Journal of Life Sciences
Volume 10, Number 4, April 2016 (Serial Number 95)
Dav i d
David Publishing Company www.davidpublisher.com
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 616 Corporate Way, Suite 2-4876, Valley Cottage, NY 10989, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Dr. Farzana Perveen (Pakistan), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Valery Lyubimov (Russia), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Dr. Wenle Xia (USA), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected].
Editorial Office
616 Corporate Way, Suite 2-4876, Valley Cottage, NY 10989, USA Tel: 1-323-9847526, Fax: 1-323-9847374
E-mail:[email protected], [email protected]
Copyright©2016 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA
Database of Cambridge Science Abstracts (CSA), USA Database of Hein Online, New York, USA
Ulrich’s Periodicals Directory, USA Universe Digital Library S/B, Proquest
American Federal Computer Library center (OCLC), USA China National Knowledge Infrastructure, CNKI, China
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China Index Copernicus, Index Copernicus International S.A., Poland
Google Scholar (scholar.google.com)
Subscription Information Price (per year): Print $680.
David Publishing Company
616 Corporate Way, Suite 2-4876, Valley Cottage, NY 10989, USA Tel: 1-323-9847526, 323-410-1082; Fax: 1-323-9847374
E-mail: [email protected]
David Publishing Company www.davidpublisher.com
JLS
Journal of Life Sciences
Volume 10, Number 4, April 2016 (Serial Number 95)
Contents
Zoology
171 Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al
Haneah Fishing Site, Mediterranean Sea, Eastern Libya
Eyman Faraj Abd Alssalam, Sayed Mohamed Ali, Mohammad El Sayed El Sayed El Mor, Ramadan
Attea Saleh Ali and Eman Salem Al Fergan
182 Tetanus in Cat: From Neglected Wound to Neuromuscular Disorder—Case Report
Maksimović Alan, Filipović Selma, Lutvikadić Ismar and Šunje-Rizvan Amila
Botany
185 Management of Insect Vectors of Viruses in Tomato Plants Using Different Densities of Yellow
Traps
Eduardo Domingos Grecco, Dirceu Pratissoli, Hugo Bolsoni Zago, Débora Ferreira Melo Fragoso and
José Romário Carvalho
192 Therapeutic Effect of Zygophyllum cornutum on Metabolic Disturbances, Oxidative Stress in
Heart Tissue and Histological Changes in Myocardium of Streptozotocin-induced Diabetic Rats
Awatif Boumaza, Samira Ferdi, Houda Sbayou, Fatima Khelifi Touhami, Mohamed Habib Belmahi and
Cherifa Benlatreche
Interdisciplinary Researches
198 A Selection Study for Sanitary Landfill Site at Basra City, South of Iraq
Wathiq A. Al-Ramdhan, Mahmood S. Thamir, Assaad F. Hamza, Abdulwahab A. Sultan, Ali G. Katea’a, Najem Al-Dean A. Al-hajaj, Ayad K. Jirri and Ekhlass B. Zubery
203 Disaster Prevention Literacy among School Administrators and Teachers: A Study on the Plan
for Disaster Prevention and Campus Network Deployment and Experiment in Taiwan
Sung-Chin Chung and Cherng-Jyh Yen
215 Behavior of a Nonlinear Difference Equation
Journal of Life Sciences 10 (2016) 171-181 doi: 10.17265/1934-7391/2016.04.001
Reproductive Biology of the Striped Seabream
Lithognathus mormyrus
(Linnaeus, 1758) from
Al-Haneah Fishing Site, Mediterranean Sea, Eastern
Libya
Eyman Faraj Abd Alssalam1, Sayed Mohamed Ali1, Mohammad El Sayed El Sayed El Mor1, 2, Ramadan Attea Saleh Ali1 and Eman Salem Al Fergani1
1. Department of Zoology, Omar Al-Mukhtar University, Al Beida, Libya
2. Department of Science, Suez Canal University, Egypt
Abstract: Reproductive biology of L. mormyrus was studied using monthly samples totaling 224 fish obtained from Al-Haneah fishing site, eastern Libya Mediterranean Sea. Lengths of the examined fish ranged between 11.5 cm and 23.4 cm. Corresponding weights were 24.5 g and 160.8 g. The minimum values of the condition factors, KF and KC, were 1.38 and 1.21 at the average fish length of 12.4 cm. These values increased with increases in length until they reached maximum values of 1.59 and 1.41 at the length of 22.1 cm. The high values of KF and KC , Gonado-Somatic Index and oocyte diameter maintained during May, June, July, and August and the monthly changes in gonadal condition, indicated that summer was the breeding season of L. mormyrus. The oocyte diameter ranged between 379 ± 25.3 µ and 1,511 ± 143.3 µ (n = 76) with an average of 895 ± 111.3 µ. The sex ratio was in favor of females during all months of the study. The overall sex ratio was 1: 1.52. Length at first maturity, L50, for L. mormyrus was found to be 14.15 cm for males and 14.45 for females. Overall average of absolute fecundity was 4,342 ± 557 egg per fish (n = 45). The overall average of relative fecundity was 234 ± 181 egg per cm. Absolute fecundity increased with increasing fish length.
Key words: Reproductive biology, length at first maturity, oocyte diameter, fecundity, striped Seabream, Lithognathus mormyrus.
1. Introduction
Lithognathus mormyrus [1], striped Seabream or sand steenbras, family Sparidae, is a marine gregarious demersal fish inhabiting sandy shallow coastal waters. It is commonly not more than 30 cm long [2, 3]. It feeds on worms, mollusks, small crustaceans and detritus. It is protandrous hermaphrodite and breeds in summer (Russell et al., 2014). The fish is subtropical, widely distributed in the eastern Atlantic Ocean, the Mediterranean sea, the Red sea and the southwestern Indian Ocean. In Libya it is present along the whole coast and is common in the artisanal catch [4]. The IUCN status of this fish is:
Corresponding author: Sayed Mohamed Ali, professor, Dr., research fields: oceanography, fisheries, chemistry, zoology and environment.
Least concern.
The objective of the present work was to study reproductive biology of L. mormyrus obtained from the artisanal catch of Al-Haneah, eastern Libya Mediterranean Sea. The data obtained will be helpful in managing the fisheries of this fish in Libya.
2. Methods
2.1 AL-Haneah Fish Landing Site
Al-Haneah and vicinity (Fig. 1) is a principal fishing ground on eastern Libyan Mediterranean Sea.
L. mormyrus is common in its artisanal catch.
2.2 The Reproductive Biology Studies
These were the condition factors, the gonado-somatic index, the maturity stages, the length at first maturity,
D
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
172
Fig. 1 Al-Haneah fishing site.
the sex ratio, the breeding season, fecundity and eggs diameter.
2.2.1 Collection and Treatment of L. mormyrus
samples
An average of 19 L. mormyrus were collected monthly from Al-Haneah artisanal catch during March 2015 to February 2016 for use in the biological studies. Altogether 224 fish were used. The monthly samples were brought to the Marine Laboratory of the Zoology Department of Omar Al-Mukhtar University. For each fish total length and corresponding weight were measured to the nearest mm and the first decimal of the gram. The abdominal cavity was then cut open with a scissor and the condition of the ovary (maturity stages) according to El-Ganainy and Buxton [5] was observed and recorded. The gonads were then taken out and weighed, ovaries with well-developed eggs were preserved in 10% formalin for later reading the oocyte diameter under the low power of a microscope fitted
with an eye piece micrometer. Corresponding eviscerated fish weights were recorded.
2.2.2 The Condition Factor
Monthly Fulton’s and Clark’s condition factors (“KF”, “KC”) for female and male L. mormyrus were obtained monthly according to Froese and Bagenal and Tesch [6, 7].
KF = 100 (W/L3)…Fulton’s condition factor. KC = 100 (EW/L3)…Clark’s condition factor. W: whole fish weight in grams
EW: eviscerated fish weightin grams L: fish length in centimeters
The factor 100 was used to bring K close to unity. 2.2.3 Gonado-Somatic Index (GSI)
GSI was determined monthly according to Anderson and Gutreuter and Akter et al. [8, 9].
GSI = 100 wt of gonad (gm)/wt of whole fish (gm) GSI: Gonado-somatic index
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
173
2.2.4 Maturity Stages
These were recorded monthly as I: immature, II: developing, III: mature, IV: regressing, V: regenerating [5].
2.2.5 Length at First Maturity (L50)
L50, the length at which 50% of individuals were mature, was obtained by plotting the percentage mature fish in each length class of the whole population versus length. From the curve, L50 was the length corresponding to 50% mature.
2.2.6 Sex Ratio
Sex ratio was determined by counting the number of males and females in the monthly samples studied.
2.2.7 The Breeding Season
The breeding season was established from analysis of the monthly variation in the condition factor, the GSI and the maturity stages.
