www.elsevier.com / locate / bres
Research report
Corticosterone inhibits generation of long-term potentiation in rat
hippocampal slice: involvement of brain-derived neurotrophic factor
*
Jianzheng Zhou , Fang Zhang, Yongxiang Zhang
Laboratory of Neuropharmacology, Beijing Institute of Pharmacology and Toxicology, Beijing 100850, PR China Accepted 29 August 2000
Abstract
In the present study, the effect of corticosterone (CORT) on the generation of long-term potentiation (LTP) and its underlying mechanism involving neurotrophin gene expression in CA1 synapses of rat hippocampal slice were examined. Our experimental results showed incubation of hippocampal slice with CORT for 3 h had no effect on either the slope or amplitude of excitatory postsynaptic potentials (EPSP) evoked in hippocampal CA1 pyramidal dentrites, indicating no marked change in basal synaptic transmission. However, when tetanic stimulation (100 pulses, 100 Hz) was delivered to the Schaffer collateral pathway, CORT application significantly attenuated the tetanus-induced increases of both EPSP slope and amplitude, demonstrating an inhibitory effect of CORT on LTP generation. In addition, CORT treatment significantly reduced both slope and amplitude ratios of the second evoked EPSP to the first one when paired-pulse facilitation (PPF) was established at different interpulse intervals from 20 to 40 ms, suggesting that a presynaptic mechanism may be involved in CORT-induced hippocampal synaptic plasticity. Reverse-transcription polymerase chain reaction (RT-PCR) analysis showed that CORT-treated hippocampal CA1 cells underwent a significant decrease in the expression of mRNA for nerve growth factor-b
(NGF-b) and brain-derived neurotrophic factor (BDNF), but not for neurotrophin-3 (NT-3) compared with those in control. Moreover, BDNF co-applied with CORT significantly antagonized CORT-induced deficit in PPF. Taken together, the present results suggest that CORT-induced inhibition of LTP may be, at least to some extent, mediated by a presynaptic mechanism and decrease in the BDNF expression in rat hippocampal CA1 cells induced by CORT may partially account for this presynaptic mechanism. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Corticosterone; Long-term potentiation; Paired-pulse facilitation; Synaptic plasticity; Synaptic transmission; Reverse-transcription-polymerase chain reaction; Neurotrophin; Nerve growth factor (NGF); Brain-derived neurotrophic factor (BDNF); Neurotrophic factor-3 (NT-3)
1. Introduction glutamate, hypoxia, etc., to the hippocampal neurons
[1,2,5]. In addition, administration of CORT to rats in vivo Glucocorticoids are series of important steroid hormones for 3 months led to significant impairment of learning and in regulating metabolism of glucose, fat and protein in memory [37]. The underlying mechanisms were believed various peripheral tissues. Recent studies indicated that to be closely related to accumulation of excitatory amino high concentration of glucocorticoids showed adverse acids, energetic deficiency or cytosolic calcium overload effects on the central nervous system (CNS), especially on [17,29,36]. More recent studies show that glucocorticoids the hippocampus [12,37]. It was reported that application may participate in regulating synaptic transmission and of corticosterone (CORT) in vitro not only impaired the play an important role in maintaining the hippocampal primary cultured hippocampal neurons directly [45] but synaptic strength [6,28]. Either adrenalectomy [34] or also exacerbated neurotoxicity of many insults, such as exogenous CORT supplementation [46] inhibited LTP generation in the hippocampal dentate gyrus. Bodnoff et al. reported that CORT application to middle-aged rats for 3 months led to impairment of learning and memory, great neuron loss and inhibition of LTP formation in the
*Corresponding author. Unit of Synaptic Development and Plasticity,
hippocampus [3]. These results implicated that the
CORT-Laboratory of Developmental Neurobiology, NICHD, NIH, Bldg. 49 / Rm.
induced deficit in hippocampal synaptic efficacy might
6A67, Bethesda, MD 20892-4480, USA.
