www.elsevier.com / locate / bres
Research report
The 4F2hc / LAT1 complex transports
L
-DOPA across the blood–brain
barrier
a b a b
Takashi Kageyama , Masaru Nakamura , Akinori Matsuo , Yasuomi Yamasaki ,
b b c d
Yoshinobu Takakura , Mitsuru Hashida , Yoshikatsu Kanai , Mikihiko Naito ,
d e a ,
*
Takashi Tsuruo , Nagahiro Minato , Shun Shimohama
a
Department of Neurology, Graduate School of Medicine, Kyoto University, 54 Shogoin-Kawaharacho, Sakyo-ku, Kyoto 606-8507, Japan
b
Department of Drug Delivery Research, Graduate School of Pharmaceutical Science, Kyoto University, Kyoto, Japan
c
Department of Pharmacology and Toxicology, Kyorin University School of Medicine, and PRESTO,
Japan Science and Technology Corporation(JST), Tokyo, Japan
d
Institute of Molecular and Cellular Biosciences, University of Tokyo, Tokyo, Japan
e
Department of Immunology and Cell Biology, Graduate School of Medicine, Kyoto University, Kyoto, Japan Accepted 25 July 2000
Abstract
L-DOPA is transported across the blood–brain barrier (BBB) by an amino acid transporter, system L. Recently, it has been
demonstrated that system L consists of two subunits, 4F2hc and either LAT1 or LAT2. 4F2hc / LAT1 and 4F2hc / LAT2 show different transport characteristics, while their distribution in the brain has not been determined. To clarify whether 4F2hc / LAT1 participates in
L-DOPA transport across the BBB, we first examined the expression of 4F2hc / LAT1 in the mouse brain capillary endothelial cell line,
MBEC4, as an in vitro BBB model. Northern hybridization and immunoblotting revealed that both 4F2hc and LAT1 are expressed and form a heterodimer in MBEC4 cells. To confirm whether 4F2hc / LAT1 acts as system L to transportL-DOPA, we characterizedL-DOPA
1
uptake into the cells. The uptake process was time-dependent, temperature-sensitive, and Na -independent. Neutral amino acids with bulky side chains and a bicyclic amino acid, 2-aminobicyclo-[2,2,1]-heptane-2-carboxylic acid (BCH), inhibitedL-DOPA uptake into
MBEC4 cells to a great extent, while an acidic amino acid, basic amino acids, and glycine had no effect. Other neutral amino acids, such as alanine, asparagine, glutamine, serine, and threonine inhibited L-DOPA uptake by 40–70% at most. These characteristics are more
compatible with those of 4F2hc / LAT1, rather than those of 4F2hc / LAT2. Finally, immunohistochemistry with anti-LAT1 antibody demonstrated that LAT1 is predominantly expressed in the microvessels of the central nervous system. This is the first report showing that the 4F2hc / LAT1 complex participates in L-DOPA transport across the BBB. 2000 Elsevier Science B.V. All rights reserved.
Theme: Cellular and molecular biology
Topic: Blood–brain barrier
Keywords: L-DOPA; Blood–brain barrier; System L; 4F2hc; LAT1
1. Introduction administration ofL-DOPA with a protein redistribution diet
[19] or with a catechol-O-methyltransferase inhibitor [16].
L-DOPA is probably the most widely used pharmaco- These methods might reduce the inhibitory effect of amino
logical agent for Parkinson’s disease, but it shows some acids or of the L-DOPA metabolite, 3-O-methyldopa, on
adverse effects, such as dyskinesia and motor fluctuation, L-DOPA transport into the brain. Therefore, it is important
especially in patients in the advanced stages of the disease. to know more about the L-DOPA transport system in the
These complications can be managed by combining oral brain so as to manage the problems in patients with advanced Parkinson’s disease.
Pharmacokinetic studies have revealed that L-DOPA is
*Corresponding author. Tel.: 181-75-751-3767; fax: 1
81-75-751-transported via an amino acid transporter, system L
9541.