2.2.8 Fecundity
Total or absolute fecundity (TF) is the total number of eggs in the ovaries of a fish prior to spawning [10]. In the present study mature ovaries were removed from their fish and placed in 10% formalin for one day to allow the eggs to harden. Each ovary was then taken out of the formalin, dried with a tissue paper and weighed. Three small sub samples were taken from the front, mid and rear parts of the ovary, weighed and atomized by rubbing gently with fingers.
The number of eggs in each sub sample was counted under the low power of a microscope. The number of eggs in the ovary based on each sub sample was calculated as:
Number of eggs in the ovary = OW (g)/SW (g) *NS [7] OW: ovary weight (g)
SW: sub sample weight (g)
NS: total number of eggs in sub sample.
Total fecundity was then obtained by averaging the numbers of eggs per ovary calculated from each of the three sub samples.
Relative fecundity (RF) is the number of eggs per unit length (cm) or the number of eggs per unit weight (g) of fish. To estimate relative fecundity individual fecundities were divided by corresponding lengths or weights [11].
2.2.9 Egg Diameter
The egg diameter (µ) was established monthly with aid of a microscope fitted with an eye piece micrometer. Each month individual dimeters of 10 non deformed and rounded oocytes taken from different parts of ovaries of individual females were measured under the low power of the microscope. The average diameter was then calculated.
3. Results
3.1 Lengths, Corresponding Weights, and Condition Factors
In the present study fish lengths ranged between 11.5 cm and 23.4 cm. Corresponding weights were 24.5 gm and 160.8 gm. All fish lengths were divided into 6 length classes with class range of 1.9 cm as shown in Table1.
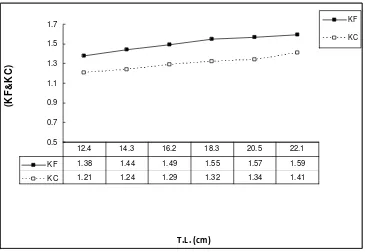
The minimum values of the condition factors KF and KC were 1.38 and 1.21 at the average observed length of 12.4 cm (Table 1 and Fig. 2). These values increased with increases in length until they reached maximum values of 1.59 and 1.41 at the maximum observed length of 22.1 cm.
Table 1 Fish lengths, weights and condition factors (KF and KC), per length class range of 224 L. mormyrus from Al-Haneah
coast. Number of fish within each class range is given between two brackets.
Length class range cm Average length cm Average weight gm KF ± S.D. KC ± S.D.
11.5-13.4 (39) 12.4 24.5 1.38 ± 0.71 1.21 ± 0.56
13.5-15.4 (37) 14.3 39.7 1.44 ± 0.99 1.24 ± 0.76
15.5-17.4 (34) 16.2 59.2 1.49 ± 1.02 1.29 ± 0.95
17.5-19.4 (41) 18.3 83.7 1.55 ± 1.09 1.32 ± 0.99
19.5-21.4 (40) 20.5 133.5 1.57 ± 1.11 1.34 ± 1.01
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
174
Fig. 2 The relationship between condition factor and length of L. mormyrus from Al-Haneah coast. The monthly variations in condition factors KF and
KC during the study period, March 2015 to February 2016 are shown in Fig. 3. During March KF and KC were 1.22 and 0.94 in order. Then they increased during April and recorded the highest values of 1.71 and 1.49 in May. High values were maintained during June, July and August. The monthly values then decreased gradually and reached a minimum of 1.27 and 0.98.
3.2 The Gonado-Somatic Index
Changes in monthly Gonado-Somatic Indices (GSI) of males of L. mormyrus during the study period are shown in Fig. 4. Highest GSI were recorded in May (4.67%), June (5.22%), July (4.76%) and August (5.81%). A sharp decrease occurred in September (1.41%). Low values were maintained during October (0.96%) to February (1.09%). The lowest value was during January (0.74%). It is, therefore, concluded that the breeding season of males of this fish is May to August, i.e. summer.
The GSI for females (Fig. 5) recorded high values during May (6.76%), June (7.61%), July (8.88%) and August (9.79%). The GSI values then decreased sharply during September (3.50%) and the following
months and reached a minimum value of 1.72 during January. From this we can conclude that the breeding season of female L. mormyrus is May to August i.e. summer.
3.3 Sex Ratio
During all the study period the ratio of Males: Females was in favor of females (Table 2). Of the 224 fish examined during the study period 89 were males (39.7%) and 135 were females (60.3%), giving an overall ratio of 1: 1.52. The highest values were recorded during the breeding season, in May (69.2%), June (64.7%), July (65%) and August (63%).
3.4 The Oocyte Diameter
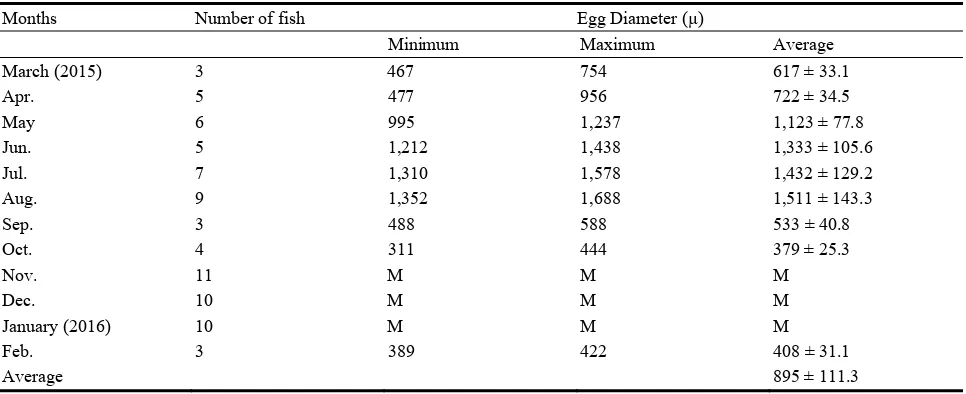
The oocyte diameter in March was 617 ± 33.1 (Table 3). It then increased during April (722 ± 34.5 µ), May (1,123 ± 77.8 µ), June (1,333 ± 105.6 µ), July (1,432 ± 129.2 µ) and reached the maximum in August (1,511 ± 143.3 µ). The oocyte diameter then dropped to the lowest value of 379 ± 25.3 µ in October. During the period November, December and January the oocytes were either not present or too small to be measured.
0.5 0.7 0.9 1.1 1.3 1.5 1.7
T.L. (cm)
(KF
&KC)
KF
KC
KF 1.38 1.44 1.49 1.55 1.57 1.59
KC 1.21 1.24 1.29 1.32 1.34 1.41
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
175
Fig. 3 Monthly variations of condition factors of L. mormyrus from Al-Haneah coast.
Fig. 4 Monthly variations of minimum, maximum and average gonado-somatic indices values of males L. mormyrus from El-Haneah coast.
Fig. 5 Monthly variations of minimum, maximum and average gonado-somatic indices of females L. mormyrus from El-Haneah coast.
0.6 0.8 1 1.2 1.4 1.6 1.8
Months
(K
F
&
K
C
)
KF
KC
KF 1.22 1.36 1.71 1.68 1.66 1.61 1.43 1.41 1.38 1.35 1.32 1.27
KC 0.94 1.22 1.49 1.41 1.39 1.37 1.22 1.19 1.17 1.15 1.11 0.98
March
(2015) Apr. May Jun. Jul. Aug. Sep. Oct. Nov. Dec.
January
(2016) Feb.
Males
0 1 2 3 4 5 6 7 8 9
Months
G
.S
.I.
(%
)
Minim um 1.18 1.33 2.69 2.87 2.99 3.22 1.11 0.84 0.73 0.57 0.49 0.81
Maxim um 1.99 2.32 7.11 7.44 6.98 8.39 1.88 1.11 1.09 1.07 0.94 1.33
Average 1.44 1.89 4.67 5.22 4.76 5.81 1.41 0.96 0.90 0.83 0.74 1.09
March
(2015) Apr. May Jun. Jul. Aug. Sep. Oct. Nov. Dec.
January (2016) Feb.
Females
0 2 4 6 8 10 12 14 16
Months
G
.S
.I. (
%
)
Minim um 2.13 2.43 3.74 3.98 4.22 4.79 2.11 1.84 1.73 1.57 1.39 1.88 Maxim um 3.49 3.78 9.18 11.24 12.98 14.39 4.89 2.99 2.49 2.27 2.04 2.73
Average 2.71 3.43 6.76 7.61 8.88 9.79 3.50 2.82 2.11 1.82 1.72 2.44 March
(2015) Apr. May Jun. Jul. Aug. Sep. Oct. Nov. Dec.
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
176
Table 2 Monthly variations in sex ratio of L. mormyrus from Al-Haneah coast.