E-mail address: [email protected] (J. Zhou). result from the structural modification of the hippocampus.
In our previous study [46], however, CORT applied 1 h tion. The hippocampus was carefully dissected in 95% before tetanus without causing any morphological modi- O / 5% CO -saturated ice-cold artificial cerebrospinal fluid2 2
fication also significantly inhibited both induction and (ACSF). A transverse hippocampal slice with thickness of maintenance phases of LTP in vivo, suggesting the inhib- 400–450 mm was prepared using a vibroslice (World itory effect may be mediated by a quick and direct Precision Instruments, USA) and recovered in warmed,
mechanism. 95% O / 5% CO -saturated ACSF for at least 1 h as2 2
Neurotrophins are traditionally identified as a family of described previously [43]. The slices were then transferred protein factors essential for neuronal survival and differen- in a submerged chamber perfused with warmed (348C) tiation. However, a series of recent studies suggested a ACSF (pH 7.4) containing (in mM) NaCl 124, KCl 3.0, novel role of neurotrophins in synaptic transmission and CaCl2 2.5, MgCl2 1.5, NaHCO3 26, KH PO2 4 1.25, plasticity [4,22,24,39]. Long-term application of the BDNF glucose 10 and ascorbic acid 2. Field EPSP was evoked in and NT-3 was found to enhance synaptic transmission at CA1 stratum radiatum by stimulating the Schaffer colla-the developing neuromuscular junction in culture teral pathway with twisted bipolar nichrome electrodes and [21,23,40]. The acute effects of neurotrophins on neuronal recorded with ACSF-filled glass pipette (,5 MV) using a activity and synaptic transmission were also observed in microelectrode amplifier (MEZ-8201, Nihon Kohden, cultured neurons and in slices by a number of laboratories Japan). Test stimuli consisted of monophasic 100-ms [15,19,20]. Kang and Schuman [13] showed that acute pulses of constant current delivered by electronic application of BDNF and NT-3 enhanced basal excitatory stimulator (SEN-3201, Nihon Kohden). Basal synaptic synaptic transmission in CA1 synapse of adult hippocam- transmission was examined by alternating, low-frequency pal slices. Tanaka [38] found BDNF did not affect basal stimulation at 0.033 Hz (one stimuli for every 30 s) of two excitatory synaptic transmission but rapidly reduced IPSC separate pathways via two stimulating electrodes at CA1 synapses in hippocampal slices. Meanwhile, BDNF positioned on both sides of the recording electrode. Only knock-out conferred deficit in generating LTP in mouse slices exhibiting EPSP of 2–3 mV in amplitude without hippocampal CA1 synapses and this defect can be rescued superimposed population spikes were used. Stimulus in-after prolonged incubation with BDNF-containing adeno- tensity was adjusted to produce an EPSP with |50% of
virus [16]. Application of exogenous BDNF promotes LTP maximum amplitude. Tetanic stimulation, which consisted induction in neonatal hippocampal slices, where the endog- of 100 pulses at 100 Hz, was used to induce LTP. LTP was enous BDNF levels are low [9]. In contrast, treatment with thought to be induced and expressed when both EPSP TrkB-IgG, a fusion protein that scavenges endogenous amplitude and slope were potentiated more than 25% BDNF, reduces the magnitude of LTP in the adult hip- above the baseline for at least 30 min after tetanus. We also pocampus [14], where the endogenous BDNF levels are employed PPF to examine the synaptic strength presynapti-high. Moreover, BDNF perfused with mice hippocampal cally. A paired stimuli with interpulse intervals from 20 to slice for 3 h enhanced PPF, suggesting that BDNF-induced 50 ms was given to the Schaffer collateral pathway and the enhancement of synaptic efficacy may be somewhat in- evoked EPSPs were recorded. The ratios of evoked EPSP
volved in a presynaptic mechanism [10]. slope and amplitude in the second stimuli to those in the
In our present study, the effect of CORT on hippocam- first one were calculated to examine presynaptic facilita-pal synaptic transmission and its underlying mechanism tion during PPF. Both slopes and amplitudes of EPSP were involving the neurotrophin gene expression are explored. digitized (10 kHz), filtered at 1 kHz (eight-pole Bessel We found that CORT application significantly inhibited filter), stored on magnetic media using an acquisition LTP, PPF as well as BDNF gene expression in rat system PClab 2.0 (Beijing PClab Instruments, Beijing, hippocampal CA1 synapses while perfusion with BDNF China) and analyzed off-line. CORT was added directly rescued the CORT-induced impairment of PPF, suggesting into the ACSF and perfused the rat hippocampal slice for 3 that the inhibitory effect of CORT on hippocampal synap- h before delivery of tetanus to the Schaffer collateral tic plasticity may be closely related to BDNF gene pathway. PPF is believed to be established when both the expression. Our experimental results provide further in- EPSP slope and amplitude in the second response is at sight in understanding endangerment of glucocorticoids on least 15% above the first one.
