 1999). The compounds detected and identi"ed (sometimes tentatively) during the combined HPLC, PC and electrophoresis analyses are presented in Table 3. The external#avonoids18}21(see Table 2) may be present in a larger quantity and in more species than is shown in Table 3, as the methods of extraction and the HPLC programmes were more suitable for#avonoid glycosides than for external#avones. Absorbance of the peaks at thej
.!9 of each#avonoid was used as a measure of the
relative amounts (see Table 3). The solvent systems used for HPLC, which contained a small percentage of HOAc, did not distinguish between glucosides and glucuronides of the same aglycone, as these had more or less the same retention times. However, the presence of both1and2, and both4and5in all taxa investigated in sectionsStoechas,
Dentata and Lavandula was con"rmed by means of paper electrophoresis of the
extracts and 2-D paper chromatography in BAW and water. Flavonoid glucuronides are very mobile in water compared with the corresponding monoglucosides and so the two are easily separated in this system.
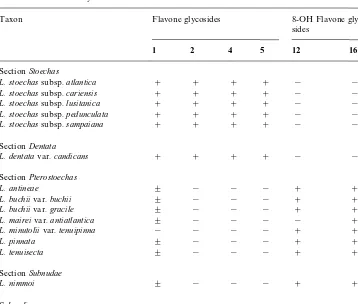
A number of accessions were investigated by means of 2-D paper chromatography only. As this technique is generally less sensitive than HPLC, only presence of the major#avonoids1,2,4and5and the 8-hydroxy#avone glycosides12and16could be scored. The latter compounds are easy to detect on paper chromatograms because they do not change colour in UV light after fuming with ammonia. Moreover, they have very characteristic UV spectra. These results are presented in Table 4 and largely agree with the"ndings of the more detailed analyses presented in Table 3.
The #avonoid pro"les observed on the 2-D chromatograms and HPLC traces showed very little variation among di!erent accessions, subspecies and varieties of the same species. Moreover, there was surprisingly little variation in major #avonoid pro"les among species belonging to the same section, any di!erences being mainly quantitative. Section Lavandulaappeared to be the most uniform group in that all
members produced#avone monoglycosides1}6,#avone diglycosides7and8, and the aglycones, apigenin (18) and genkwanin (19). Again, in sectionStoechasthe di!erences between the three taxa were very minor. However, in sectionPterostoechasonly two of the seven taxa fully investigated (Table 3) had identical#avonoid pro"les, namelyL.
maroccanaandL. rotundifolia, although in all seven species 8-hydroxy#avone
glyco-sides were the major#avonoid constituents.Lavandula maireivar.maireidi!ered from
all other members of this group in the presence of a chrysoeriol 7-glycoside (3) and apigenin glycosides (4and5) and the absence of 8-hydroxy#avone glycosides12}15. Again,L. minutoliivar.minutoliiwas distinguished by the absence of simple#avone glycosides and the presence of#avone aglycones. Within sectionSubnudae,L.
aristib-racteataandL. subnudahave almost identical leaf#avonoid pro"les with the absence
of the simple#avone glycosides found inL. dhofarensisandL. nimmoi.
Table 3
Presence of#avonoids in species, subspecies and varieties ofLavandulaandSabaudiabased on HPLC, PC and electrophoresis results%
Table 3*continued
Flavone glycosides 6-OH Flavone glycosides
Fla. C-gly.
8-OH Flavone 8- glycosides
8-OH Flavone 7-glycosides
Flavone aglycones (external)
Compound 1/2 3 4/5 6 7/8 9 10 11 12 13 14 15 16 17 18 19 20 21
Texon
SectionSubnuda
L. aristibracteata% ! ! ! ! ! ! ! ! # ## # $ $ $ ! ! ! #
L. dhofarensis% $ ! ! ! ! ! ! ! ## # ## ! $ ! ! ! ! !
L. subnuda ! ! ! ! ! ! ! ! ## # # ## $ $ ! ! ! !
SectionChaetostachys
L. bipinnata% $ ! ! ! ! ! ! ! ## ## # $ $ $ ! ! ! $
Sabaudiagroup
S. atriplicifolia $ ! $ # ! ! ! ! ! ! ! ! # ## ! ! ! !
%Extract subjected to electrophoresis; Fla. C-Gly.: FlavoneC-glycoside;!Flavonoid not detected by HPLC;$,#,##Relative amounts of#avonoids present (low- medium- or high-UV absorbance of the#avonoid peak, respectively).
1002
T.M.
Upson
et
al.