Months No. of fish Males Females Sex ratio
No. % No. %
March (2015) 21 8 38.1 13 61.9 1: 1.63
Apr. 22 10 45.5 12 54.5 1: 1.20
May 13 4 30.8 9 69.2 1: 2.25
Jun. 17 6 35.3 11 64.7 1: 1.83
Jul. 20 7 35.0 13 65.0 1: 1.86
Aug. 27 10 37.0 17 63.0 1: 1.70
Sep. 18 8 44.4 10 55.6 1: 1.25
Oct. 17 6 35.3 11 64.7 1: 1.83
Nov. 21 10 47.6 11 52.4 1: 1.10
Dec. 18 8 44.4 10 55.6 1: 1.25
January (2016) 17 7 41.2 10 58.8 1: 1.43
Feb. 13 5 38.5 8 61.5 1: 1.60
Total 224 89 39.7 135 60.3 1: 1.52
Table 3 Monthly variations of oocyte diameters (µ) of L. mormyrus from El-Hanea coast.
Months Number of fish Egg Diameter (µ)
Minimum Maximum Average
March (2015) 3 467 754 617 ± 33.1
Apr. 5 477 956 722 ± 34.5
May 6 995 1,237 1,123 ± 77.8
Jun. 5 1,212 1,438 1,333 ± 105.6
Jul. 7 1,310 1,578 1,432 ± 129.2
Aug. 9 1,352 1,688 1,511 ± 143.3
Sep. 3 488 588 533 ± 40.8
Oct. 4 311 444 379 ± 25.3
Nov. 11 M M M
Dec. 10 M M M
January (2016) 10 M M M
Feb. 3 389 422 408 ± 31.1
Average 895 ± 111.3
M = The eggs were either not present or were too small for their diameters to be measured. During February the oocyte diameter was (408 ± 31.1
µ). The average oocyte diameter for all the examined 45 fish was 895 ± 111.3 µ.
3.5 Maturity
11.2% and 8.2% of males and females L. mormyrus
in order in the class length 11.5-13.4 cm were mature (Table 4). Percentage maturity for males and females increased in the following class ranges such that all males and females in the class ranges 19.5-21.4 cm and 21.5-23.4 cm in order were mature.
3.6 Length at First Maturity
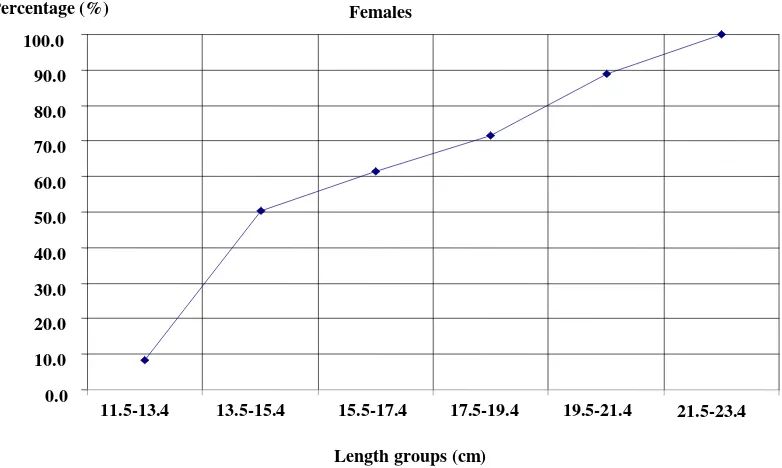
Length at first maturity L50 is the length at which half the population is mature and the other half is not. For L. mormyrus L50 was found to be 14.15 cm for males (Fig. 6) and 14.45 for females (Fig. 7).
3.7 Absolute and Relative Fecundity
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
177
Table 4 The percentage of mature and immature fishes at different length ranges of L. mormyrus from Al-Haneah coast.
Total length (cm) Males Females
Range Average % Immature % Mature % Immature % Mature
11.5-13.4 12.4 88.8 11.2 91.8 8.2
13.5-15.4 14.3 45.8 54.2 49.8 50.2
15.5-17.4 16.2 32.5 67.5 38.5 61.5
17.5-19.4 18.3 18.6 81.4 28.6 71.4
19.5-21.4 20.5 _ 100 11.1 88.9
21.5-23.4 22.1 _ 100 _ 100
Remarks: Data expressed as percentage, (_) No fish in length group occurred.
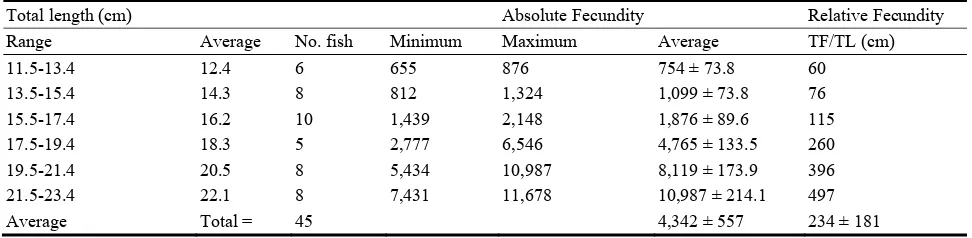
Table 5 Relationship between fecundity and total body length (cm) of L. mormyrus from El-Hanea coast.
Total length (cm) Absolute Fecundity Relative Fecundity
Range Average No. fish Minimum Maximum Average TF/TL (cm)
11.5-13.4 12.4 6 655 876 754 ± 73.8 60
13.5-15.4 14.3 8 812 1,324 1,099 ± 73.8 76
15.5-17.4 16.2 10 1,439 2,148 1,876 ± 89.6 115
17.5-19.4 18.3 5 2,777 6,546 4,765 ± 133.5 260
19.5-21.4 20.5 8 5,434 10,987 8,119 ± 173.9 396
21.5-23.4 22.1 8 7,431 11,678 10,987 ± 214.1 497
Average Total = 45 4,342 ± 557 234 ± 181
TF: Total Fecundity. TL: Total fish length in cm.
Fig. 6 Length at first maturity of males L. mormyrus from Al-Hanea coast. Males
0.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 100.0
11.5-13.4 13.5-15.4 15.5-17.4 17.5-19.4 19.5-21.4 21.5-23.4
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
178
Fig. 7 Length at first maturity of females L. mormyrus from Al-Haneah coast. Absolute fecundity increased with increasing fish
length from 754 ± 73.8 egg per fish at the average total fish length of 12.4 cm to 10,987 ± 214.1 egg per fish at the average total length of 22.1 cm.
The same trend was observed for the relative fecundity. It ranged from a minimum of 60 egg per centimeter at the average total length of 12.4 cm to 497 egg per centimeter at the average total length of 22.1 cm.
4. Discussion
In the present study lengths of L. mormyrus studied ranged between 11.5 cm and 23.4 cm. corresponding to the weights 24.5 gm and 160.8 gm. Previous studies pointed that L. mormyrus can grow to a maximum length of about 55 cm, with a weight of around 1 kg, but the common size is 20-30 cm [12, 13], it grows relatively fast during the first few years of life attaining approximately 50% of its maximum length during the second year [13-20].
In the present study the condition factors KF and KC increased with increasing fish length from 1.38 and 1.21 in order at the class range 11.5-13.4 cm to 1.59
and 1.41 at the class range 21.5-23.4 cm. The larger the fish is the larger the condition factor. Matić-Skoko [21] studied growth of juvenile L. mormyrus from the DućeGlava, eastern Adriatic Sea. The obtained condition factor of 1.245 is close to the range of condition factors observed in the present study. Monthly variation of KF and KC was also determined in the present study. KF and KC recorded high values during May, June, July and August suggesting that summer is the breeding season of L. mormyrus. Same conclusion was also deduced from changes in monthly Gonado-Somatic Indices (GSI) of males and females. The highest GSI were recorded in May, June, July and August. A sharp decrease occurred in September. Low values were maintained during October to February. This was also supported by the observation that gravid females were encountered during summer. Previous studies agree that the reproductive season of L. mormyrus is summer [20, 22-25] or spring and summer [26-30].
During all the study period the ratio of Males: Females was in favor to females. The overall ratio was 1: 1.52. Kraljević et al. reported the ratio for the Females
0.0 10.0 20.0 30.0 40.0 50.0 60.0 70.0 80.0 90.0 100.0
11.5-13.4 13.5-15.4 15.5-17.4 17.5-19.4 19.5-21.4 21.5-23.4
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
179
western Istrian coastal waters as 1: 1.62. Ramos and Lorenzo et al. [20, 25], off the Canary islands reported 1: 0.85 and EMRE et al. [31], in the Beymelek Lagoon (Antalya, Turkey) reported 1: 1.84. L. mormyrus is protandric hermaphrodite [20, 25, 31, 32]. It starts its adult life as a male and later changes its sex to female at a length of about 21 to 30 cm (4-9 years) according to Bizsel et al. [13], or 25 cm to 35 cm according to the Malawi home page and Wikipedia, internet.