E-mail address: [email protected] (S. Shimohama). [11,37]. System L prefers branched-chain amino acids,
aromatic amino acids and the nonmetabolizable analog established according to the method of Williams et al. [36] 2-aminobicyclo-[2,2,1]-heptane-2-carboxylic acid (BCH) with some modifications [31]. The cells were maintained [5]. This system does not transport any acidic or basic in Dulbecco’s modified Eagle’s medium (DMEM, obtained amino acids and its activity is not altered by the depletion from Nissui Co. Ltd., Tokyo) supplemented with 10% fetal
1
of Na from the extracellular fluid. bovine serum (FBS), 100 units / ml of penicillin and 100 Recently, system L has been cloned and found to be mg / ml of streptomycin at 378C under 95% O / 5% CO .2 2 identical to the T-cell surface antigen, 4F2 (of which the
murine homologue is CD98) [4,14,18]. 4F2 is a
hetero-dimeric protein consisting of two subunits. One of these is 2.2. Northern hybridization analysis an 80-kDa glycosylated protein, 4F2hc (the heavy chain of
4F2 cell surface antigen), which has one membrane-span- The cDNA fragment corresponding to 792–1165 bp of ning domain. The other is a 40-kDa nonglycosylated 4F2hc cDNA [23] was used as a probe for Northern protein with 12 membrane-spanning domains, named hybridization. To prepare the probe for LAT1, mouse LAT1 (L-type amino acid transporter 1) [3,14,20,26] or LAT1 cDNA (GeneBank, EMBL, DDBJ accession No. LAT2 (L-type amino acid transporter 2) [25,28]. 4F2hc AB023409) cloned into pSPORT1 (GibcoBRL) was di-and either LAT1 or LAT2 bind to one another by disulfide gested with Pst 1. The cDNA fragment corresponding to bonds. LAT1 or LAT2, rather than 4F2hc, might determine 147–707 bp of mouse LAT1 was separated on agarose gel the properties of system L because 4F2hc binds to an electrophoresis and purified using the phenol-chloroform alternative light chain showing the transport activities of extraction method.
1 1
system y L or of the cystine / glutamate exchanger For Northern analysis, poly (A) RNA was isolated [24,27,32]. While LAT1 and LAT2 show 50% identity in from the total RNA of MBEC4 cells using OligotexE
-1
the cDNA sequence, there are some differences in the dT30 (Roche). Two microgram of poly (A) RNA was substrate selectivity between 4F2hc / LAT1 and 4F2hc / then blotted onto a GeneScreen Plus (NENE Life Science LAT2 [25,28]. In Xenopus oocytes expressing 4F2hc / Products). The probes for LAT1 cDNA or 4F2hc cDNA LAT1, alanine, asparagine, glutamine, serine, and were labelled with alkaline phosphatase, hybridized and threonine inhibit leucine uptake by 40–90% of control detected using the AlkPhos Direct system for chemi-values [14]. When 4F2hc / LAT2 is overexpressed in oo- luminescence (Amersham Pharmacia Biotech).
cytes, however, these amino acids, as well as large neutral
amino acids, inhibit L-leucine uptake by less than 20% of 2.3. Immunoblotting 1
control values in the absence of Na [25,28]. Furthermore,
glycine, which does not compete with leucine at 4F2hc / MBEC4 cells were lysed with lysis buffer (1% Nonidet LAT1, inhibits leucine uptake by 4F2hc / LAT2 by less P-40, 50 mM Tris–HCl, pH 7.4, 150 mM NaCl, 2 mM than 50% [25,28]. To date, it has not been determined phenylmethylsulfonyl fluoride, 1 mM Na VO , 2 mM2 4 which isoform of system L, 4F2hc / LAT1 or 4F2hc / LAT2, NaF, 5 mM EDTA). An equal volume of sample buffer
transportsL-DOPA across the BBB. (124 mM Tris–HCl, pH 6.8, 4% SDS, 10% glycerol,
In the present study, we examined the expression of 0.02% bromophenol blue), with or without 4% 2-mercap-4F2hc and LAT1 in the mouse brain capillary endothelial toethanol, was then added to the lysate. The mixture was cell line, MBEC4 [29,31]. This cell line is regarded as an heated for 5 min at 1008C, separated by 7.5% SDS in vitro BBB model because it forms tight junctions, shows polyacrylamide gel electrophoresis (SDS–PAGE), and then high alkaline phosphatase and g-glutamyl transpeptidase blotted onto a polyvinylidine difluoride membrane. The activity [1,9,21], and transports substrates for P-glycopro- membrane was incubated with LAT1 [20] or anti-tein unidirectionally as seen in vivo [6]. To confirm 4F2hc (Santa Cruz Biotechnology Inc.) antibody followed whether 4F2hc / LAT1 contributes to L-DOPA transport in by horseradish peroxidase-conjugated second antibody,
MBEC4 cells, we further characterized theL-DOPA uptake and developed with the ECL Western blotting detection
into the cells. Finally, we demonstrated that LAT1 is system (Amersham Pharmacia Biotech). expressed in the microvessels in the central nervous system
3
(CNS) of adult mice. These results suggest that the 4F2hc / 2.4. Cellular uptake of [ H]-L-DOPA LAT1 complex transportsL-DOPA across the BBB in vivo.
