www.elsevier.com / locate / bres
Research report
The effects of protein kinase C and calmodulin kinase II inhibitors on
vestibular compensation in the guinea pig
a a ,
*
b aAndrew J. Sansom , Paul F. Smith
, Cynthia L. Darlington , Richard Laverty
a
Vestibular Research Group, Department of Pharmacology, School of Medical Sciences, University of Otago Medical School and Neuroscience Research Centre, University of Otago, Dunedin, New Zealand
b
Vestibular Research Group, Department of Psychology, School of Medical Sciences, University of Otago Medical School and Neuroscience Research Centre, University of Otago, Dunedin, New Zealand
Accepted 2 August 2000
Abstract
Previous studies have demonstrated that vestibular compensation, the process of behavioural recovery which occurs following unilateral deafferentation of the vestibular labyrinth (UVD), is correlated with changes in in vitro phosphorylation of various protein substrates in the brainstem vestibular nucleus complex (VNC). The aim of the present study was to investigate the possible causal relationship between protein kinase activity and the induction of the vestibular compensation process, by delivering inhibitors of protein kinase C (PKC) or
21
Ca / calmodulin-dependent kinase II (CaMKII) into the ipsilateral VNC at the time of the UVD and determining their effects on three static symptoms of UVD, spontaneous nystagmus (SN), yaw head tilt (YHT) and roll head tilt (RHT) in guinea pigs. Infusion of the PKC inhibitor, 3-[1-(3-dimethylaminopropyl)-1H-indol-3-yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione, HCl (bisindolylmaleimide I, HCl / GF 109203X, HCl) (‘Bis I’), at a concentration of 5 or 50mM, significantly increased SN frequency at the earliest time points (6 and 8 h post-UVD) compared to vehicle controls and the less selective analogue, 2,3-bis(1H-indol-3-yl)-N-methylmaleimide (bisindolylmaleimide V) (‘Bis V’). However, the compensation of YHT and RHT was unaffected by the PKC inhibitor. By contrast, the cell-permeable CaMKII inhibitor, myristoylated autocamtide-2 related inhibitory peptide (N-Myr-Lys-Lys-Ala-Leu-Arg-Arg-Gln-Glu-Ala-Val-Asp-Ala-Leu-OH) (‘myr-AIP’) or the cell-impermeable analogue, autocamtide-2 related inhibitory peptide (N-Lys-Lys-Ala-Leu-Arg-Arg-Cln-Glu-Ala-Val-Asp-Ala-Leu-OH) (‘AIP’), failed to alter the compensation of SN, YHT or RHT at any dose compared to vehicle controls. These results implicate PKC-, but not CaMKII-, signal transduction pathways in the initiation of SN compensation in guinea pig. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Auditory, vestibular, and lateral line: periphery
21
Keywords: Protein kinase C; Vestibular compensation; Ca / calmodulin-dependent kinase II; Vestibular nuclei; Neural plasticity
1. Introduction spontaneous nystagmus (SN), yaw head tilt (YHT) and roll
head tilt (RHT)] and therefore relate to the imbalance in Unilateral deafferentation of the peripheral vestibular resting activity between neurons in the ipsilateral and labyrinth (UVD) results in a complex syndrome of eye contralateral vestibular nucleus complexes (VNC). The movement and postural disorders which arises from the dynamic symptoms are those which are observed during imbalance in tonic and dynamic neuronal activity in the head movement [e.g., reduced gain of the vestibulo-ocular vestibulo-ocular and vestibulo-spinal reflex pathways [43]. reflex (VOR)] and are related to the reduced sensitivity of The static symptoms of UVD have been referred to as VNC neurons to head movement stimuli (see Ref. [6] for a those which persist in the absence of head movement [e.g., review). Over time, the severity of some static symptoms gradually decreases in a process of behavioural recovery known as ‘vestibular compensation’. Although a partial
*Corresponding author. Tel.:164-3-479-5747; fax: 164-3-479-5747
recovery of resting activity in the VNC ipsilateral to the
or 9140.