In the present study the oocyte diameter increased gradually through the months March, April, May, June, July and August where it reached the maximum value of 1,511 ± 143.3 µ. The oocyte diameter then dropped to the lowest value of 379 ± 25.3 µ in October. During the period November, December and January the oocytes were either not present or too small to be measured. This indicated that the reproductive season of L. mormyrus was summer. Firat et al. [33], found that the average diameter of mature L. mormyrus egg was 0.71 ± 0.1286 mm. They also mentioned that generally, striped sea bream eggs have a diameter of 0.55-1.02 mm. In the present study the average oocyte diameter of L. mormyrus was 895 ± 111.3 µ.
In the present study in the class length 11.5-13.4 cm, 11.2% and 8.2% of male and female L. mormyrus in order were mature. Length at first maturity, L50, is the length at which half the population is mature and the other half is not. In the present study L50 for L.
mormyrus was found to be 14.15 cm for males and 14.45 cm for females. Sexual maturity for this species was reported by Suau, UNESCO, Kraljević et al. [28, 34], and Wikipedia, internet, to occur at a minimum length of 14.1 cm for males at age two. FAO [27], reported that this species matures at lengths of 13.3 cm. Lorenzo et al. [20] and Kallianiotis et al. [35], reported that the lengths at 50% maturity for males and females were 16.2-20.7 cm and 19.0-24.6 cm consecutively. According to Monteiro et al. [36], (Algarve, south Portugal), the length at first maturity was similar for males and females and the value for both sexes combined was 16.08 cm corresponding to an age
between 1 and 2 years.
In the present study the overall average absolute fecundity and relative fecundity were 4,342 ± 557 egg per fish and 234 ± 181 egg per cm in order. Both absolute fecundity and relative fecundity increased with increasing fish length. We did not find any previous studies on fecundity of L. mormyrus.
References
[1] Linnaeus, 1758. “Lithognathus mormyrus—Linnaeus, 1758.” In Fish Base. Retrieved 2015.
[2] Bauchot, M. L., and Smith, J. L. B. 1985. “Sparidae in FAO Species Identification Sheets from Fishery Purposes.” Western Indian Ocean Fishing Area 51 (4) (Fisher, W. and Bainchi, G.) eds. FAO. Rome.
[3] Guidetti, P. 2000. “Differences among Fish Assemblages Associated with Nearshore Posidonia Oceanica Seagrass Beds, Rocky-algal Reefs and Unvegetated Sand Habitats in the Adriatic Sea.” Estuar. Cstl. Shelf Sci. 50: 515-29.
[4] Khamis, E. 2008. The Analysis of the Catch Trawling Net along Ben Gghazi, M. Sc, Thesis. Coast Fac. of Nat. Res. and Envi, 89, Omar El Mukhtar University.
[5] El-Ganainy, A. A. 2010. “Some Biological Aspects of the Filefish Setphanolepis diaspros (Family: Monacanthidae) from the Gulf of Suez, Egypt.” Researcher 2 (10). [6] Froese, R. 2006. “Cube Law, Condition Factor and
Weight-length Relationships: History, Meta-analysis and Recommendations.” J. Appl. Ichthyol. 22: 241-53, Journal Compilation 2006 Blackwell Verlag, Berlin, ISSN 0175-8659.
[7] Bagenal, T. B., and Tesch, F.W. 1978. Age and Growth.
In: Methods for Assessment of Fish Production in
Freshwaters, 101-36 (Bagenal, T., ed.). IBP Handbook
No. 3. Blackwell Scientific Publications, London. [8] Anderson, R. O., and Gutreuter, S. J. 1983. Length,
Weight, and Associated Structural Indices. In: Fisheries Techniques (Eds. Nielsen, L. A. and Johnson, D. L.), 283-300. American Fisheries Society, Bethesda.
[9] Akter, H., Islam, M. R., and Belal Hossain, M. 2012. “Fecundity and Gonadosomatic Index (GSI) of Corsula,
Rhinomugil corsula Hamilton, 1822 (Family: Mugilidae)
from the Lower Meghna River Estuary, Bangladesh.”
Global Veterinaria 9 (2): 129-32, ISSN 1992-6197. ©
IDOSI Publications, 2012. DOI: 10.5829/idosi.gv.2012.9.2.6431.
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
180
[11] Hossain, Y., Mosaddequr, R., and Elgorban, M. A. 2012. “Relationships between Body Size, Weight, Condition and Fecundity of the Threatened Fish Puntius ticto
(Hamilton, 1822) in the Ganges River, Northwestern Bangladesh.” Sains Malaysiana 41 (7): 803-14.
[12] Bauchot, M. L., and Hureau, J. C. 1990. Sparidae. In FAO Species Rome.
[13] Bizsel, C., Kara, M. H., Pollard, D., Yokes, B., Goren, M., and Francour, P. 2011. Lithognathus mormyrus. The IUCN Red List of Threatened Species 2011: e.T170160A6727018. Downloaded on 02 October 2015. [14] Besseau, L., and Brusl-Sicard, S. 1991. Sex Inversion in a
Protandric hermophrodite Model Lithognathus mormyrus L. (1758) (Teleostei: Sparidae): Histological Peculiarities, 95. In: Proceedings of the 4th International Symposium
on the Reproductive Physiology of Fish (Eds. Scott, P. A.,
Sumpter, J. P., Kime, D. E. and Rolfe, M. S.). University of East Anglia, Norwich.
[15] Besseau, L., and Brusl-Sicard, S. 1995. “Plasticity of Gonad Development in Hermaphroditic Sparids: Ovotestis Ontogeny in Protandric Species, Lithognathus
mormyrus.” Env. Biol. of Fishes 43: 255-67.
[16] Kraljevic, M., Dulcùic, J., Pallaoro, A., Cetinic, P., and Jug-Dujakovic, J. 1995. “Sexual Maturation, Age and Growth of Striped Sea Bream, Lithognathus mormyrus L., on the Eastern Coast of the Adriatic Sea.” J. Appl.
Ichthyol. 11: 1-8.
[17] Kraljevic, M., Dulcùic, J., Cetinic, P., and Pallaoro, A. 1996. Age, Growth and Mortality of the Striped Sea Bream, Lithognathus mormyrus L., in the Northern Adriatic. Fisheries Research 28: 4361-70.
[18] Pajuelo, J. P., Lorenzo, J. M., Mendez, M., Coca, J., and Ramos, A. G. 2002. “Determination of Age and Growth of the Striped Seabream Lithognathus mormyrus
(Sparidae) in the Canarian Archipelago by Otolith Readings and Backcalculation.” Sci. Mar. 66 (1): 27-32. [19] Mann, B. Q., and van der Elst, R. P. 2000. Lithognathus
mormyrus. In: Mann, B. Q. (ed.), Southern African
Marine Linefish Status Reports. Special Publication No. 7 Oceanographic Research Institute, Durban.
[20] Lorenzo, J. M., Pajuelo, J. G., Méndez-Villamil, M., Coca, J., and Ramos, A. G. 2002. “Age, Growth, Reproduction and Mortality of the Striped Sea Bream, Lithognathus
mormyrus (Pisces, Sparidae), off the Canary Islands (Central-East Atlantic).” J. Appl. Ichthyol. 18 (3): 204-9. [21] Matić-Skoko, S., Josipa, F., Miro, K., and Jakov, D. 2007.
“Growth of Juvenile Striped Seabream, Lithognathus
mormyrus (Teleostei: Sparidae) in the Adriatic Sea.”
Institute of Oceanography and Fisheries, Meštrovićevo Šet. 63, P.O.Box. 500, 21000 Split, Croatia. Fax: +38521358650.
[22] Bini, G. 1968. Atlante dei pesci delle coste Italiane. Vol.
IV. Osteiti. Mondo Sommerso Editrice.
[23] Tortonese, E. 1975. Osteichthyes (Pesci ossei), II. Fauna dÕ Italia, 11. Ed. Calderini, Bologna.
[24] Grubisùic, F. 1982. Ribe, rakoviisùkoljkejadrana. ITRO Naprijed, Zagreb-GRO Liburnija, Rijeka.
[25] Ramos, A. G. 2002. “Age, Growth, Reproduction and Mortality of the Striped Sea Bream, Lithognathus
mormyrus (Pisces, Sparidae), off the Canary Islands
(Central-East Atlantic).” Journal of Applied Ichthyology
18 (3): 204-9. DOI: 10.1046/j.1439-0426.2002.00318.x (Impact Factor: 0.87).