MBEC4 cells were seeded into Corning 24-well plates at 4
a concentration of 6310 / ml, and L-DOPA uptake was
2. Materials and methods measured 2 days later. Cultures were equilibrated for more
than 20 min in a preincubation buffer containing 125 mM 2.1. Preparation of the mouse brain endothelial cell line NaCl, 5.6 mMD-glucose, 4.8 mM KCl, 1.2 mM MgSO ,4
(MBEC4) 1.2 mM KH PO , 1.3 mM CaCl , and 25 mM HEPES2 4 2
1
[33]. To evaluate the Na -dependency of the uptake, 1
chloride for NaCl. The pH of the buffer was adjusted to 7.4 using Tris base. L-DOPA uptake was initiated by
draining the cultures and adding 0.5 ml of buffer con-taining 10–1000mML-DOPA with 0.5mCi ofL
-3,4-[ring-3 3
2,5,6- H]-dihydroxyphenylalanine ([ H]-L-DOPA, 60 Ci /
mmol, ICN / Moravek). To examine the inhibitory effects on L-DOPA uptake, 1 mM of each amino acid or amino
3
acid derivative was added to a 10 mM [ H]-L-DOPA
solution. Uptake was terminated by washing the cultures three times with 0.5 ml ice-cold buffer. The cells were lysed with 0.3 M NaOH containing 0.1% Triton-X and the radioactivity of the lysate was measured by liquid scintilla-tion counting. The protein content was determined by the method of Lowry et al. [17], using bovine serum albumin as the protein standard. The uptake values were expressed as uptake rate (nmol / mg protein / min) after correction for extracellularly adsorbed L-DOPA, estimated from the
ap-14
parent uptake of [ C(U)]-sucrose (Dupont NEN). The uptake rate of L-DOPA influx was estimated based on the
uptake over 1 min, because linearity of uptake was sustained over this period (Fig. 2A). The kinetic constants, Km and Vmax, for L-DOPA uptake were calculated by
non-linear regression analysis, using MULTI [37]. All experiments were repeated with more than three different cell preparations to demonstrate that the results were qualitatively reproducible.