E-mail address: [email protected] (P.F. Smith). UVD (ipsi-VNC) is believed to be largely responsible for
the compensation of the static symptoms, the biochemical selective CaMKII inhibitor was not employed. The present basis of this resting activity recovery, and the other experiment attempted to further investigate the role of neuronal changes responsible for compensation, are poorly protein kinases in static compensation by comparing the understood (see Refs. [6,38] for a review). effects of PKC and CaMKII inhibitors, delivered into the
21
Protein kinase C (PKC) and Ca / calmodulin-depen- ipsi-VNC at the time of the UVD in guinea pig. dent kinase II (CaMKII) are major serine / threonine
pro-tein kinases found in particularly high concentrations in
brain [42]. There is good evidence that these kinases play 2. Methods important roles in several types of neuronal plasticity (e.g.,
[3,13]; see Ref. [40] for a recent review). A number of 2.1. Animals studies have shown that vestibular compensation in frog
and guinea pig is accompanied by changes in the phos- Fifty-four adult male and female pigmented guinea pigs phorylation of specific brain proteins in vitro [10,22– (Cavia porcellus; 290–725 g) were used in these experi-24,35]. In guinea pig, the phosphorylation of at least one ments, twenty four each for the PKC inhibitor and CaMKII probable PKC substrate in the bilateral medial vestibular inhibitor experiments in which cannulae were placed nucleus / prepositus hypoglossi, increased following com- stereotaxically in the right VNC (see Table 1). In addition, pensation of the static symptoms compared to sham-oper- data for the two doses of the PKC inhibitor were obtained
21
ated controls [35]. This contrasts with a lack of Ca / from six animals (n53 for each dose) in which the cannula calmodulin-stimulated protein phosphorylation changes in was placed in the IVth ventricle as an additional control. the same tissue. There is also some evidence that the in Although the animals used in these experiments varied in vitro phosphorylation changes that occur in frog whole weight, they were all adult guinea pigs and in our previous brains, removed at different stages of compensation, are studies we have not observed differences in vestibular mediated by an endogenous PKC and CaMKII [21,22]. compensation for adult animals of different weight [39]. These results suggest that the phosphorylation state of
specific endogenous proteins in areas of the brain involved 2.2. Cannula implantation in processing vestibular information, is changed during
vestibular compensation in vivo. Several potential mecha- All surgical procedures were approved by the University nisms could account for these changes, including de novo of Otago Committee on Ethics in the Care and Use of synthesis of substrate proteins, and / or a change in kinase Laboratory Animals. The cannula implantation procedure activity. Direct evidence that the initial stages of static has been described in detail elsewhere [34]. Briefly, compensation in guinea pig are not associated with a animals were anaesthetised with fentazin (0.4 ml / kg, i.m.; change in de novo protein synthesis was recently reported fentazin contains 0.4 mg / ml fentanyl citrate, 58.3 mg / ml by Ris et al. [31], who found that systemic injections of a xylazine HCl and 3.2 mg / ml azaperone; Parnell Lab-protein synthesis inhibitor (cycloheximide) had no effect oratories, Auckland) and a 21-gauge metal cannula was upon the early stages of resting activity recovery in the lowered through the cerebellar vermis into the right VNC ipsi-VNC of the guinea pig. However, the possibility that
later stages of compensation might involve de novo protein Table 1
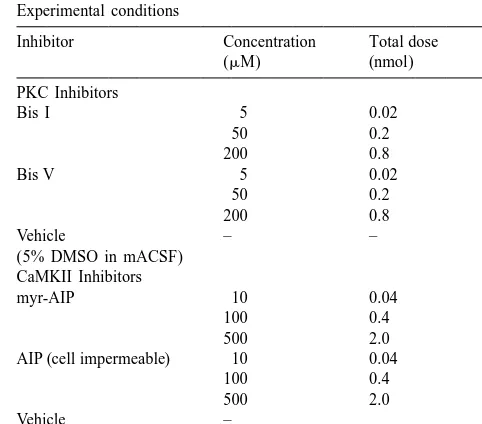
synthesis cannot be excluded [4]. The hypothesis that Experimental conditions
mammalian vestibular compensation might involve a Inhibitor Concentration Total dose N change in PKC activity is further supported by recent (mM) (nmol)
immunohistochemical experiments, which have shown that PKC Inhibitors
early stages of compensation in rat are accompanied by Bis I 5 0.02 4
changes in the expression of vestibulo-cerebellum PKC- 50 0.2 4
200 0.8 2
alpha, -gamma and / or -delta [2,12] or VNC PKC-delta
Bis V 5 0.02 4
[28]. Taken together, these data suggest that vestibular
50 0.2 4
compensation of the static symptoms is correlated with 200 0.8 2
changes in PKC / CaMK pathways in the VNC and / or Vehicle – – 4
cerebellum; however, it is not clear whether these changes (5% DMSO in mACSF) CaMKII Inhibitors
are causally involved in the compensation process or
myr-AIP 10 0.04 4
merely coincident with it.
100 0.4 4
Despite the correlative evidence implicating protein 500 2.0 3
phosphorylation in the VNC in the mechanisms of vestibu- AIP (cell impermeable) 10 0.04 3
lar compensation [23,35], to date there has been only one 100 0.4 4
500 2.0 3
study (in rat [1]) which has examined the effects of kinase
Vehicle – 3
inhibitors on the compensation process, and in this case
(mACSF)
complex (stereotaxic co-ordinates: 12.5 mm caudal; 1.5 autocamtide-2 related inhibitory peptide (AIP; N-Lys-Lys-mm lateral; 10.8 N-Lys-Lys-mm ventral to the skull surface) and Ala-Leu-Arg-Arg-Gln-Glu-Ala-Val-Asp-Ala-Leu-OH; MW secured to the skull with dental cement. Animals (n56) 1498), were purchased from Biomol Research Laboratories with IVth ventricle placements were used as additional (USA). These peptides are analogs of the synthetic CaM-controls; in these animals the cannula had penetrated the KII substrate, autocamtide-2. myr-AIP is reputedly cell-cerebellum and there was clear evidence of dye infusion permeable due to the addition of a myristoyl group (a (see below) in that region, suggesting that the drug may saturated fatty acid) on the N-terminus [16,19,20]. Since have also diffused to some extent into the cerebellum. AIP lacks this myristol group it was used as a cell-Some animals displayed transient SN and mild postural impermeable control. The concentrations of AIP used in asymmetries during the first few hours after cannula the present experiments were based upon those used in an implantation. As a necessary part of the cannula implanta- in vitro phosphorylation assay of post-synaptic density tion procedure, some part of the cerebellum and VNC must proteins [15], since to the best of our knowledge, there are be lesioned in order to position the cannula in the VNC. In no published reports of its use in vivo. Each peptide was our experience, transient vestibular symptoms indicate reconstituted in sterile filtered millipure water to a stock only a minor lesion and our previous studies indicate that solution of 1000 mM, and stored as frozen aliquots at such minor damage, while confirming that the cannula is in 2808C for a maximum of 1 month to ensure optimal the right place, does not significantly affect vestibular stability. On the day of each experiment, frozen aliquots function thereafter [5,34]. However, animals which failed were thawed at room temperature and diluted to working to compensate for these symptoms in less than 24 h were concentrations with sterile mACSF (pH 7.0). Vehicle excluded from the experiment. controls for both the Bis (5% DMSO in mACSF) and AIP (mACSF) groups were run in the same way as the