the hippocampus.
2.2. Determination of the expression of mRNA for
NGF-2. Materials and methods b, BDNF and NT-3 by RT-PCR
2.1. Electrophysiological recording After termination of electrophysiological experiments,
NGF-b, BDNF as well as NT-3 in the present study. RT-PCR 3. Results
analysis was conducted according to the standard
ex-perimental protocols in our laboratory [42]. Briefly, total 3.1. CORT treatment had no effect on basal RNA was firstly extracted from the dissected rat hip- hippocampal synaptic transmission
pocampal CA1 tissues by the RNAzol reagents (Life
Technologies, USA). Then, reverse-transcription was im- Single low-frequency stimulation was delivered to the plemented with a total reaction volume of 12.5 ml. Schaffer collateral pathway to evoke the field EPSP.
26
Approximately 1 mg of total RNA was reverse-tran- Incubation of hippocampal slice with CORT (10 M and
25
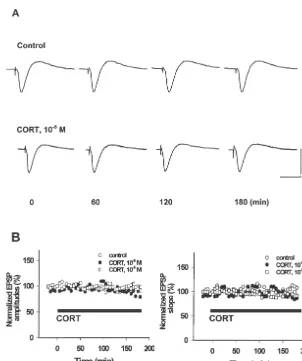
scribed into cDNA at 458C for 30 min with a mixture of 10 M) for 3 h had no effect on both the slopes and 50 mM Tris–HCl (pH 8.3), 5 mM MgCl , 5 mM DTT,2 amplitudes of evoked EPSPs recorded in rat hippocampal 50 mM KCl, 0.5 mM each dNTP (Boehringer, Mann- CA1 dendrites (Fig. 1, P.0.05).
heim), 0.25 mg of oligo(dT)12–18 primers (Pharmacia,
Piscataway, NJ) and 5 U avian myeloblastosis virus 3.2. CORT treatment inhibited the induction and (AMV) reverse transcriptase (Life Technologies). Ac- maintenance of LTP in CA1 synapses of the cording to the published sequence of cDNA encoding rat hippocampus
NGF-b, BDNF and NT-3 and with the assistance of
PCR primers design software, the primers used for PCR Before induction of LTP, we pre-screened the hippocam-amplification were designed and synthesized as follows: pal slices using the PPF approach. Only those slices NGF-b, 59-CCAAGGACGCAGCTTTCTAT-39 (sense), showing potentiated response in the second stimuli during
59-CTCCGGTGAGTCCTGTTGAA-39 (antisense); PPF were used while the hippocampal slices displaying
BDNF, 59-CTGGATGAGGACCAGAAGGT-39 (sense), paired-pulse depression (PPD) were discarded. In our
59-TATCCATAGTAAGGGCCCGA-39 (antisense); NT-3, present study, high frequency stimulation (100 Hz3100
59-TTGATCCAGGCGGATATCTT-39 (sense), pulses) was used to induce LTP in hippocampal CA1
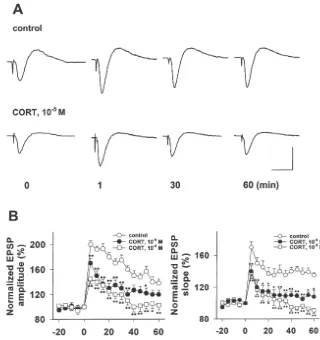
59-TGTCAATGGCTGAGGACTTG-39(antisense). Ampli- synapses. After delivery of tetanus, both the slope and fication with primers for constitutively expressed b-actin amplitude of EPSP were instantly facilitated with the served as an internal control as published elsewhere [42]. maximum increases of 70% and 90% above the baseline, PCR was performed in a total volume of 25 ml containing respectively, and this potentiation was persistent during the 1-ml aliquots of RT products, 0.5 mM of each sense and experimental period (Fig. 2), indicating that LTP was antisense primers, 125mM each dNTP, 13PCR buffer (50 induced and expressed in hippocampal slices. Though mM KCl, 1.5 mM MgCl and 10 mM Tris–HCl, pH 9.0)2 tetanus also induced great increases of both slope and and 1.25 U of Taq DNA Polymerase (Life Technologies). amplitude of EPSP evoked in CORT-treated hippocampal After determining the linear amplification ranges, the slices, this promotion cannot persist within 60 min, thermal cycle profiles used were: 132 min at 948C, 30 suggesting only short-term potentiation (STP) was gener-cycles for neurotrophin gene amplification with each cycle ated (Fig. 2). After calculation, we found that CORT