[26] Suau, P. 1970. Contribución al studio de la biologia de
Lithognathus (= Pagellus) mormyrus L. (Pecesespáridos).
Inv. Pesq. 34: 237-65.
[27] FAO. 1982. ConseilG.n.ral des Pches pour la M.diterran.e, Rapport de la premi.re Consultation Technique sur l'Evaluation des Stocks dans la M.diterran.e Centrale. FAO Rapp. P.ches 266, FAO, Rome.
[28] UNESCO. 1984/1986. “Fishes of the North-eastern Atlantic and the Mediterranean” (FNAM). UNESCO Publication. Edited by Whitehead, P. J. P., Bauchot, M.-L., Hureau, J.-C., Nielsen, J., and Tortonese, E.. [29] Erzini, K., Bentes, L., Lino, P. G., Riberio, J., Coelho, R.,
Monteiro, P., Correia, C., and Goncalves, J. M. S. 2001a. “Age and Growth of Seven Sparid Species of the South Coast of Portugal.” Tenth European Congress on Ichthyology ECI X, Prague, Czech Republic.
[30] Erzini, K., Bentes, L., Lino, P. G., Riberio, J., Coelho, R., Monteiro, P., Correia, C., and Goncalves, J. M. S. 2001b. Reproductive Aspects of Seven Sparid Species of the Southern Coast of Portugal (Algarve). Tenth European Congress on Ichthyology ECI X, Prague, Czech Republic.
[31] Emre, Y., İsmet, B., Cetin, S. D., Aytuğ, O., and Ozgur YEŞİLCİMEN, H. 2010. “Age, Growth, Length-weight Relationship and Reproduction of the Striped Seabream
(Lithognathus mormyrus L., 1758) (Sparidae) in the
Beymelek Lagoon (Antalya, Turkey).” Turk. J. Zool. 34: 93-100 c TUBİTAK doi:10.3906/zoo-0808-13.
[32] Bailly, N. 2015. Lithognathus mormyrus (Linnaeus, 1758). In: Froese, R. and Pauly, D.. Editors. FishBase. Accessed through: World Register of Marine Species at http://www.marinespecies.org/aphia.php?p=taxdetails&id =127055 on 2015-04-29.
[33] Firat, K., Sahin, S., and Okan Kamac, H. 2005. EMBRYONIC AND LARVAL DEVELOPMENT OF STRIPED SEABREAM.
[34] Suau, P. 1970. “Contribuci.n al estudio de la biologiaLithognathus (= Pagellus) mormyrus L. especialmente de la sexualidad.” Invest. Pesq. 1: 59-66. [35] Kallianiotis, A., Michele, T., and Anna, A. 2005. “Age,
Reproductive Biology of the Striped Seabream Lithognathus mormyrus (Linnaeus, 1758) from Al-Haneah Fishingsite, Mediterranean Sea, Eastern Libya
181
the Striped Seabream, Lithognathus mormyrus (Pisces: Sparidae) in the Coastal Waters of the Thracian Sea, Greece.” Scientia Marina 69 (3): 391-404. doi:10.3989/scimar.2005.69n3391.
[36] Monteiro, P., Luis, B., Rui, C., Carla, C., Karim, E., Pedro, G. L., Joaquim, R., and Jorge, M. S. G. 2010. “Age and
Growth, Mortality and Reproduction of the Striped Sea Bream, Lithognathus mormyrus Linnaeus 1758, from the South Coast of Portugal (Algarve).” Marine Biology
Research 6 (1): 53-65.
Journal of Life Sciences 10 (2016) 182-184 doi: 10.17265/1934-7391/2016.04.002
Tetanus in Cat: From Neglected Wound to
Neuromuscular Disorder—Case Report
Maksimović Alan, Filipović Selma, Lutvikadić Ismar and Šunje-Rizvan Amila
Department of Surgery, Veterinary Faculty University of Sarajevo, Sarajevo 71000, Bosnia and Herzegovina
Abstract: Tetanus is caused by the bacterium, Clostridium tetani, and can infect both domestic animals and man. The disease is rarely diagnosed in cats, as a consequence of their increased resistance to the neurotoxin, tetanospasmin. Mortality in animals is generally high (80%). To date these authors have not been able to locate any reports of mortality rates specific to cats. Clinical diagnosis is based on clinical signs and a history of an untreated penetrating wound. This case report describes the development of moderate generalized tetanus in an approximately six month old female stray cat, found with an untreated wound on the side of its neck. This report describes clinical signs, treatment and recovery of the animal.
Key words: Feline, neglected wound, tetanus, therapy.
1. Clinical Presentation
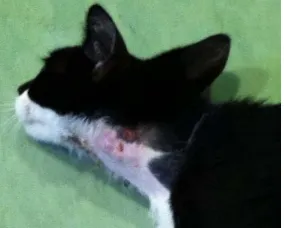
An approximately six month old, domestic shorthaired, female cat was presented in lateral recumbency with extensor rigidity of the neck, shoulders and elbows and partial flexion of the carpal joints. An old wound, already crusted over, was noted on the left side of the neck. Interestingly, rigid extension and caudal displacement was more pronounced on the right thoracic limb. The owner reported the cat had been found five days prior, and the cat did not initially display any signs of weakness. The owner treated the wound by daily cleansing with iodine. On the sixth day, the cat developed stiffness in its neck, inability to eat and lameness in the left thoracic limb. This condition rapidly progressed during that day, to the extent that cat became unable to vocalize. On physical examination the cat displayed extension and rigidity of its front limbs, more pronounced in the right forelimb, right hind limb, tail and neck (Fig. 2). Heart rate and respiratory rate were normal, and the rectal temperature was 39.9 °C. Abdominal palpation revealed a distended urinary
Corresponding author: Maksimović Alan, DVM, M.Sc., Ph.D., research field: small animal surgery analgesia and anesthesia.
bladder and feces- filled intestines. X-ray examination confirmed these findings and an absence of radiographic indicators of spinal trauma or other pathological conditions was noted (Fig. 1).
2. Therapy
The wound was debrided and irrigated with hydrogen peroxide (3% concentration) and chlorhexidine (0.5 % concentration) after removal of the crust. Metronidazole (Efloran, Krka, Slovenia) at a dose of 15 mg/kg and vitamin B complex (vitamins B complex + C, Norbrook, Northern Ireland) were dissolved in saline 0.9% and administered in a slow intravenous infusion (rate 2 mL/kg/h), once daily. In addition, amoxicillin with clavulanic acid (Synulox, Pfizer) was given subcutaneously at a dose of 8.75 mg/kg/24 h. Diazepam (0.2 mg/kg (IV)) was administered every 12 h, resulting in a minimal reduction of muscle rigidity. During the first five days of treatment, manual expression of the urinary bladder was necessary, due to external urethral sphincter hypertonus. During the same period the cat was unable to prehend and chew canned food, but was able to swallow. Therefore, assisted feeding was prescribed with high-fibre food combined with lactulose at dose
D
Tetanus in Cat: From Neglected Wound to Neuromuscular Disorder—Case Report 183
Fig. 1 Rigid extension of the neck and wound presentation after scab removing.
Fig. 2 Clinical presentation of the cat at the second day of treatment.
Fig. 3 Distended urinary bladder and intestines filled with feces are shown.
of 1 mL/4.5 kg/12 h. The cat was sent home with specific instructions regarding bedding, feeding and minimizing CNS stimulation.
After five days of therapy the cat began to vocalize. The rigidity of neck, limbs and tail decreased and minimal movement of the head was observed. Five days later she was able to stand with mild motor activity difficulties. Following day 15 of treatment the cat has been able to walk on a level surface without
visible ataxia. On the 23rd day the client reported the cat had successfully jumped from floor to sofa and back for the first time.
3. Discussion
Although cats are known to be resistant to the effects of the Clostridium tetani exotoxin, cases of tetanus in this species have been reported [1, 2]. Signs can be limited to a single limb, causing monoparesis associated with extensor rigidity [1] or manifest in all four limbs [2]. Rapid onset and generalization of clinical signs is more likely if the wound is located near the head [3]. This phenomenon reflects the typical course of tetanus, as the majority of the toxin is absorbed by the peripheral nerve terminals near the wound and transported intra-axonally to the spinal cord [2]. In the present case, the entry point for the clostridial organisms was the wound on the left side of the neck. Consequently, muscle rigidity was evident first in the region of the neck and in thoracic limbs, subsequently involving the pelvic limbs and the tail. Carpal joints were in partial flexion. Thoracic limb rigidity with elbow extension and carpal flexion has been previously described in cats with tetanus, and this seems to be a feature of this disease in cats, while dogs with tetanus present with carpal extension [2].