2.3. Drugs experimental groups (see Table 1).
The PKC inhibitor 3-[1-(3-dimethylaminopropyl)-1H- 2.4. Drug delivery indol-3 -yl]-4-(1H-indol-3-yl)-1H-pyrrole-2,5-dione, HCl
(bisindolylmaleimide I, HCII GF 109203X, HCl) and the Guinea pigs were randomly assigned to one of four less selective analogue, 2,3-bis(1H-indol-3-yl)-N- experimental groups (see Table 1). Two 25-ml Hamilton methylmaleimide (bisindolylmaleimide V), were purchased syringes were attached to a 1m length of polyethylene from LC Laboratories (USA). Bisindolylmaleimide I HCl tubing and placed into a syringe pump (Harvard Instru-(Bis I) is selective for PKC in the nM range in vitro ments, MA, USA). Distal to the syringes, each infusion (IC50531 nM) [41] but will inhibit other protein kinases at line was attached to an infusion needle constructed of much higher concentrations; for example, the IC ’s of Bis50 27-gauge stainless steel tubing. Drug or vehicle solutions I for phosphorylase kinase, PKA, the endothelial growth were backfilled into the tubing prior to the infusion needle factor receptor and the insulin receptor are: 0.7mM, 2mM, being placed inside the guide cannula in the animal. The
.100mM,.100mM, respectively [26,41]. In contrast, the syringe pump delivered drug solutions at a constant rate of affinity of bisindolylmaleimide V (Bis V) for PKC is 2ml / h for a total of 2 h, beginning 1 h pre-, and finishing greatly reduced (IC50.100 mM) [8] and was used as a 1 h post-UVD. The total infusion volume was verified after negative control for possible non-specific effects of Bis I. each infusion. The group mean infusion volumes ranged The doses of Bis I were chosen based upon data from from 4.21 to 4.34ml. Each animal was given a single dose whole cell preparations in vitro ([40] see Ref. [16] for a of the sedative / muscle relaxant, xylazine (12 mg / kg, i.m.), review). Bis I and V were dissolved in 100% dimethylsul- 10 min prior to the start of the cannula infusion. Approxi-phoxide (DMSO) to stock solutions of 1 mM and stored at mately 30 min after the start of the infusion, animals were
2208C. On the day of each experiment, frozen stock anesthetised for UVD surgery. For PKC inhibitor experi-solutions were diluted to working concentrations with ments, all drug and vehicle solutions were administered sterile filtered modified artificial cerebrospinal fluid blind; solutions were coded by a third party and codes (mACSF) [34] and pH adjusted to approximately 7.0 if were not revealed to the experimenter until after the required (with 0.1 M HCl). The concentration of DMSO in experiments were completed. Drug and control conditions working solutions was 5%, except for 200mM solutions in were run together on the same day where possible. which case the DMSO concentration was raised to 10% CaMKII inhibitor experiments were not run blind because due to solubility problems. For this reason, the 200 mM AIP peptides have a limited shelf-life in solution (approxi-groups were not compared with other dose (approxi-groups in mately 1 month at 2808C), therefore control and ex-statistical analysis. perimental conditions could not be run in parallel.
drug infusion (2 ml / h) means that drug concentrations at In addition, no statistical comparisons were made between sites distant from the target nuclei will be low compared to Bis I and V at the 200 mM dose, because of the small the injection site [36,44]. The infusion rate used in this sample sizes involved (n52 per group).
study is comparable to that of some osmotic minipumps.