2.5. Immunohistochemistry
BALB / c mice (2 male, 2 female, 8–10 weeks old) were anesthetized with pentobarbital and perfused intracardially with 4% paraformaldehyde and 0.2% picrate in 0.1 M phosphate buffer, pH 7.4. The brains were removed and
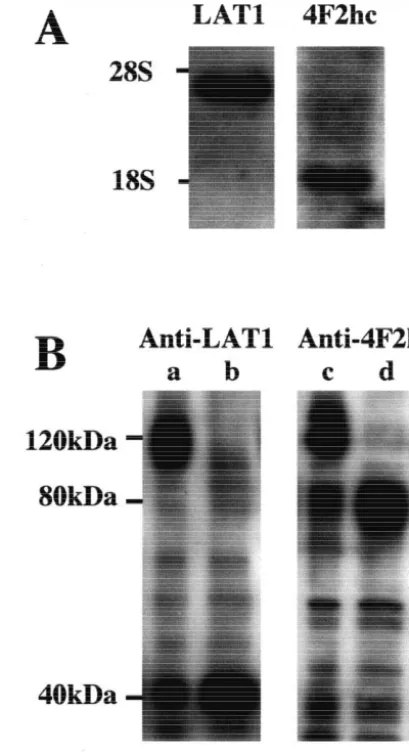
Fig. 1. Expression of LAT1 and 4F2hc in MBEC4 cells. (A) Northern
fixed overnight in 4% paraformaldehyde, transferred to 1
hybridization of poly (A) RNA from MBEC4 cells with cDNA probes
20% (w / v) sucrose containing 0.2% (w / v) sodium azide in
for LAT1 and 4F2hc. The LAT1 and 4F2hc cDNA probes hybridized to
0.1 M phosphate buffer, pH 7.4, and stored at 48C for at 3.5-kb and 1.8-kb transcripts, respectively. (B) Immunoblotting of LAT1 least 2 days. The brains were then rapidly frozen and cut and 4F2hc. Under reducing conditions, bands of|40 kDa and|80 kDa were detected with anti-LAT1 (b) and anti-4F2hc (d) antibody,
respec-into 16 mm slices. The slices were preblocked, incubated
tively. Under nonreducing conditions, both antibodies bound to a band at
with anti-LAT1 monoclonal antibody, and then with
|120 kDa (a, c). 2-ME, 2-Mercaptoethanol. biotin-conjugated goat anti-hamster IgG which was
de-tected using the ABC kit (Vector, Burlingame, CA).
cells, respectively (Fig. 1A). Immunoblotting exhibited a
2.6. Statistical analysis band of |40 kDa and |80 kDa with anti-LAT1 and
anti-4F2hc antibody, respectively, in the presence of 2-All data are expressed as mean6standard error (S.E.M.). mercaptoethanol. Both antibodies recognized a band of Statistical analysis was carried out using the Student’s |120 kDa on SDS–PAGE under nonreducing conditions
t-test and the Dunnett’s test. P,0.05 was considered to be (Fig. 1B). statistically significant.
3.2. L-DOPA uptake into MBEC4 cells
3
3. Results At 378C, [ H]-L-DOPA was taken up into MBEC4 cells
in a time-dependent manner and reached a plateau after 20 3.1. The expression of LAT1 and 4F2hc in MBEC4 min at a concentration of 100mM both in the presence and
1 1
3
Fig. 2. Kinetics ofL-DOPA uptake in MBEC4 cells. (A) Time-course of [ H]-L-DOPA uptake at a concentration of 100mM in the presence (j,d) and the
1
absence (h,s) of Na . L-DOPA uptake was significantly suppressed at 48C (d,s), compared with that at 378C (j,h). (B) Relationship between
1
L-DOPA concentration and the initialL-DOPA uptake by a saturable carrier in the absence of Na . The saturable component ofL-DOPA uptake was calculated by subtracting values at 48C from those at 378C.
L-DOPA uptake was concentration-dependent at 378C, 80–90% by non-radiolabelled L-DOPA, histidine,
iso-and was apparently suppressed at 48C. The uptake values leucine, leucine, methionine, phenylalanine, tryptophan, at 48C increased linearly in proportion to the concentration tyrosine, valine, and BCH. In contrast, there was little or of L-DOPA applied, suggesting the existence of a non- negligible inhibition by proline, glycine, glutamate, lysine,
saturable component ofL-DOPA uptake (data not shown). or arginine. Other amino acids, such as alanine, asparagine,
The values obtained by subtracting the uptake at 48C from glutamine, serine, and threonine suppressed L-DOPA
up-that at 378C were taken as the saturable component (Fig. take by 40–70% of the control value. These inhibition 2B). The Km and Vmax of the saturable component in the profiles were not altered by the presence of extracellular
1 1
absence of Na were 88.2610.4 mM and 3.6360.32 Na (data not shown). nmol / mg protein / min, respectively. These values were not
1
significantly changed in the presence of Na (Km 3.3. The expression of LAT1 in the mouse CNS 95.465.6 mM, Vmax 5.5461.21 nmol / mg protein / min).