26 25
containing 1 min at 958C, 1 min at 558C, 1.5 min at 728C. incubation (10 M and 10 M) with rat hippocampal Eight-ml aliquots of the PCR products (402 bp for NGF-b, slices for 3 h significantly attenuated the tetanus-facilitated 453 bp for BDNF, 393 bp for NT-3 and 462 bp for increments of both EPSP slopes and amplitudes (Fig. 2, b-actin) were electrophoresed on a 1.9% agarose gel, P,0.05, or P,0.01). In addition, LTP in 90% (9 / 10) of stained by ethidium bromide and photographed under UV hippocampal slices were successfully induced after the light. The intensity of the PCR products generated by the pre-screening step (Table 1). After CORT treatment, only b-actin and neurotrophins were quantitated by a computer- 30% of hippocampal slices (3 / 10) developed LTP while assisted, linear scanning densitometric analysis of the 70% (7 / 10) hippocampal slices displayed short-term photograph in a reflectance mode (Kodak 120, Kodak potentiation after tetanus. After statistics, we found CORT-Digital Science, USA). A ratio of neurotrophins tob-actin treated hippocampal slices showed a great decrease of PCR product units was used to calculate the relative successful rate for generating LTP (P,0.05, or P,0.01, amount of neurotrophin mRNA expression in rat hip- Table 1) in comparison to control.
pocampal CA1 cells.
3.3. CORT treatment inhibited PPF in rat hippocampal
CA1 synapses 2.3. Statistical analysis
Paired pulses at different frequencies from 20 to 50 Hz Data are presented as the means6S.E. (standard error). were employed to induce the PPF and the ratios of EPSP Statistical significance was determined by ANOVA, fol- slope or amplitude evoked by the second stimuli to that by lowed by Duncan analysis as a post hoc test. A non- the first one were figured out. In the present study, the parametric Mann–Whitney U-test was applied alternatively ratios showed a frequency-dependent change and paired-when indicated. Differences were considered significant at pulse stimulation at 25 Hz produced the maximum ratios
26 25
Fig. 1. CORT (10 M and 10 M) treatment for 3 h had no effect on basal hippocampal synaptic transmission. Single low-frequency stimulation was delivered to rat hippocampal Schaffer collateral pathway to evoke the field EPSP. The amplitudes and slopes of EPSP were measured every 30 s before and during application of CORT to a submerged chamber (black bar, up to 3 h). Each point represents the average amplitude and slope of 10 consecutive EPSPs (5-min interval) normalized to the average of the EPSPs acquired before CORT administration in control (20 min baseline; n56). (A) Two sets of
25
traces represent the evoked EPSPs in control (upper) and CORT (10 M)-treated (lower) hippocampal slice before (t50 min) and 60, 120 and 180 min after CORT treatment. The calibration scale stands for 15 ms of horizontal bar and 2 mV of vertical bar. (B) Two figures showing the changes of both slopes and amplitudes of EPSPs recorded in rat hippocampal CA1 dentrites after perfusion with CORT-treated ACSF for 3 h. No significance (P.0.05) was observed during the experiments.