Second, the diameter of the injection needle and inner 2.8. Histology cannula were identical in order to minimise drug backflow
up the sides of the injection needle. Cannula tip positions were verified for each animal in these experiments. At the conclusion of the experiment, 2.5. Unilateral vestibular deafferentation (UVD) animals were given an overdose of pentobarbital followed by a 0.5–1.0 ml cannula injection of dye (Alcian blue One week following cannula surgery, all animals were 8GX, 5% w / v) to mark the infusion site. Animals were given a right global surgical labyrinthectomy (UVD), as then transcardially perfused with 0.9% saline followed by described in detail elsewhere [34]. Briefly, guinea pigs a phosphate-buffered formaldehyde (10%) solution. The were anesthetised and prepared for surgery in an identical brainstem and cerebellum were removed and stored in a manner to that described for the cannula surgery above. A formaldehyde solution (see above) until sectioned. The horse-shoe-shaped incision was made over the right ear fixed tissue was mounted on a microtome stage, frozen and the temporal bone exposed following blunt dissection with CO and 80–902 mm coronal sections cut serially with of the temporalis muscle and its subsequent removal with a Leitz microtome. The coronal sections were mounted on surgical scissors. The bony labyrinth was exposed with a gelatin-coated slides and air dried for 24 h before staining high speed drill, the horizontal and anterior semicircular with thionin blue and coverslipping. At least one slide canal ampuliae and otoliths were subsequently destroyed from each animal was photographed (Kodak Ektachrome under microscopic control using a fine burr, and their 64T film).
contents aspirated. Our previous studies have shown that this UVD procedure results in complete destruction of the
vestibular receptors [5,11,23,33–35,39]. 3. Results
2.6. Behavioural measurements 3.1. Histological verification of cannula tips
Spontaneous nystagmus (SN), roll head tilt (RHT) and Alcian blue dye spots were used to mark the injection yaw head tilt (YHT) were measured for each animal at 6, site and hence the position of the cannula tip, but were not 8,10, 12, 20, 25, 30, and 50 h post-UVD. Measurements intended to measure drug spread from the injection site. could not be carried out earlier than 6 h post-UVD due to Cannula tips of all animals were located within the ipsi-the possibility of lingering anesipsi-thetic affects. All behav- VNC or on its border (Fig. 1A–B) or in the IVth ventricle ioural measurements for experiments using PKC inhibitors in the case of the additional control animals described were carried out double-blind. SN, YHT and RHT were above (data not shown).
measured using video techniques and the procedures we
have described in detail previously (e.g., [5,11]). Mean SN, 3.2. Spontaneous nystagmus compensation YHT and RHT were calculated for each measurement time
within each group of animals. Animals treated with the PKC inhibitor Bis I (5 or 50
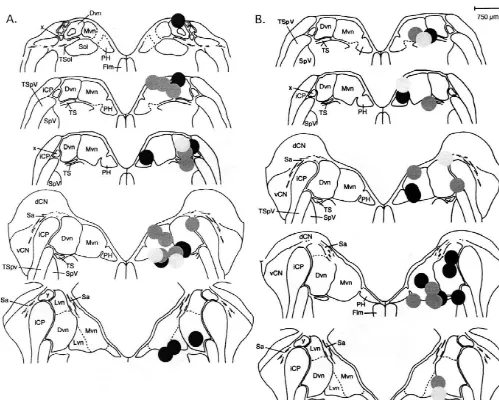
Fig. 1. Schematic illustration of cannula placements in the ipsi-VNC. Animals were infused with either (A) myr-AIP (black circles), AlP (grey circles) or vehicle (light grey circles); (B) Bis I (black circles), Bis V (grey circles) or vehicle (light grey circles). Schematic adapted from Ref. [14]. Dvn, descending vestibular nucleus; Mvn, medial vestibular nucleus; PH, prepositus hypoglossi; TS; tractus solitarius; TSpV, spinal tract of the trigeminal nerve; SpV, spinal nucleus of the trigeminal nerve; iCP, inferior cerebellar peduncle; x, cell group x; dCN, dorsal cochlea nucleus; vCN, ventral cochlea nucleus; Sa, stria acoustica; Flm, medial longitudinal fasciculus.
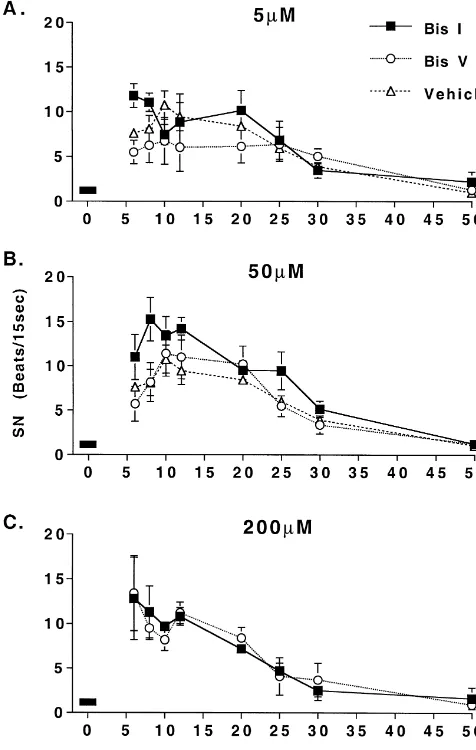
Fig. 3. The change in spontaneous nystagmus (SN) frequency expressed as % of vehicle (100%) produced by either 5mM (A) or 50mM (B) Bis I or Bis V over time post-UVD. Symbols represent the ratio of two means. When 5 and 50mM dose groups were combined, the difference between Bis I and Bis V at 6 and 8 h post-UVD was statistically significant (P,0.05).