In this case, the presentation of rapidly progressive extensor rigidity could be due to the cat’s age. Younger animals generally demonstrate a more severe form of tetanus as a result of their immature natural immunity [4]. Diagnosis is based on clinical signs and a history of a recent or old wound [2, 4, 5]. The first clinical sign of tetanus in cats occurs between two days and three weeks post injury [6]. In this case, the incubation period was unknown, because the cat was found injured. The incubation period was known to be longer than 5 days (period from adoption to first clinical sign), though.
Tetanus in Cat: From Neglected Wound to Neuromuscular Disorder—Case Report
184
the spasticity [5]. We did not use tetanus antitoxin, in consideration of the onset of symptoms and the time of patient presentation. Antitoxin neutralizes only unbound circulating toxin; it has no effect on bound toxin. Therefore, it should be administered as soon as possible, though it does not necessarily speed the rate of recovery [3]. The benefit of tetanus antitoxin in cats remains uncertain, insofar as there is too little information in the literature regarding its safety and efficacy [7]. Resolving tetanus in cats without the use of antitoxin has been previously reported [6, 8]. Prompt presumptive diagnosis and beginning of adequate therapy potent recovery.
References
[1] Polizopoulou, Z., Kazakos, G., and Georgiadis, G., et al. 2002. “Presumed Localized Tetanus in Two Cats.”
Journal of Feline Medicine and Surgery 4: 209.
[2] De Risio, L., and Gelati, A. 2003. “Tetanus in the Cat—an Unusual Presentation.” Journal of Feline
Medicine and Surgery 5: 237-40.
[3] Grace, S. F. 2011. Tetanus. In: Gary D. Norsworthy (ed)
The Feline Patient. 498-9, 4th edition. Wiley-Blackwell.
[4] Greene, C. E. 2012. Tetanus. In: Greene Infectious
Diseases of the Dog and Cat. 423-31, 4th edition.
Georgia: Athens.
[5] Garosi, L. 2012. “Neurological Lameness in the Cat: Common Causes and Clinical Approach.” Journal of
Feline Medicine and Surgery 14: 85-93.
[6] Tomek, A., Kathmann, I., Faissler, D., Cizinauskas, S., Timmann, D., Reimer, Y., Moser, J., and Jaggy, A. 2004. “Tetanus in Cats: 3 Case Descriptions.” Schweiz Arch
Tierheilkd 146: 295-302.
[7] Little, S. 2012. The Cat, Clinical Medicine and
Management. 764, St. Louise, Missouri.
[8] Langner, K. F., Schenk, H. C., Leithaeuser, C., Tholen, H., and Simon, D. 2011. “Localised Tetanus in a Cat.”
Journal of Life Sciences 10 (2016) 185-191 doi: 10.17265/1934-7391/2016.04.003
Management of Insect Vectors of Viruses in Tomato
Plants Using Different Densities of Yellow Traps
Eduardo Domingos Grecco, Dirceu Pratissoli, Hugo Bolsoni Zago, Débora Ferreira Melo Fragoso and José Romário Carvalho
Department of Plant Production, Center of Agricultural Sciences and Enengineering (CCAE), Universidade Federal do Espírito
Santo (CCA-UFES), Alegre, Espírito Santo, Brazil
Abstract: The initial phase of tomato is critical to the infestation of insect vectors of viruses. Therefore, this study aimed to test the use of yellow card traps around the crop to manage insect vectors of viruses and test the best density of traps/tomato plants. Yellow card traps were placed on the border of the crop plot to capture adult insect vectors. Density of trap/tomato plant was assessed in 10 blocks at the following levels: 1/25; 1/50; 1/75; 1/100; 1/125; 1/150. The monitoring was carried out in 1% of the crop during 60 days in 2011 and 2012 crop. The evaluated systems were Conventional and Phytosanitary Pest Management (PPM). During 2011 season the Conventional system received 14 insecticide applications whereas only 6 insecticide applications were made on the PPM, representing a reduction of 133%. In 2012, the crop under Conventional system was subjected to 15 applications of insecticides, over 8 on PPM, with a reduction of 87.5%. The PPM allowed a 90% reduction in application cost for this insects, obtaining a reduction of R$1,345.00/ha. The highest density was 60 plants/trap. We can conclude that the yellow card traps in tomato crop decreased infestations of insect vectors of viruses.
Key words: Viruses vectors, Solanumlycopersicum, yellow card trap.
1. Introduction
The tomato (Lycopersicon esculentum Miller), is a solanaceous with socioeconomic benefits, but the implementation of this crop is considered as a high risk one due the occurrence of pests and diseases throughout the cycle [1, 2].
Large leaf exchange area and staggered tomatoes planting in nearby areas are considered the main factors to insect vectors of viruses onset. The formation of an ideal microclimate and constant availability of food for long periods allows a concurrency of several generations in high population levels of insect vectors of viruses in tomato [3, 4].
The first stage of crop development, which lasts up to 60 days, is considered critical to virus vectors. These insects are classified as aphids, Myzus persicae
Corresponding author: Débora Ferreira Melo Fragoso, doctor, research fields: entomology, viruses vectors, solanum lycopersicum and yellow card trap.
Sulzer Macrosiphum euphorbiae Thomas (Homoptera: Aphididae), which transmit four types of viruses, tomato yellow top and tomato mosaic, the “Y” and virus yellow botton. The insects known as thrips,
Frankliniella schultzei Trybom and Thrips palmi
Karny (Thysanoptera: Thripidae), also fit in this category, responsible for transmitting the virus complex called tomato spotted wilt. The whitefly, Bemisia tabaci
Gennadius (Hemiptera: Aleyrodidae), is another viruses transmitter, which is responsible for spreading four viruses, the most common cause of tomato golden mosaic and Tospoviruses. These viruses are considered limiting factors because infected plants cannot produce fruits, or when produced, they do not meet market requirements [5, 6].
In any pest management program it is essential to monitor arthropod pests and pests which do not occur in an agricultural system, since they facilitate decision making concerning the introduction of control measures. Therefore, it is important to use colored
D
Management of Insect Vectors of Viruses in Tomato Plants Using Different Densities of Yellow Traps
186
yellow card traps and their correct distribution in the planting area.
The objective of this research was to test the use of yellow card traps surrounding the crop to manage insect vectors and assess the best density of trap/tomato plants.
2. Materials and Methods
The experiments were conducted by the Nucleus of Scientific and Technological Development in Phytosanitary Management of Pests and Diseases (NUDEMAFI), Federal University of Espírito Santo (CCA-UFES), in 2011 and 2012 harvests in Cachoeiro de Itapemirim, geographical coordinates 20° 50′ 56″ south and 41° 06′ 46″ west (Distrito Córrego dos Monos)—Espírito Santo in tomato plantations treated with Conventional and Phytosanitary Pest Management (PPM). The experiment was conducted during May-September of both crop the variety used was Ibatã (recommended for the region). The cultivation, transplanting, staking, tying, designated rig and thinning were performed following crop recommendations [7].
We adopted the guidance system with two stems per plant in vertical staking, the most used by producers of staked tomatoes in the State of Espírito Santo. This method was based on the conduction of plants on bamboo stakes on which plants were tied
every 7 days. We used a 1.3 × 0.6 m spacing (lines × plants).
Systems management reviews. The systems evaluated were: (1) Phytosanitary Pest Management (PPM) [8] and adapted monitoring pests [9] (Table 1) and in decision making to the application of insecticides based on the level of infestation in the field. (2) Conventional (producer), who adopted calendar sprays drawn up by producers where applications started seven days after transplanting and continued to be held twice a week regardless of the level of pest infestation in the field (Table 2). Pesticides that producers used to control insect vectors of viruses were used.
Biocontrol® yellow card traps (100 cm × 30 cm) with adhesive glue were placed around the tomato PPM for adults insects vectors of viruses were captured and did not cause damage to tomato crop. These traps were changed every 20 days because they lie with the area of adhesive glue without empty spaces for capturing or young adults because of the loss of the chemical structure of the adhesive insects, because the field receives solar rays that degrade the adhesive, making traps less sticky.
The experiment was conducted in a plot with 2,000 plants consisting in 4 repetitions. The monitoring reviews were made 7 days after planting, in 1% of the crop. For each repetition 1 random spot was assessed
Table 1 Pest, sampling methods and action level adopted in the Phytosanitary Pest Management (PPM) system [9].
Pest Vector Sampling Method Action level
Vectors
Whitefly—Bemisia tabaci (Tospoviruses) Aphids—Mysus perssicae and
Macrosiphum euphorbiae (Mosaics)
Thrips—Frankliniella schultzei (winter cropping tomato)
Knock rod in PVC boxes with blue background
1 vector by rod in mean and/or 0.5 thrips/rod in tomato
Table 2 Insecticides used by producers in Phytosanitary Pest Management (PPM) and conventional.