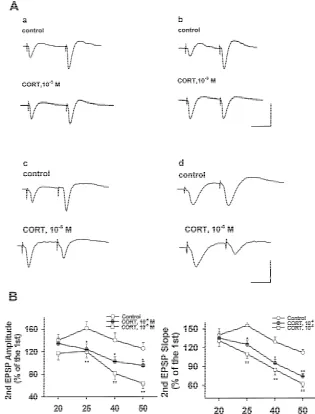
than 55% above the first response. The ratios showed a sion between control and CORT-treated hippocampal frequency-dependent decrease (Fig. 3) when interpulse slices. The ratios of neurotrophin gene expressions to those frequencies from 25 to 50 Hz were alternated. PPF was ofb-actin were obtained. It showed that mRNA expression successfully established in 80% (8 / 10) of hippocampal of neurotrophins, including NGF, BDNF as well as NT-3,
25 26
slices in control while CORT (10 M and 10 M) expressed abundantly in rat hippocampal CA1 cells (Fig.
26 25
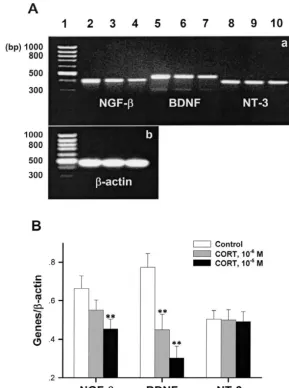
application reduced the successful rate to 20% (2 / 10) and 4). However, CORT (10 M and 10 M) incubation 10% (1 / 10), respectively, significantly smaller than those with hippocampal slice for 3 h dose-dependently
dimin-26 25
in control (Table 1). In addition, CORT (10 M and 10 ished the mRNA expression of NGF-band BDNF with the M) application for 3 h markedly decreased the ratios maximum decrease of 40 and 60%, respectively. Anyway, during PPF evoked at interpulse frequencies from 25 to 50 no effect on NT-3 mRNA expression was found in the
Hz (Fig. 3, P,0.05 or P,0.01). present study (Fig. 4, P,0.05, or P,0.01).
3.4. CORT treatment reduced the expression of mRNA 3.5. BDNF co-applied with CORT antagonized the
for NGF and BDNF in CA1 region of rat hippocampal CORT-induced reduction of EPSP slope and amplitude
slice ratios during PPF
26 25
Fig. 2. CORT (10 M and 10 M) treatment inhibited the induction and maintenance of long-term potentiation (LTP) induced by high frequency stimulation (HFS) in rat hippocampal slice. A tetanus containing 100 testing stimulations at 100 Hz was used to induce LTP. (A) Two sets of traces showing the time-course changes of EPSP slope and amplitude during the LTP generation in CA1 dentrites of rat hippocampal slice. Four traces in both
25
control and CORT (10 M)-treated slices displayed changes of EPSP slope and amplitude before (t50 min) and after tetanus (t51, 30, 60 min) delivery to the Schaffer collateral pathway. The calibration scale indicates 15 ms in horizontal bar and 2 mV in vertical bar. (B) Two graphs showing inhibition of both
26 25
induction and maintenance of LTP in hippocampal CA1 synapses after CORT (10 M and 10 M) was applied to ACSF and perfused the hippocampal slice for 3 h. The amplitudes and slopes of EPSP were measured every 30 s before and during application of CORT to a submerged chamber. Each point represents the average amplitude and slope of 10 consecutive EPSPs (5-min interval) normalized to the average of the EPSPs acquired before tetanus.
n58–9, means6S.E., *P,0.05, **P,0.01, compared with control.