Fig. 2. Effect of 5mM (A), 50 mM (B) or 200mM (C) of either the selective PKC inhibitor (Bis I), the less selective PKC inhibitor (Bis V),
or vehicle on the compensation of spontaneous nystagmus (SN). Symbols in the compensation patterns of RHT or YHT between represent means61 S.E. When 5 and 50mM dose groups were combined, animals treated with the PKC inhibitor, Bis I (5 or 50mM) the difference between Bis I and Bis V at 6 and 8 h post-UVD was
and controls (data not shown). As has been reported in
statistically significant (P,0.05). Bar represents the drug infusion period.
numerous other studies, the compensation of RHT and YHT within groups was extremely variable precise cannula placement within the VNC. It is conceiv- [9,33,34,37,39].
able that differences in the effects of the anesthesia were A number of animals in both the mAIP (2 / 11 animals) responsible; however, we have not observed such effects in and AIP (5 / 10) groups, and one animal (1 / 3) in the previous studies (e.g. [5]). vehicle group, could not be measured for either RHT or Comparison of 5 and 50mM Bis I infused into the right YHT at early time points because they could not stand VNC versus the IVth ventricle indicated no significant unassisted. Since a greater number of animals in the AIP difference in the effects on SN (P.0.05), suggesting that group were affected, statistical comparisons were made Bis I injected into the right VNC might be diffusing to between mAIP and vehicle (mACSF) data only, beginning some extent into other areas of the brainstem and cere- at 10-h post-UVD. There were no statistically significant bellum (data not shown; see Discussion). differences in RHT or YHT between myr-AIP (at any dose)
Infusion of the CaMKII inhibitor, myr-AIP, into the and vehicle groups (data not shown). ipsi-VNC at the time of the UVD had no significant effect
upon the compensation of SN, at doses ranging from 10 to
500 mM (Fig. 4). 4. Discussion
possibility that the behavioral effects observed were due to drug action on the VNC.
The exact mechanism(s) by which Bis I produced its effects upon SN frequency cannot be determined from the present experiment. It is possible that Bis I affected SN indirectly, for example, by blocking one or more com-ponents of the anaesthetic (fentazin), resulting in a greater expression of behavioural symptoms at early time points. However, this seems unlikely given that: (1) only SN was affected and not postural symptoms; (2) animals showed no obvious signs of sedation at the earliest measurement time (6 h post-UVD); and (3) similar results have recently been obtained with Bis I using a different anaesthetic in a different species [2].
As many previous reports have shown, RHT and YHT are highly variable in mammals (9,29,32,34,37,39; see Ref. [6] for a review). However, it seems unlikely that the lack of statistically significant effects on posture shown in the present study was simply due to the inherent variability since a number of laboratories (including our own) have reported statistically significant drug effects on postural compensation in guinea pig [5,18,27,33]. This finding is consistent with the idea that the induction of postural compensation in guinea pig does not involve PKC or CaMKII activity in the ipsi-VNC.
During completion of the present study, Balaban et al. [2] reported the effects of various serine / threonine protein kinase inhibitors on the induction of SN compensation in male Long–Evans rats. Each rat was given a single intraventricular bolus injection of one of the following kinase inhibitors: Bis I (10 mM), A-3 (inhibits cAMP-dependent protein kinase (PKA) and cGMP-cAMP-dependent
Fig. 4. Effect of 10 mM (A), 100 mM (B) or 500 mM (C) of the
cell-permeable CaMKII inhibitor peptide (myr-AIP), the cell-imperme- protein kinase (PKG; 1 mM)), H-7 (a non-selective
able CaMKII inhibitor peptide (AIP), or vehicle (mACSF) on the inhibitor of PKC, PKA and PKG; 1 or 10 mM), Iso-H-7 compensation of spontaneous nystagmus (SN). Symbols represent
(an inhibitor of PKC and cyclic nucleotide-dependent
means61 S.E. Bar represents drug infusion period.
protein kinases; 10 or 50 mM), or distilled water (control). Injections were given 15 min pre-UVD. SN frequency was post-UVD compared to controls. However, the compensa- measured every hour from 1 to 8 h, then at 24, 48 and 72 h tion patterns of RHT and YHT were unaffected by the post-UVD. The highest dose of H-7 increased SN levels at PKC inhibitor. In contrast, the cell-permeable CaMKII 4, 5, 6 and 48 h post-UVD compared to controls, while Bis inhibitor (myr-AIP) failed to alter the compensation pattern I and Iso-H-7, increased SN levels from 1 to 8 h post-UVD of SN, RHT or YHT, compared to controls. These results only. SN levels were highest at 7 and 8 h post-UVD in the show that inhibition of PKC, but not CaMKII, in the group treated with the highest dose of Iso-H-7. In contrast, ipsi-VNC changes the pattern of SN compensation in SN levels in rats given the non-selective PKA and PKG guinea pig, and suggest that a causal link may exist inhibitor, A-3, were not significantly different from con-between PKC activity and the initiation of SN compensa- trols at any time point. Therefore, these results are similar tion. It should be noted that while Bis I affected the to those of the present study in that the same PKC inhibitor compensation of SN at early time points, it did not prevent at a similar dose, increased SN frequency during the early its compensation altogether. This suggests that PKC might stages of its compensation. In addition, these results show not be the only signalling pathway involved. that Bis I produced its effects as early as 1 h post-UVD (1 Despite our attempts to limit drug administration to the h and 15 min drug). The fact that a pre- and post-VNC, it is probably inevitable that some drug would have treatment paradigm (as used in the present study) produced spread beyond the injection site into surrounding areas (if behavioural effects that were qualitatively similar to those only by simple diffusion). This possibility is supported by produced by pre-treatment alone [2] is further evidence to the fact that IVth ventricle infusions of Bis I produced suggest that molecular events at the time of the UVD play similar effects to infusions of the same drug into the an important role in compensation [33].