Product Active Ingredient MAPA Register Chemical Dose mL/ha
Actara 250 WG Thiamethoxam 10,098 Neonicotinoid 500-1,000
Platinum Neo Thiamethoxam +
Lambda-cialotrina 5,110
Neonicotinoid +
Pyrethoid 50-100
Connect Imidacloprid +
Beta-ciflutrin 4,804
Neonicotinoid +
500-1,000 Pyrethoid
Oberon Espiromesifen 1,706 Keto-enol 500-600
Management of Insect Vectors of Viruses in Tomato Plants Using Different Densities of Yellow Traps
187
with 5 plants in a row, being evaluated the top of the plants [10]. These evaluations were performed making knock rod in a plastic tray (40 cm × 20 cm × 10 cm) of blue color, which accounted adult insects present and alive. The index for application of insecticide, if necessary, a first vector is rod. The data were analyzed through Shapiro-Wilk test of normality and absence by non-parametric Mann-Whitney P ≤ 0.05.
The density experiment of yellow card trap/plants was in an area with 8,000 plants, the following densities were used: 1/25 (= 0.04); 1/50 (= 0.02); 1/75 (= 0.13); 1/100 (= 0.01); 1/125 (= 0.008); 1/150 (= 0.007) trap/plants. As control treatment, yellow traps were not used and the experimental unit was composed of 1,000 (= 0.001) tomato plants over conventional planting. The experiment was conducted in 10 blocks, and the evaluations were done twice a week for 60 days, which is the most critical period for viruses in tomato. Yellow card traps (100 cm × 30 cm) were distributed equidistantly among the plants according to its scope and so they did not interfere one over the other. Data were subjected to analysis of exponential regression. To estimate the best density the linearization curve using a logarithmic transformation was performed.
3. Results and Discussion
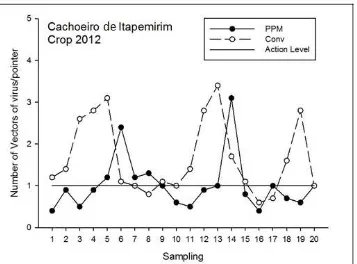
In Table 3 (2011 harvest) is shown that average infestation of thrips and aphids were not significant to crops and conventional tomato PPM. However, to whitefly and the sum of the vectors, the PPM was significant compared to Conventional. For whitefly in PPM, the average values of adults have not reached the level of action being 60% below the average obtained by conventional tillage. When summed the viruses vectors, we observed that the PPM was below the action level, unlike conventional that achieved a 56% higher rate of adults in tomato. A reduction in fly vectors population in PPM due to the use of yellow card traps around the crop reduced the use of specific insecticides to control insect vectors of viruses.
The level of infestation observed in Fig. 1, in 2011 shows that only assumed whitefly vector values greater than 1 vector/rod to the PPM and Conventional systems. For the sum of the vectors of viruses, conventional was above or equal to the action level in 80% of samples, compared to 30% to PPM. It can be seen the occurrence of population peaks even when there were no sprays, which can be attributed to climatic factors such as humidity and temperature. In the 2011 harvest, for conventional tillage, there were 14 applications of insecticides listed in Table 2, compared with 6 applications of PPM in the same season.
In Table 4 (2012 harvest) is shown that the average infestation of thrips was not significant to crops and conventional tomato PPM, no application is recommended to insect vectors. However, to aphids and whitefly vector, the PPM infestation was significant compared with the conventional one. Population reduction of aphids and whitefly vectors in PPM occurred due the use of yellow card traps around the crop, which allowed the capture of adult vectors. The average adult whiteflies number in the conventional system was 61% higher than that obtained in the PPM. When summed viruses vectors, it is observed that conventional system achieved a 63% higher rate of adult vectors in the field.
The level of infestation observed in Fig. 2 (2012 harvest) shows that only whitefly assumed values exceeding a vector/rod in both treatments. In the sum of viruses vectors, conventional was above or equal the action level in 85% of samples, versus only 40% of PPM. A whitefly was the most abundant species caught in yellow card traps. In the 2012 harvest, using conventional tillage, there were 15 insecticides applications listed in Table 2, compared with 8 PPM applications in the same harvest.
Management of Insect Vectors of Viruses in Tomato Plants Using Different Densities of Yellow Traps
188
Table 3 Average number (± SEM) of adult thrips, aphids, whitefly vectors and samples collected in tomato planting Conventional and Phytosanitary Pest Management systems (PPM) Cachoeiro de Itapemirim, 2011 harvest.
Insect pests
Systems thrips aphids whitefly vectors
Conventional 0.075 ± 0.017a 0.230 ± 0.0371a 1.175 ± 0.141a 1.495 ± 0.168a
PPM 0.090 ± 0.019a 0.145 ± 0.0312a 0.735 ± 0.131b 0.955 ± 0.157b
Means followed by the same letter in the column do not differ by Mann-Whitney test P≤ 0.05.
Fig. 1 Infestation of insect vectors of viruses in the 2011 index from tomato crops in conventional Phytosanitary Pest Management (PPM) in Cachoeiro de Itapemirim.
Table 4 Average number (± SEM) of adult thrips, aphids, whitefly, vectors collected in tomato planting Conventional and Phytosanitary Pest Management systems (PPM) in Cachoeiro de Itapemirim, 2012 harvest.
Insect pests
Systems thrips aphids whitefly vectors
Convencional 0.105 ± 0.017a 0.236 ± 0.034a 1.295 ± 0.166a 1.660 ± 0.200a
PPM 0.075 ± 0.018a 0.140 ± 0.024b 0.805 ± 0.129b 1.020 ± 0.147b
Means followed by the same letter in the column do not differ by the Mann-Whitney P ≤ 0.05. because of visual and olfactory characteristics. The
high reflectance of yellow color promotes whitefly attractiveness. Studies have shown that the yellow color for traps are more attractive [11, 12]. Mainali and Lim found a reduction in whitefly number in tomato grown in greenhouses using yellow traps. Therefore, one can correlate the high incidence of catching adult whitefly in this study with the yellow
color of the traps of previous studies, which could increase the cost/benefit to the producer and of the utmost importance for consumers and the environment [13].
Management of Insect Vectors of Viruses in Tomato Plants Using Different Densities of Yellow Traps
189
Fig. 2 Fluctuation of insect vectors of viruses population in 2012 tomato harvest in Conventional and Phytosanitary Pest Management (PPM) in Cachoeiro de Itapemirim.
injecting a toxin that causes anomalies in ripe fruits, leaving them with yellowish bands, making them depreciable to be consumed “In nature” (Fig. 3). Therefore, monitoring is required throughout the cycle to avoid significant increase of the population of vectors, which may cause considerable damage to the following crops. Another relevant factor is the wind that spreads the vectors to new crops, so the importance of having traps with traps and color if possible borders with plants that are not vectors hosts of viral diseases and/or pests of tomato.
To handle vectors of viruses in tomato, it is necessary to eliminate all vectors of host weeds before planting an early crop. It is also of great importance and puts them seedlings are still protected by the sowing anti-aphid screens to reach stronger the field, thus enduring greater pest attack. The sooner the plant is infected by viruses, more damage will be observed that will directly affect productivity.
One adult whitefly per plant is enough to cause incidence of the virus of 100% under field conditions, if such an insect is infected by some virus, may cause
total losses [14]. Reported that only 0.3 adult whitefly per plant quickly spreads the virus on tomato [15]. Where tospoviruses are present, control measures must conform to the level of action indicated by the constant sampling in the field [16].
Considering this evidence, the visual attraction of insects may be used in integrated pest management in order to reduce the use of pesticides in the environment and in food. Therefore, it is important to make it an indispensable component in monitoring and management of whitefly, thrips and aphids on vegetable crops.
It can be observed in Fig. 3, the density of traps followed an exponential regression to whitefly, aphids, thrips vectors and viruses. The ideal amount of traps per plant ranked 60 plants/trap.
Management of Insect Vectors of Viruses in Tomato Plants Using Different Densities of Yellow Traps
190
Fig. 3 Density of traps to insect vectors of plant viruses in Cachoeiro de Itapemirim.
Table 5 Cost/benefit/ha on Phytosanitary Pest Management (PPM) and Conventional (Conv.) systems in Cachoeiro de Itapemirim.