/
Biochemical
Systematics
and
Ecology
28
(2000)
991
}
Table 4
The distribution of#avonoids in additional species, subspecies and varieties ofLavandulaandSabaudia based on PC results only
Taxon Flavone glycosides 8-OH Flavone
glyco-sides
1 2 4 5 12 16
SectionStoechas
L. stoechassubsp.atlantica # # # # ! !
L. stoechassubsp.cariensis # # # # ! !
L. stoechassubsp.lusitanica # # # # ! !
L. stoechassubsp.pedunculata # # # # ! !
L. stoechassubsp.sampaiana # # # # ! !
SectionDentata
L. dentatavar.candicans # # # # ! !
SectionPterostoechas
L. antineae $ ! ! ! # #
L. buchiivar.buchii $ ! ! ! # #
L. buchiivar.gracile $ ! ! ! # #
L. maireivar.antiatlantica $ ! ! ! ! #
L. minutoliivar.tenuipinna ! ! ! ! # #
L. pinnata $ ! ! ! # #
L. tenuisecta $ ! ! ! # #
SectionSubnudae
L. nimmoi $ ! ! ! # #
Sabaudiagroup
S. erythreae # # ! ! ! #
was restricted to certain sections. For example, the #avone diglycosides, luteolin 7,4@-diglucuronide (7) and 7-glucoside-4@-glucuronide (8) were found only in section
Lavandula and the #avone C-glycoside, vitexin (11), only in sections Stoechas and
Dentata. 8-Hydroxy#avone glycosides occurred widely in sections Pterostoechas,
Subnudaand Chaetostachys and in Sabaudia, whereas 6-hydroxy#avone glycosides
were restricted to sectionDentata. As to leaf surface #avonoids, apigenin (18) and genkwanin (19) were regularly detected in members of sectionsStoechas, Dentataand
Lavandula, whereas the highly methoxylated external#avones20and21were found in
sectionsStoechas, Pterostoechas, SubnudaeandChaetostachys.
4. Discussion
The combined use of 2-D paper chromatography and HPLC analysis has provided both a detailed and comprehensive survey of the leaf#avonoids inLavandula. The
results show a strong correlation between the accumulation of certain #avonoid classes and groupings of species, which is congruent with the present sectional classi"cation. The variation found at the lower taxonomic ranks was either not great enough to be informative or appeared to be random and not correlated to any taxonomic entity presently recognised. However,#avonoid patterns do appear to be of systematic use at the sectional level and above inLavandula. The distribution of the
di!erent#avonoid classes in the sections ofLavandulaand inSabaudiais presented in
Table 5. In this Table accumulation rather than presence of a#avonoid class was often taken as a character, because chemotaxonomically accumulation of a chemical feature is more important than mere presence. The sensitivity of HPLC can reveal presence of trace compounds which serve as precursors of the ones that accumulate. For instance, small amounts of a#avone 7-monoglycoside were present in sectionPterostoechas,
SubnudaandChaetostachys, but only 8-hydroxylated#avone glycosides were
accumu-lated in these taxa.
Three major groupings of taxa can be identi"ed using #avonoid features as characters. The"rst group corresponds to sectionsStoechas,DentataandLavandula,
which are characterised by the accumulation of#avone 7-monoglycosides. However, each of these sections can be distinguished by its overall#avonoid pro"le (see Table 5). Thus, Lavandula is unique in producing #avonoid diglycosides, whereas sections
DentataandStoechasboth contain#avone C-glycosides. But sectionDentata di!ers
from section Stoechas in the presence of 6-hydroxylated #avone 7-glycosides. The second chemical group within the genus contains sectionsPterostoechas,Subnudaand
Chaetostachys, which are characterised by an accumulation of 8-hydroxylated#avone
7-and 8-glycosides. There are small individual di!erences among the#avonoid pro-"les of the species in these sections, but no overall distinction in#avonoid patterns among the three sections.
The third group corresponds to those taxa assigned to the genusSabaudia, which accumulate both 8-hydroxylated#avone 7-glycosides and#avone 7-glycosides. It is therefore characterised by the presence of compounds which otherwise are accumu-lated only in group one or group two. Flavone 7-glycosides are the most common type of#avonoid in the Lamiaceae, and therefore are not very informative about relation-ships within this family (TomaHs-BarberaHn et al., 1988). 8-Hydroxylated#avone glyco-sides, on the other hand, are uncommon in the family. Indeed, in subfamily Nepetoideae they have only been found in the genusLavandula(El Garf et al., 1999),
so that the presence of this class of#avonoid inSabaudiais a very strong indication that this taxon is very closely related to Lavandula, at least to those sections that
contain 8-hydroxy#avone glycosides. It is possible that Sabaudia is basal to all sections ofLavandula, but that the species of sectionsStoechas,DentataandLavandula
have all without exception lost the ability to synthesise the 8-hydroxy#avones. However, this does not seem very likely, as the ability to produce these#avonoids has not been lost in any of the taxa belonging to the other three sections. It seems more probable that a predecessor of Sabaudia has evolved the ability to produce 8-hydroxy#avone 7-glycosides and that this taxon is also basal to sections
Ptero-stoechas,SubnudaandChaetostachys, but not to the other three sections ofLavandula.
If this is the case, the immediate predecessor ofPterostoechas,Subnudaand
Table 5
The distribution of di!erent#avonoid classes in sections ofLavandulaand inSabaudia
tachys must also have evolved separately the ability to produce 8-hydroxy#avones
8-glycosides.