inhibitors in vivo has the potential to limit their selectivity, such as modulating ion channels involved in generating and therefore, it is possible that the effects of Bis I in the pacemaker activity ([7]; see Ref. [30] for contrary evi-present study were due to inhibition of kinases other than dence) or a change in the efficacy of synaptic inputs which PKC and / or non-specific actions. The following evidence could involve, among other things, a change in transmitter suggests that this was not the case. First, the behavioural release (see Ref. [25] for a recent review). Interestingly, effects produced by Bis I were different to those produced Qian and Barmack [28] have shown a decrease in the by the same dose of the less potent analogue, Bis V, or the transport of PKC-delta to Purkinje cell axon terminals cell permeable CaMKII inhibitor, myr-AIP. Second, the within the ipsilateral medial vestibular nucleus and pre-effects of Bis I showed a trend toward dose-dependency. positus hypoglossi following UVD in rat. The authors Both of these factors suggest that Bis I was producing its speculated that these changes might contribute to com-effects by binding to a specific receptor, probably PKC, pensation by reducing GABA release onto the ipsi-VNC, rather than having a non-specific action. Third, protein thereby disinhibiting the spontaneous firing rate of these phosphorylation changes which have been demonstrated in neurons. This hypothesis is consistent with the results of the guinea pig medial vestibular nucleus / prepositus hypo- the present experiment, in that PKC-delta inhibition by Bis glossi in vitro following UVD, were probably mediated by I would be expected to prevent this disinhibition, resulting an endogenous PKC rather than CaMKII, PKA or PKG in the exacerbation of UVD symptoms.
[23,35]. Furthermore, in the Balaban et al. [2] study, the Finally, it will be important for future experiments to increased SN produced by the non-selective protein kinase confirm that the effects produced by Bis I were mediated inhibitor, H-7, occurred over a different time course through PKC; for example, whether the increase in SN can compared to that observed following Bis I in rat. Fourth, it be reversed by acute administration of PKC activators, seems unlikely that the behavioural effects of Bis I in the and / or mimicked by chronic administration of PKC ac-present experiment can be explained by PKA or PKG tivators through down-regulation of endogenous PKC inhibition (despite the fact that the IC50of Bis I for PKA is activity. The use of antisense oligonucleotides to ‘knock-relatively low, 2mM in vitro), for the following reasons: down’ specific PKC isozymes in the VNC, as has already (a) Balaban et al. [2] have shown that a PKA and PKG been used successfully elsewhere, may also assist in inhibitor had no effect on SN compensation in rat; (b) the identifying which isozymes are involved in these effects. endogenous activities of PKA and PKG in the guinea pig
medial vestibular nucleus / prepositus hypoglossi [23,35]
are particularly low compared to other brain areas; (c) Acknowledgements biochemical experiments have shown that 5mM Bis I did
not affect cAMP-dependent phosphorylation in vitro [41]. This research was supported by research grants from the The transient nature of the behavioural effects produced Health Research Council of New Zealand and the New by Bis I and the fact that it affected only one UVD Zealand Neurological Foundation. The authors wish to symptom–SN–suggest that its effects were not due to thank Dr. Phillip Marley (Department of Pharmacology, toxicity. Furthermore, all of the animals receiving Bis I University of Melbourne, Victoria, Australia) and Assoc. compensated to the same extent and within the same time Prof. John Rostas (Department of Medical Biochemistry, period as the control group. There was no behavioural University of Newcastle, NSW, Australia) for advice on evidence of ill-health in the Bis-treated animals and protein kinase inhibitors; Dr. Brian Niven (University of histology did not indicate any obvious toxic effects on the Otago) for statistical advice and Dr. Arata Horii (Depart-morphology of VNC neurons. Finally, if the effect of Bis I ment of Otolaryngology, Osaka University Medical on SN was due to toxicity, it would be expected that higher School) for helpful comments on a draft version of this doses would produce even greater behavioural effects; manuscript.
however, the effect of the highest dose of Bis I (200mM) was not significantly different to the vehicle control.
We know of no other studies in which the CaMKII
References inhibitors myr-AIP or AIP have been used in vivo.
Consequently, it is important to note that the in vivo
[1] C.D. Balaban, M.I. Freilino, G.G. Romero, Protein kinase C
mechanism of drug action is not necessarily the same as
inhibition blocks the early appearance of vestibular compensation,
that in cell-free, or whole cell preparations in vitro. Brain Res. 845 (1999) 97–101.
Therefore, the conclusion that CaMKII might not be [2] C.D. Balaban, G.G. Romero, A role of climbing fibres in regulation
involved in static compensation must be a tentative one at of flocculonodular lobe protein kinase C expression during vestibu-lar compensation, Brain Res. 804 (1998) 253–265.
this stage.