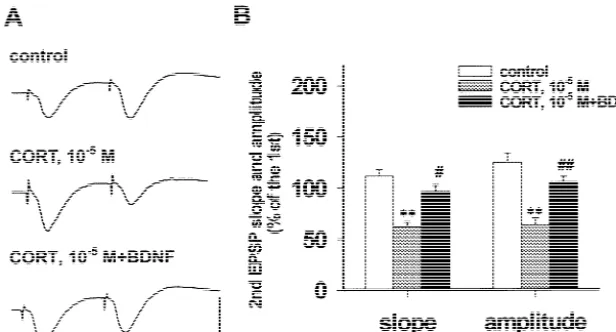
25
(10 M)-induced PPF impairment in hippocampal slices. stimuli during PPF with more than 45% decreases of ratios
25
CORT (10 M) incubation with rat hippocampal slice for of both EPSP slope and amplitude compared with those in 3 h significantly impaired the response to the second control (Fig. 5, P,0.05 or P,0.01). BDNF, at the final
Table 1
a
CORT application for 3 h reduced the generation probability of LTP and PPF in rat hippocampal CA1 synapses
CORT Hippocampal slices induced Hippocampal slices induced PPF
concentration LTP/ total hippocampal slice at 25 Hz / total hippocampal slice
(M) tested tested
0 9 / 10 8 / 10
26
10 4 / 10** 2 / 10**
25
10 4 / 10** 1 / 10**
a
26 25
Fig. 3. CORT (10 M and 10 M) incubation with rat hippocampal slice for 3 h inhibited the paired-pulse facilitation (PPF). (A) Four sets of traces showing the changes of EPSP slopes and amplitudes during generation of paired-pulse facilitation (PPF) at different interpulse intervals from 50 ms|20 ms. a. Paired-pulse facilitation was induced with interpulse interval of 50 ms. b. Paired-pulse facilitation was induced with interpulse interval of 40 ms. The calibration scale for a and b indicates 25 ms in horizontal bar and 2 mV in vertical bar. c. Paired-pulse facilitation was induced with interpulse interval of 25 ms. d. Paired-pulse facilitation was induced with interpulse inverval of 20 ms. The calibration curve for c and d indicates 10 ms in horizontal bar and 2
26 25
mV in vertical bar. (B) CORT (10 M and 10 M) treatment for 3 h significantly reduced the ratios of the second evoked EPSP slope and amplitude to the first one during PPF. n58–9, means6S.E., *P,0.05, **P,0.01, compared with that in control.
29
concentration of 10 M, applied half an hour before in vivo [7,32]. Our previous study [46] found that acute CORT addition to ACSF could antagonize these decreases application of CORT in single dose duplicated this effect, and basically rescued the PPF impairment (Fig. 5). suggesting that elevated circulating CORT level might be responsible for the deficit of LTP generation in stressed animals. Our present results showed that CORT incubation
4. Discussion with rat hippocampal slice for 3 h significantly affected the
26 25
Fig. 4. CORT (10 M and 10 M) incubation with rat hippocampal slice significantly reduced mRNA levels of NGF-band BDNF, but not NT-3 expressed in hippocampal CA1 cells. (A) Two photographs of RT-PCR products electrophoresed on 1.9% agarose gels stained with ethidium bromide. (a)
26 25
Lanes 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, correspond to DNA marker, NGF-bmRNA RT-PCR products in control, CORT-treated (10 M and 10 M) groups,
26 25
BDNF mRNA RT-PCR products in control group, CORT-treated (10 M and 10 M) groups as well as NT-3 mRNA RT-PCR products in control,
26 25
CORT-treated (10 M and 10 M) groups, respectively. (b) Lanes 1, 2, 3, 4, correspond to DNA marker,b-actin mRNA RT-PCR products in control
26 25
and CORT-treated (10 M and 10 M) groups, respectively. The lengths of NGF-b, BDNF, NT-3 andb-actin PCR products were 402, 453, 393 and 462 bp, respectively. (B) The ratios of neurotrophin mRNA expression to that of the internal control,b-actin, were calculated and changes of neurotrophin
26 25
mRNA expressed in hippocampal CA1 cells treated with CORT were observed. CORT (10 M and 10 M) incubation with rat hippocampal slices for 3 h significantly reduced the NGF-band BDNF mRNA levels expressed in hippocampal CA1 region. Means6S.E., n53, **P,0.01, compared with control.