Fig. 3 summarizes the inhibitory effects of various Anti-LAT1 monoclonal antibody recognized microves-1
amino acids onL-DOPA transport in Na -depleted buffer. sels in the mouse brain (Fig. 4A and 4B) and spinal cord
3
[ H]-L-DOPA uptake in MBEC4 cells was inhibited up to (data not shown). Microvessels were not stained in the
1 3
Fig. 3. Inhibition ofL-DOPA uptake by amino acids in the absence of Na . Initial uptake of 10mM [ H]-L-DOPA was measured in the presence of 1 mM non-radiolabelled L-amino acids and a system L-specific substrate, BCH. Values are expressed as % of control uptake (nmol / mg protein / min). At pH 7.4,
3
contributes to L-DOPA uptake in MBEC4 cells. Although L-DOPA was taken up into the cells by both saturable and
non-saturable mechanisms, we consider that the saturable component is essential for L-DOPA transport. This is
because this in vitro BBB model lacks astrocytes which cooperate with endothelial cells to tighten the BBB [2,13], resulting in the simple diffusion of substrates occurring more easily than through the BBB in vivo. Previous studies have also suggested thatL-DOPA is transported in a
saturable manner in the brain [22,35]. The present study showed that substitution of choline chloride for NaCl in the uptake buffer did not influence the Km and Vmax of the saturable component. L-DOPA uptake was almost
completely blocked by 100 times excess of histidine, isoleucine, leucine, methionine, phenylalanine, tryptophan, tyrosine, valine, and BCH. In contrast, either an acidic amino acid (glutamate), basic amino acids (lysine and arginine) or glycine did not compete withL-DOPA. Other
amino acids such as alanine, asparagine, glutamine, serine and threonine did not inhibit L-DOPA uptake as much as
neutral amino acids with bulky side chains. These charac-teristics of L-DOPA uptake in MBEC4 cells were very
similar to those of L-leucine uptake seen in oocytes
expressing 4F2hc / LAT1 [14], suggesting that 4F2hc / LAT1 is the main complex participating inL-DOPA uptake
into MBEC4 cells.
LAT1 mRNA is region-specifically expressed in the
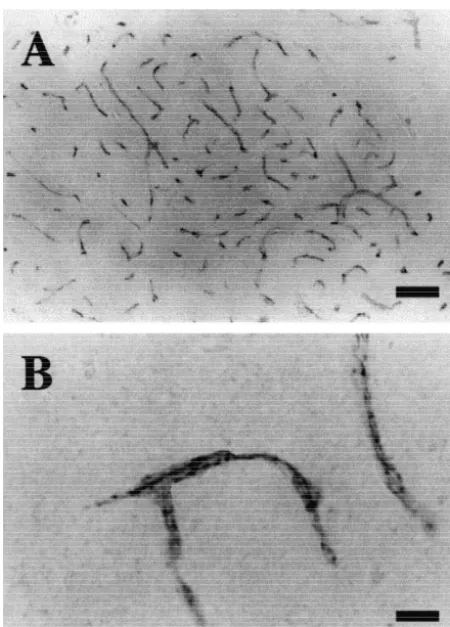
Fig. 4. The expression of LAT1 in the mouse brain. Immunohisto- brain, spleen, placenta and testis, while 4F2hc mRNA is chemistry revealed that LAT1 is mainly expressed in the microvessels in diffusely distributed in many organs [14,20]. Boado et al. the brain parenchyma. (A) Lower magnification (3100). Scale bar5100
showed a high expression of LAT1 mRNA in the bovine
mm. (B) Higher magnification (31000). Scale bar510mm.
BBB [3]. Using immunohistochemistry with anti-LAT1 monoclonal antibody, we first demonstrated that LAT1 is sections incubated with only the second antibody or expressed on the microvessels both in the brain and the preimmune serum (data not shown). Neurons in the spinal cord. Together with the previous finding that 4F2hc substantia nigra, striatum, locus ceruleus, or raphe nuclei, is expressed in the cerebral microvessels of the mouse were not stained (data not shown). [20], we conclude that the 4F2hc / LAT1 complex operates as a major component of system L in the BBB in vivo.