[3] X. Bi, S. Standley, M.I. Baudry, Posttranslational regulation of
It is unclear precisely how PKC-mediated signalling
ionotropic glutamate receptors and synaptic plasticity, in:
Interna-pathways in the ipsi-VNC may contribute to the initiation tional Reviews in Neurobiology, Vol. 42, Academic Press, New of SN compensation. Potential mechanisms could involve York, 1998, pp. 227–285.
synthesis after unilateral labyrinthectomy: natural one-month evolu- hypoglossi and medial vestibular nuclei of labyrinthine-intact and tion and EGb 761 treatment effect, in: Y. Christen, J. Costentin, M.I. labyrinthectomized guinea pigs, J. Vest. Res. 10 (2000) 107–117. Lacour (Eds.), Advances in Ginko Biloba Extract Research. Effects [24] T. Kitahara, N. Takeda, T. Kubo, H. Kiyama, An implication of of Ginko Biloba Extract (EGb 761). On the CNS, Elsevier, Paris, protein phosphatase 2A-b in the rat flocculus for lesion-induced 1992, pp. 57–74. vestibular plasticity, Acta Otolaryngol (Stockh) 118 (1998) 685– [5] C. Bolger, A.J. Sansom, P.F. Smith, C.L. Darlington, An antisense 691.
oligonucleotide to brain-derived neurotrophic factor delays postural [25] H. MIajewski, L. lannazzo, Protein kinase C: a physiological compensation following unilateral labyrinthectomy in guinea pig, mediator of enhanced transmitter output, Prog. Neurobiol. 55 (5)
NeuroReport 10 (1999) 1485–1488. (1998) 463–475.
[6] I.S. Curthoys, G.M.I. Halmagyi, Vestibular compensation: a review [26] D. Moclily-Rosen, L.M.I. Kauvar, Modulating protein kinase C of the oculomotor, neural, and clinical consequences of unilateral signal transduction, Adv. Pharmacol. (New York) 44 (91) (1998) vestibular loss, J. Vest. Res. 5 (1995) 67–107. 91–145.
[7] C.L. Darlington, P.F. Smith, The recovery of static vestibular [27] L. Petrosini, M.E. Dell’Anna, Vestibular compensation is affected by function following peripheral vestibular lesions in mammals: the treatment with dopamine active agents, Arch. Ital. Biol. 131 (1993) intrinsic mechanism hypothesis, J. Vest. Res. 6 (3) (1996) 185–201. 159–171.
[8] P.D. Davis, C.H. Hill, G. Lawton, J.S. Nixon, S.E. Wilkinson, S.A. [28] Z.-Y. Qian, R.S. Barmack, Distribution of protein kinase C-delta in Hurst et al., Inhibitors of protein kinase C.1. 2,3-Bisarylmaleimides, cerebellar purkinje cells following unilateral labyrinthectomy in rat, J. Med. Chem. 35 (1992) 177–184. Soc. Neurosci. Abstr. 22 (1996) 720.7.
[9] C. De Waele, W. Graf, P. Josset, P.-P. Vidal, A radiological analysis [29] L. Ris, B. Capron, C. de Waele, P.-P. Vidal, E. Godaux, Dissociations of the postural syndromes following hemilabyrinthectomy and between behavioural recovery and restoration of vestibular activity selective canal and otolith lesions in the guinea pig, Exp. Brain Res. in the unilabyrinthectomized guinea pig, J. Physiol. 500.2 (1997)
77 (1989) 166–182. 509–522.
[10] H. Flohr, U. Luneburg, C. Richter-Landsberg, ACTH / MSH-like [30] L. Ris, E. Godaux, Spike discharge regularity of vestibular neurons neuropeptides and lesion-induced plastic processes, in: E. Keller, in labyrinthectomized guinea pigs, Neurosci. Letts. 253 (1998) D.S. Zee (Eds.), Adaptive Processes in Visual and Oculomotor 131–134.
Systems, Pergamon Press, Oxford, 1986, pp. 409–416. [31] L. Ris, R. Wattiez, C. de Waele, P.-P. Vidal, E. Godaux, Reappear-[11] D.P.D. Gilchrist, C.L. Darlington, P.F. Smith, A dose–response ance of activity in the vestibular nucleus neurones of labyrinthec-analysis of the beneficial effects of the ACTH-(4–9) analogue, Org tomized guinea-pigs is not delayed by cycloheximide, J. Physiol. 2766, on behavioural recovery following unilateral labyrinthectomy 512.2 (1998) 533–541.
in guinea-pig, Br. J. Pharmacol. 111 (1) (1994) 358–363. [32] L. Ris, B. Capron, C. de Waele, P.-P. Vidal, E. Godaux, Neck muscle [12] M.M. Goto, G.G. Romero, C.D. Balaban, Transient changes in activity after unilateral labyrinthectomy in the alert guinea pig, Exp.
flocculonodular lobe protein kinase C expression during vestibular Brain Res. 124 (1999) 159–165.