The major groupings of sections suggested by the #avonoid data are highly congruent with the major groupings suggested by other data sets such as gross morphology, carpology, molecular sequence data and palynology (Upson, 1997). At present, this grouping of sections is not re#ected in any classi"cations of the genus, but evidently these data need to be incorporated. The results obtained here provide further compelling evidence to support the recognition of these groupings. The rank at which to recognise these groups is a more complex question and in the taxonomic hierarchy they probably represent either subgenera or even distinct generic groupings.
Acknowledgements
The University of Reading is gratefully acknowledged for funding to T.M.U. through a postgraduate scholarship. We thank the following people for their help in supplying plant material for use in this study: J. Head (National Plant Collection holder ofLavandulaunder the National Council for the Conservation of Plants and
Gardens Scheme), W. Lobin (Botanischer Garten der UniversitaKt Bonn), A.G. Miller (Royal Botanic Garden, Edinburgh), H.D.V. Prendergast (Royal Botanic Gardens, Kew), N.J. Singh (Botanical Survey of India) and the Curators of the following herbaria: BM, C, E, M and O. Thanks to Dr. Stephen Jury of the University of Reading for help and advice in undertaking this work.
References
Boelens, M.H., 1995. Chemical and sensory evaluation ofLavandulaoils. Perfumer & Flavorist 20, 23}51. Brummit, R.K., Powell, C.E., 1992. Authors of Plant Names. Royal Botanic Gardens, Kew, UK. Cantino, P.D., Harley, R.M., Wagsta!, S.J., 1992. Genera of Labiatae: status and classi"cation. In: Harley,
R.M., Reynolds, T. (Eds.), Advances in Labiate Science. Royal Botanic Gardens, Kew, UK, pp. 511}522. Chaytor, D.A., 1937. A taxonomic study of the genusLavandula. J. Linnaean Soc. Botany 51, 153}204. Cufodontis, G., 1962. Enumeratio plantarum Aethiopiae spermatophyta. Bulletin du Jardin Botanique de
l'EDtat. Bulletin van den Rijksplantentuin. Brussels, 32 (Suppl.) pp. 806}807.
El-Garf, I., Grayer, R.J., Kite, G.C., Veitch, N.C., 1999. Hypolaetin 8-O-glucuronide and related#avonoids fromLavandula coronopifoliaandL. pubescens. Biochem. Syst. Ecology 27, 843}846.
Erdtman, G., 1945. Pollen morphology and plant taxonomy IV. Labiatae, Verbenaceae and Avicenniaceae. Svensk Botanisk Tidskrift 39, 279}285.
Ferreres, F., BarberaHn, F.A.T., TomaHs, F., 1986. Flavonoids from Lavandula dentata. Fitoterapia 57, 199}200.
Grayer, R.J., Kite, G.C., Goldstone, F.J., Bryan, S.E., Paton, A., Putievsky, E., 1996. Infraspeci"c taxonomy and essential oil chemotypes in sweet basil.Ocimum basilicum. Phytochemistry 43, 1033}1039. Harborne, J.B., 1998. Phytochemical Methods, 3rd Edition, Chapman & Hall, London.
Hegnauer, R., 1989. Chemotaxonomie der P#anzen, Vol. 8. BirkhaKuser, Basel.
Kaufmann, M., Wink, M., 1994. Molecular systematics of the Nepetoideae (Labiatae): phylogenetic implications fromrbcL gene sequences. Zeitschrift fuKr Naturforschung C49, 635}645.
Mabry, T.J., Markham, K.R., Thomas, M.B., 1970. The Systematic Identi"cation of Flavonoids. Springer, New York.
Miller, A.G., 1985. The genusLavandulain Arabia and Tropical N.E. Africa. Notes of the Royal Botanic Garden. Edinburgh 42, 503}528.
Ross, J.D., Sombrero, C., 1991. Environmental control of essential oil production in Mediterranean plants. In: Harborne, J.B., TomaHs-BarberaHn, F.A. (Eds.), Ecological Chemistry and Biochemistry of Plant Terpenoids. Clarendon Press, Oxford, pp. 83}94.
TomaHs-BarberaHn, F.A., Grayer-Barkmeijer, R.J., Gil, M.I., Harborne, J.B., 1988. Distribution of 6-hydroxy-, 6-methoxy- and 8-hydroxy#avone glycosides in the Labiatae, the Scrophulariaceae and related families. Phytochemistry 27, 2631}2645.
Upson, T.M., 1997. Systematics of the genusLavandulaL. (Lamiaceae). Ph.D. Thesis, University of Reading. Wagsta!, S.J., Olmstead, R.G., Cantino, P.D., 1995. Parsimony analysis of chloroplast DNA variation in
subfamily Nepetoideae. Am. J. Botany 82, 886}892.
Xaver, H., Andary, C., 1988. Polyphenols ofLavandula stoechasL. Bulletin Liaison Group Polyphenols 133, 624}626.