The 4F2hc / LAT1 complex prefers phenylalanine,
4. Discussion tryptophan, tyrosine, and L-DOPA, which are the
pre-cursors of catecholamine and serotonin. However, the The present study has demonstrated for the first time expression of LAT1 was not prominent in specific neurons that both LAT1 and 4F2hc are expressed and form a such as catecholaminergic and serotonergic neurons. This heterodimer by disulfide bonds in MBEC4 cells. The suggests that other neutral amino acid transporters might cDNA probes for LAT1 and 4F2hc were hybridized with function in neuronal cells taking up amino acids for mRNAs of 3.5-kb and 1.8-kb length, which, respectively, neurotransmitter synthesis. Indeed, LAT2 mRNA is also corresponds to full-length messages of these proteins detected in the brain [25,28]. Furthermore, it was reported
1
described previously [20,23]. Immunoblotting also ex- that L-DOPA accumulates in a Na -dependent manner in
1 hibited the presence of these proteins. In non-reducing the brain [12,15]. Therefore, 4F2hc / LAT2 or other Na
-1 0,1 0
conditions, both anti-LAT1 and anti-4F2hc antibodies dependent transporters such as y L [7,8], B [34], or B recognized a|120 kDa band. When the cRNAs of 4F2hc [10,30], might also participate in transporting large neutral
and LAT1 are cotransfected into COS cells or oocytes, amino acids in the CNS.
these two proteins form a heterodimer of |120 kDa by In conclusion, the present study is the first to
demon-disulfide bonds [18,20]. Therefore, these results suggest strate that the 4F2hc / LAT1 complex participates in L
-that LAT1 and 4F2hc bind to one another by disulfide DOPA transport in the in vitro BBB model. Furthermore,
bonds in MBEC4 cells. we demonstrated that LAT1 is expressed in the brain in
[13] R.C. Janzer, M.C. Raff, Astrocytes induce blood–brain barrier
complex transports L-DOPA across the BBB in vivo.
properties in endothelial cells, Nature 325 (1987) 253–257.
Although further study is needed to clarify whether the
[14] Y. Kanai, H. Segawa, K. Miyamoto, H. Uchino, E. Takeda, H.
impairment of the blood-to-brain transfer of L-DOPA is
Endou, Expression cloning and characterization of a transporter for
associated with motor fluctuation in patients with advanced large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98), J. Biol. Chem. 273 (1998) 23629–23632.
Parkinson’s disease, the 4F2hc / LAT1 complex might be a
[15] K. Kawai, H. Ohta, A. Kubodera, M.A. Channing, W.C. Eckelman,
key molecule in making the L-DOPA treatment more
Synthesis and evaluation of radioiodinated 6-iodo-L-DOPA as a
appropriate in patients with Parkinson’s disease.
cerebral L-amino acid transport marker, Nucl. Med. Biol. 23 (1996) 251–255.
[16] the Tolcapone Fluctuator Study Group I, M.C. Kurth, C.H. Adler, M.St. Hilaire, C. Singer, C. Waters, P. LeWitt, D.A. Chernik, E.E.
Acknowledgements
Dorflinger, K. Yoo, Tolcapone improves motor function and reduces levodopa requirement in patients with Parkinson’s disease
ex-This work was supported by a Grant-in-Aid for Sci- periencing motor fluctuations: a multicenter, double-blind, random-entific Research from the Ministry of Education, Science, ized, placebo-controlled trial, Neurology 48 (1997) 81–87.
Sports and Culture, Japan and grants from the Ministry of [17] O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, Protein measurement with the folin phenol reagent, J. Biol. Chem. 193
Welfare of Japan. We thank Dr Hiroshi Shibasaki for
(1951) 265–275.
reading this manuscript and providing some useful
com-[18] L. Mastroberardino, B. Spindler, R. Pfeiffer, P.J. Skelly, J. Loffing,
ments. C.B. Shoemaker, F. Verrey, Amino-acid transport by heterodimers of
4F2hc / CD98 and members of a permease family, Nature 395 (1998) 288–291.