compensation, J. Neurosci. 17 (11) (1997) 43674381. [33] A.J. Sansom, C.L. Darlington, P.F. Smith, Pretreatment with MK-[13] E. Gozal, A.L. Roussel, G.A. Holt, L. Gozal, Y.M. Gozal, J.E. 801 reduces spontaneous nystagmus following unilateral
labyrin-Torres et al., Protein kinase C modulation of ventilatory response to thectomy, Eur. J. Pharmacol. 220 (1992) 123–129.
hypoxia in nucleus tractus solitarii of conscious rats, J. Appl. [34] A.J. Sansom, C.L. Darlington, P.F. Smith, D.P.D. Gilchrist, C.J. Physiol. 84 (6) (1998) 1982–1990. Keenan, R. Kenyon, Injections of calinidazolium chloride into the [14] W. Gstoettner, M.I. Burian, Vestibular nuclear complex in the guinea ipsilateral medial vestibular nucleus or IVth ventricle reduce sponta-pig: A cytoarchitectonic study and map in three planes, J. Comp. neous ocular nystagmus following unilateral labyrinthectomy in Neurol. 257 (1987) 176–188. guinea pig, Exp. Brain Res. 93 (1993) 271–278.
[15] Y. Hayashi, A. Ishida, H. Katagiri, M.I. Mishina, H. Fujisawa, T. [35] A.J. Sansom, V.A. Brent, P.E. Jarvie, C.L. Darlington, P.F. Smith, R. Manabe et al., Calcium- and calmodulin-dependent phosphorylation Laverty et al., In vitro phosphorylation of medial vestibular nucleus of AMPA type glutamate receptor subunits by endogenous protein and prepositus hypoglossi proteins during behavioural recovery from kinases in the post-synaptic density, Mol. Brain Res. 46 (1997) unilateral vestibular deafferentation in the guinea pig, Brain Res.
338–342. 778 (1) (1997) 166–177.
[16] H.C. Hemmings (Ed.), Protein Kinase and Phosphatase Inhibitors, [36] R.L. Schroeder, M.B. Weinger, L. Vakassian, G.F. Koob, Vol. 30, Humana Press, New Jersey, 1997. Methylnaloxonium diffuses out of the rat brain more slowly than [17] D.C. Howell, Statistical Methods for Psychology, 3rd Edition, naloxone after direct intracerebral injection, Neurosci. Lett. 121
Duxbury Press, Belmont, CA, 1992. (1991) 173–177.
[18] R.T. Hung, S.A. Harvey, S.J. Mullen, Vestibular compensation in [37] D.W. Sirkin, W. Precht, J.H. Courjon, Initial, rapid phase of recovery guinea pigs given intravenous lidocaine after unilateral labyrinthec- from unilateral vestibular lesions in rat is not dependent on survival tomy, Otolaryngol. Head Neck Surg. 120 (1999) 12–16. of central portion of vestibular nerve, Brain Res. 302 (1984) [19] A. Ishida, I. Kameshita, S. Okuno, T. Kitani, H. Fujisawa, A novel 245–256.
highly specific and potent inhibitor of calmodulin-dependent protein [38] P.F. Smith, C.L. Darlington, The contribution of NMDA receptors to kinase II, Biochem. Biophys. Res. Commun. 212 (3) (1995) 806– lesion-induced plasticity in the vestibular nucleus, Prog. Neurobiol.
812. 53 (1997) 517–531.
[20] A. Ishida, H. Fujisawa, Stabilization of calmodulin-dependent [39] P.F. Smith, C.L. Darlington, I.S. Curthoys, The effect of visual protein kinase through the autoinhibitory domain, J. Biol. Chem. deprivation on vestibular compensation in the guinea pig, Brain Res.
270 (5) (1995) 2163–2170. 364 (1986) 195–198.
[21] U. Janssen, C. Richter-Landsberg, H. Flohr, Vestibular compensation [40] M.I. Tokuda, O. Hatase, Regulation of neuronal plasticity in the affects endogenous phosphorylation of frog brain proteins, J. central nervous system by phosphorylation and dephosphorylation, Neurochem. 58 (1) (1992) 65–71. Mol. Neurobiol. 17 (1998) 137–156.
[22] U. Janssen, C. Richter-Landsberg, A.B. Oestreicher, P.N.E. De [41] D. Toullec, P. Pianetti, H. Coste, P. Bellevergue, T. Grand-Perret, Graaf, W.H. Gispen, H. Flohr, Identification of a B-50-like protein in M.I. Ajakane, V. Baudet, P. Boissin, E. Boursier, F. Loriolle, L. frog brain synaptosomes, Brain Res. 570 (1992) 21–26. Duhamel, D. Chyaron, J. Kirilovsky, The bisindolylmaleimide GF [23] D.R. Kerr, A.J. Sansom, P.F. Smith, C.L. Darlington, Comparison of 109203X is a potent and selective inhibitor of protein kinase C, J.
3
[42] S.I. Walaas, A.C. Nairn, P. Greengard, Regional distribution of [44] L.I. Wolfson, L.L. Brown, Intrastriatal injection of [ H]dopamine calcium- and cyclic adenosine 39:59-monophosphate-regulated pro- through a chronic cannula to produce rotation: distribution and tein phosphorylation systems in mammalian brain. I. Particulate concentration of the tracer in specific brain regions, Brain Res. 262 systems, J. Neurochem. 3 (2) (1983) 291–301. (1983) 205–212.