2011 harvest
Inseticides R$ ha PPM Conv. R$ PPM R$ Conv. Saving
Platinum Neo 125.00 2 4 250.00 500.00 250.00
Actara 27.00 1 2 27.00 54.00 27.00
Connect + Oberon 186.00 2 2 372.00 372.00 -
Mospilan 49.00 1 6 49.00 294.00 245.00
Sum total 698.00 1,220.00 522.00
2012 harvest
Inseticides R$ ha PPM Conv. R$ PPM R$ Conv. Saving
Platinum Neo 125.00 2 5 250.00 625.00 375.00
Actara 27.00 1 2 27.00 54.00 27.00
Connect + Oberon 186.00 2 4 372.00 744.00 372.00
Mospilan 49.00 3 4 147.00 196.00 49.00
Sum total 796.00 1,619.00 82.00
Total Crops 1,494.00 2,839.00 1,345.00
The Table 5 (2011 harvest), 14: 06 insecticide applications were held respectively in the conventional area and PPM to control vectors of virus diseases in tomato. In 2012 (Table 5) 15: 08 insecticide applications were held respectively in the conventional area and PPM. There was an economy in the insecticides used on PPM, R$522.00 during 2011
harvest and R$823.00 in 2012, totaling an overall saving of R$1,345.00/ha.
This saving only on vectors of viruses is very important, because the cost of tomato production is around R$3.50/plant. One hectare holds an average of 12,000 plants, so the cost of an acre is worth R$42,000.00.
1.0
0.8
0.6
0.4
0.2
0.0
Management of Insect Vectors of Viruses in Tomato Plants Using Different Densities of Yellow Traps
191
4. Conclusions
Yellow card traps can be employed as a physical barrier to insect vectors of viruses;
The monitoring of plants showed that yellow card traps reduced the population of insect vectors of viruses;
A reduction in insecticides usage increases the producer profit;
The optimal trap density, as a physical barrier should be 0.017 traps/plant or 60 plants/trap.
References
[1] Filgueira, F. A. R. 2000. Tomate: a hortaliça cosmopolita, In: Filgueira, F. A. R. (ed.). Novo manual de olericultura: agrotecnologia moderna na produção e comercialização de hortaliças, 189-234, 402. Viçosa, UFV.
[2] Luz, J. M. Q., Shinzato, A. V., and Silva, M. A. D. 2007. “Comparação dos sistemas de produção de tomate convencional e orgânico em cultivo protegido.”
Bioscience Journal 23: 7-15.
[3] Ávila, A. C., Inoue-Nagata, A. K., Costa, H., Boiteux, L. S., Neves, L. O. Q., Prates, R. S., and Bertini, L. A. 2004. “Ocorrência de viroses em tomate e pimentão na região serrana do estado do Espírito Santo.” Horticultura
Brasileira 22: 655-8.
[4] Silva, A. C., and Carvalho, G. A. 2004. Manejo Integrado de Pragas. In: Alvarenga, M. A. R. (Ed.). Tomate:
Produção em campo, em casa-de-vegetação e em hidroponia. Lavras, 309-66, UFLA, 400.
[5] Gallo, D., Nakano, O., Silveira Neto, S., Carvalho, R. P. L., Baptista, G. C. de, Berti Filho, E. B., Parra, J. R. P., Zucchi, R. A., Alves, S. B., Vendramim, J. D., Marchini, L. C., Lopes, J. R. S., and Omoto, C. 2002. Entomologia
Agrícola. Piracicaba: FEALQ, 920.
[6] Lebedenco, A. 2006. “Eficiência de métodos de controle de pragas do tomateiro (Lycopersiconesculentum Mill.) na região de Presidente Prudente-SP”. Dissertação de Mestrado, UNOESTE, Presidente Prudente.
[7] Abaurre, M. E. O. 2010. Práticas culturais. In: Instituto
Capixaba de Pesquisa, Assistência Técnica e Extensão
Rural. Eds. Tomate. 133-48, Vitória: Incaper.
[8] Alves, F. R., Jesus Junior, W. C., Pratissoli, D., Polanczyk, R. A., Zanuncio Junior, J. S., Holtz, A. M., and Vianna, U. R. J. 2007. Manejo fitossanitário de doenças e plantas—novas perspectivas. In: (Ed) Jesus Junior, W. C., Polanczyk, R. A., Pratissoli, D., Pezzopane, J. E. M., Santiago, T.. Atualidades em Defesa
Fitossanitária: 383-415.
[9] Gravena, S., and Benvenga, S. R. 2003. Manual prático
para manejo de pragas do tomate. Jaboticabal: Gravena.,
143.
[10] Gravena, S. 1991. Encontro nacional de produção e
abastecimento de tomate, 105-57, 2. Ed. Jaboticabal:
FUNEP.
[11] Van Lenteren, J. C., and Noldus, L. P. J. J. 1990. Whitefly-plant relationships: behavioural and ecological aspects. In: Gerling, D. (ed.), 47-89. Whiteflies: their Bionomics, Pest Status and Management. Intercept Ltd, Hants, UK.
[12] Byrne, D. N., and Bellows, T. S. 1991. Whitefly Biology.
Annual Review of Entomology 36: 431-57.
[13] Mainali, B. P., and Lim, U. T.. “Evaluation of Chrysanthemum Flower Model Trap to Attract Two
Frankliniella thrips (Thysanoptera: Thripidae).” Journal
of Asia-Pacific Entomology 11: 171-4.
[14] Hilje, L. 1997. Possibilidades para el manejo integrado del complejo mosca blanca-geminivirus en tomate, na America Central. In: Congresso Brasileiro De Entomologia, 16., Salvador. Resumos... Salvador: SEB; EMBRAPA-CNPMF, 9.
[15] Cubillo, D., Sanabria, G., and Hilje, L. 1999. Eficacia de coberturas vivas para el manejo de Bemisia tabaci como vector de geminivirus, en tomate. Manejo Integrado de
Plaga (51): 10-20. Turrialba.
[16] Mattos, M. A. de A. 2001. Bemisia argentifolii
Journal of Life Sciences 10 (2016) 192-197 doi: 10.17265/1934-7391/2016.04.004
Therapeutic Effect of
Zygophyllum cornutum
on
Metabolic Disturbances, Oxidative Stress in Heart
Tissue and Histological Changes in Myocardium of
Streptozotocin-induced Diabetic Rats
Awatif Boumaza1, 2, Samira Ferdi3, Houda Sbayou4, Fatima Khelifi Touhami2, Mohamed Habib Belmahi3 and
Cherifa Benlatreche3
1. Laboratory of Biology, Water and Environment, University of 8 Mai 1945, Guelma 24000, Algeria
2. Department of Animal Biology, University of Mentouri-Constantine 1, Constantine 25000, Algeria
3. Faculty of Medicine, University of Mentouri, Constantine 25000, Algeria
4. University of Hassan 1er, Faculty of Sciences and Technology, Department of Biology, Settat, Morocco
Abstract: The present study depicts the therapeutic effect of Zygophyllumcornutum methanolic extract (ZCME) on metabolic disturbances, oxidative stress in heart and myocardium histological changes in streptozotocin (STZ)-induced diabetic rats. Three days after diabetes induction, ZCME was administered orally for six weeks (700 mg/kg bw/day). Serum glucose and lipid profile were evaluated. Reduced glutathione (GSH), catalase (CAT) and thiobarbituric acid reactive substances (TBARS) were measured in heart tissue. The results showed increased levels of blood glucose, total cholesterol (TC), LDL cholesterol (LDL-C) and triglyceride (TG) in the diabetic rats. On the other hand, the level of HDL cholesterol (HDL-C) decreased. Compared to the control normal rats, the level of TBARS in heart tissue was markedly increased while GSH and CAT were significantly modified in the diabetic rats. Oral administration of ZCME has normalized serum glucose and lipid profile. TBARS were significantly reduced in heart while CAT and GSH were markedly normalized. Myocardium sections showed the absence of histological changes observed in the diabetic rats. The study suggests that Zygophyllum cornutum may provide a useful therapeutic option in the reversal of metabolic disturbances and oxidative stress-induced cardiac dysfunction in diabetes mellitus.
Key words: Diabetes mellitus, myocardium, oxidative stress, Zygophyllum cornutum coss.
1. Introduction
Diabetes mellitus is one of the most costly burdensome chronic diseases of our time and is a
condition that is increasing in epidemic population in the whole word. Diabetes mellitus is regarded as a group of metabolic diseases characterized by an elevated
blood glucose level resulting from defects in insulin secretion, insulin action or both [1]. Hyperglycemia,
resulting from uncontrolled glucose regulation, is widely recognized as the causal link between diabetes, oxidative stress and diabetic complications [2]. Elevated
Corresponding author: Awatif Boumaza, Ph.D., assistant professor, research field: cellular and molecular toxicology.
glucose levels may cause a wide range of metabolic disturbances in vascular cells and organ tissues of
diabetic patients [3]. More research is directed towards medicinal plants that are considered as an important source of many herbal substances with antidiabetic
and antioxidant activities. The present study depicts
the therapeutic effect of Zygophyllum cornutum on
metabolic disturbances and oxidative stress in heart as one of the target organs in diabetes mellitus.
2. Materials and Methods
2.1 Preparation of Zygophyllum cornutum Methanolic Extract (ZCME)
The preparation method of the methanolic extract