[19] I. Mena, G.C. Cotzias, Protein intake and treatment of Parkinson’s
References
disease with levodopa, N. Engl. J. Med. 292 (1975) 181–184. [20] E. Nakamura, M. Sato, H. Yang, F. Miyagawa, M. Harasaki, K. [1] Z. Albert, M. Orlowski, Z. Rzucidlo, J. Orlowska, Studies on Tomita, S. Matsuoka, A. Noma, K. Iwai, N. Minato, 4F2 (CD98) gamma-glutamyl transpeptidase activity and its histochemical locali- heavy chain is associated covalently with an amino acid transporter zation in the central nervous system of man and different animal and controls intracellular trafficking and membrane topology of 4F2 species, Acta Histochem. 25 (1966) 312–320. heterodimer, J. Biol. Chem. 274 (1999) 3009–3016.
[2] F.E. Arthur, R.R. Shivers, P.D. Bowman, Astrocyte-mediated induc- [21] M. Orlowski, G. Sessa, J.P. Green, Gamma-glutamyl transpeptidase tion of tight junction in brain capillary endothelium: an efficient in in brain capillaries: possible site of a blood–brain barrier for amino vitro model, Brain Res. 433 (1987) 155–159. acids, Science 184 (1974) 66–68.
[3] R.J. Boado, J.Y. Li, M. Nagaya, C. Zhang, W.M. Pardridge, Selective [22] W.M. Pardridge, W.H. Oldendorf, Kinetic analysis of blood–brain expression of the large neutral amino acid transporter at the blood– barrier transport of amino acids, Biochim. Biophys. Acta 401 (1975) brain barrier, Proc. Natl. Acad. Sci. USA 96 (1999) 12079–12084. 128–136.
¨ ¨
[4] S. Broer, A. Broer, B. Hamprecht, The 4F2hc surface antigen is [23] M.S. Parmacek, B.A. Karpinski, K.M. Gottesdiener, C.B. Thomp-necessary for expression of system L-like neutral amino acid- son, J.M. Leiden, Structure expression and regulation of the murine transport activity in C6-BU-1 rat glioma cells: evidence from 4F2 heavy chain, Nucl. Acids Res. 17 (1989) 1915–1931. expression studies in Xenopus laevis oocytes, Biochem. J. 312 [24] R. Pfeiffer, G. Rossier, B. Spindler, C. Meier, L. Khhn, F. Verrey,
1
(1995) 863–870. Amino acid transport of y L-type by heterodimers of 4F2hc / CD98 [5] H.N. Christensen, M.E. Handlogten, I. Lam, H.S. Tager, R. Zand, A and members of the glycoprotein-associated amino acid transporter
bicyclic amino acid to improve discriminations among transport family, EMBO J. 18 (1999) 49–57.
´ ´ ´
systems, J. Biol. Chem. 244 (1969) 1510–1520. [25] M. Pineda, E. Fernandez, D. Torrents, R. Estevez, C. Lopez, M. ´
[6] C. Cordon-Cardo, J.P. O’Brien, D. Casals, L. Rittman-Grauer, J.L. Camps, J. Lloberas, A. Zorzano, M. Palacın, Identification of a Biedler, M.R. Melamed, J.R. Bertino, Multidrug-resistance gene membrane protein, LAT-2, that co-expresses with 4F2 heavy chain, (P-glycoprotein) is expressed by endothelial cells at blood–brain an L-type amino acid transport activity with broad specificity for barrier sites, Proc. Natl. Acad. Sci. USA 86 (1989) 695–698. small and large zwitterionic amino acids, J. Biol. Chem. 274 (1999)
´
[7] R. Deves, P. Chavez, C.A.R. Boyd, Identification of a new transport 19738–19744.
system (y1L) in human erythrocytes that recognizes lysine and [26] P.D. Prasad, H. Wang, W. Huang, R. Kekuda, D.P. Rajan, F.H. leucine with high affinity, J. Physiol., Lond. 454 (1992) 491–501. Leibach, V. Ganapathy, Human LAT1, a subunit of system L amino
´ ´
[8] R. Deves, S. Angelo, P. Chavez, N-ethylmaleimide discriminates acid transporter: molecular cloning and transport function, Biochem. between two lysine transport systems in human erythrocytes, J. Biophys. Res. Commun. 255 (1999) 283–288.