level. It has been basically accepted that the circulating mechanism involved in neurotrophin gene expression in glucocorticoid levels tend to increase during the aging hippocampal CA1 synapses was investigated.
process in both animals and humans [18], especially when
some mental disorders, such as Alzheimer’s disease, occur 4.1. CORT application had no effect on basal [18,27,35]. So it is important to understand the effect of hippocampal synaptic transmission
CORT and its underlying mechanism on synaptic
transmis-sion when its concentration is increased. The physiological The in vivo experiment showed that exogenous applica-total CORT concentrations in rodents determined in our tion of CORT had no effect on basal synaptic transmission previous papers were around 50–300mg / l, approximately [46]. Our present results also indicated no basal synaptic
27 26
equivalent to 10 –10 M [44]. The goal of the present plasticity was affected by CORT application in vitro. Since
26
study is to determine whether CORT in high levels (10 basal synaptic transmission mainly involved in excitatory
25
25
Fig. 5. BDNF co-applied with CORT (10 M) antagonized the impaired EPSP slope and amplitude during PPF. PPF was induced by paired-pulse stimulation delivered to the Schaffer collateral pathway at an interpulse interval of 20 ms. (A) Three traces showing that BDNF application diminished the reduction of both slope and amplitude of the second EPSP referring to the first one during PPF. The calibration scale indicates 10 ms of horizontal bar and
25
2 mV of vertical bar. (B) BDNF co-applied with CORT (10 M) significantly increased the diminished slope and amplitude in the second EPSP referring
[ 25
to the first one during PPF. n57–9, means6S.E., **P,0.01, compared with control; P,0.05, compared with CORT (10 M)-treated hippocampal slice.
on either exocytosis process or AMPA / kainate receptor — PPF — to further address the possible presynaptic functions. Anyway, previous studies showed that CORT mechanism.
could enhance glutamate release in both vivo [26] and PPF is one kind of manifestation of synaptic plasticity. vitro [43] while it did not alter NMDA and non-NMDA Paired pulse stimulation reaching presynapse in a certain glutamate receptors in the hippocampus [41]. Therefore, it interval usually induces a potentiated response in the appears that CORT application to the hippocampal slice postsynaptic membrane [31]. No report so far gives should somewhat speed up the basal synaptic transmission. concerns to the effect of CORT on PPF. In our present However, the present experiment does not support previ- study, CORT incubation with rat hippocampal slice for 3 h ous results obtained by neurochemical approaches. diminished the facilitated ratios, suggesting that CORT impaired the PPF. This result provides further evidence 4.2. CORT application inhibited LTP generation in rat supporting a presynaptic mechanism underlying
CORT-hippocampal CA1 synapses induced LTP impairment in the hippocampal CA1 circuits.