Physiol., Lond.) 468 (1993) 753–766. [27] H. Sato, M. Tamba, T. Ishii, S. Bannai, Cloning and expression of a [9] B.M. Djuricic, B.B. Mrsulja, Enzymic activity of the brain: mi- plasma membrane cystine / glutamate exchange transporter com-crovessels vs. total forebrain homogenate, Brain Res. 138 (1977) posed of two distinct proteins, J. Biol. Chem. 274 (1999) 11455–
561–564. 11458.
[10] F.A. Doyle, J.D. McGivan, The bovine renal epithelial cell line [28] H. Segawa, Y. Fukasawa, K. Miyamoto, E. Takeda, H. Endou, Y.
1
NBL-1 expresses a broad specificity Na -dependent neutral amino Kanai, Identification and functional characterization of a Na1 -acid transport system (System B0) similar to that in bovine renal independent neutral amino acid transporter with broad substrate brush border membrane vesicles, Biochim. Biophys. Acta 1104 selectivity, J. Biol. Chem. 274 (1999) 19745–19751.
(1992) 55–62. [29] A. Shirai, M. Naito, T. Tatsuta, J. Dong, K. Hanaoka, K. Mikami, T. [11] P. Gomes, P. Soares-da-Silva, L-DOPA transport properties in an Oh-hara, T. Tsuruo, Transport of cyclosporin A across the brain immortalised cell line of rat capillary cerebral endothelial cells, capillary endothelial cell monolayer by P-glycoprotein, Biochim. RBE4, Brain Res. 829 (1999) 143–150. Biophys. Acta 1222 (1994) 400–404.
[31] T. Tatsuta, M. Naito, T. Oh-hara, T. Sugawara, T. Tsuruo, Func- broad-scope system in mouse blastcysts, J. Biol. Chem. 260 (1985) tional involvement of P-glycoprotein in blood–brain barrier, J. Biol. 12118–12123.
Chem. 267 (1992) 20383–20391. [35] L.A. Wade, R. Katzman, Synthetic amino acids and the nature of
´ ´
[32] D. Torrents, R. Estevez, M. Pineda, E. Fernandez, J. Lloberas, Y.-B. L-dopa transport at the blood–brain barrier, J. Neurochem. 25 ´
Shi, A. Zorzano, M. Placın, Identification and characterization of a (1975) 837–842.
1
membrane protein (y L amino acid transporter-1) that associates [36] S.K. Williams, J.F. Gillis, M.A. Matthews, R.C. Wagner, M.W.
1
with 4F2hc to encode the amino acid transporter activity y L, J. Bitensky, Isolation and Characterization of brain endothelial cells: Biol. Chem. 273 (1998) 32437–32445. Morphology and enzyme activity, J. Neurochem. 35 (1980) 374– [33] M.J. Tsai, E.H.Y. Lee, Characterization of L-DOPA transport in 381.
cultured rat and mouse astrocytes, J. Neurosci. Res. 43 (1996) [37] K. Yamaoka, Y. Tanigawara, T. Nakagawa, T. Uno, A
phar-490–495. macokinetic analysis program (MULTI) for microcomputer, J.
1
![Fig. 2. Kinetics of L-DOPA uptake in MBEC4 cells. (A) Time-course of [ H]-3L-DOPA uptake at a concentration of 100 mM in the presence (j, d) and theabsence (h, s) of Na .1 L-DOPA uptake was significantly suppressed at 48C (d, s), compared with that at 378C](https://thumb-ap.123doks.com/thumbv2/123dok/3140560.1382966/4.612.123.475.64.237/kinetics-uptake-concentration-presence-theabsence-signicantly-suppressed-compared.webp)