Our previous study showed that acute application of 4.3. CORT-induced impairment of hippocampal synaptic CORT to rats significantly inhibited LTP in both induction plasticity is closely related to BDNF gene expression in
and maintenance phases in the hippocampus [46]. In the CA1 synapses of the hippocampus present study, CORT application to hippocampal slice also
inhibited LTP generation and induced a short-term poten- NGF, BNDF as well as NT-3 gene expression and tiation (STP) in CA1 synapses. Therefore, the inhibitory protein translation in the hippocampus were all signifi-effect of CORT in vivo may result from a direct interaction cantly inhibited by application of glucocorticoids while between CORT and the hippocampus. Interestingly, CORT exogenous supplementation with NGF and BDNF to had no effect on basal synaptic transmission whereas it hippocampal culture prevented CORT-induced hippocam-significantly inhibited LTP formation when the hippocam- pal neuron death [11,30,33], suggesting that decreased pal synaptic transmission was strengthened by tetanus, neurotrophin expression may participate in glucocorticoid-suggesting that the inhibitory effect of CORT on synaptic s-induced endangerment to the hippocampus. In the
pres-transmission is activity-dependent. ent study, CORT treatment for 3 h significantly reduced
As CORT treatment inhibited hippocampal LTP in both the gene expression of NGF and BDNF, but not NT-3 in induction and early maintenance phases in the present the hippocampal CA1 region. Anyway, only the high
25
study, so it seems that CORT may impair synaptic efficacy concentration of CORT (10 M) significantly decreased in both pre- and postsynaptic manner. However, since the the NGF mRNA expression in the hippocampus while
26
observation period in our experiment is not long enough, CORT application in the concentrations of both 10 M
25
[8] D.M. Diamond, G.M. Rose, Stress impairs LTP and
hippocampal-relationship with the threshold for LTP induction. LTP
dependent memory, Ann. NY Acad. Sci. 746 (1994) 411–414.
cannot be generated in the hippocampal slices from both
[9] A. Figurov, L. Pozzo-Miller, P. Olafsson, T. Wang, B. Lu,
Regula-neonatal mouse where the BDNF level is very low [10] tion of synaptic responses to high-frequency stimulation and LTP by and adult mouse treated with TrkB-IgG [14]. So we used neurotrophins in the hippocampus, Nature 381 (1996) 706–709.
[10] W. Gottschalk, L.D. Pozzo-Miller, A. Figurov, B. Lu, Presynaptic
adult rats for preparation of hippocampal slices in our
modulation of synaptic transmission and plasticity by brain-derived
present study and found that incubation of the hippocampal
neurotrophic factor in the developing hippocampus, J. Neurosci. 18
slices with CORT for 3 h greatly reduced the expression of (1998) 6830–6839.
mRNA for BDNF, suggesting that BDNF may mediate the [11] M. Hahn, H. Lorez, G. Fischer, Effect of calcitriol in combination with corticosterone, interleukin-1beta, and transforming growth
CORT-modified synaptic plasticity. Meanwhile, we found
factor-beta1 on nerve growth factor secretion in an astroglial cell
that BDNF co-applied with CORT to ACSF basically
line, J. Neurochem. 69 (1997) 102–109.
rescued the impairment of PPF in the hippocampus. It has [12] H. Hortnagl, M.L. Berger, L. Havelec, O. Hornykiewicz, Role of been known that BDNF modulated hippocampal synaptic glucocorticoids in the cholinergic degeneration in rat hippocampus transmission by increasing the numbers of docked vesicles induced ethylcholine aziridinum (AF64A), J. Neurosci. 13 (1993)
2939–2945.
with the presynaptic membrane [10], our study also found
[13] H. Kang, E.M. Schuman, Long-lasting neurotrophin-induced
en-that CORT affected synaptic plasticity presynaptically.
hancement of synaptic transmission in the adult hippocampus,
Taken together, BDNF may be the exclusively suitable Science 267 (1995) 1658–1662.
candidate among the three tested neurotrophins for im- [14] H. Kang, A.A. Welcher, D. Shelton, E.M. Schuman, Neurotrophins and time: different roles for TrkB signaling in hippocampal
long-plicating CORT-induced impairment of LTP. Anyway,
term potentiation, Neuron 19 (1997) 653–664.
since BDNF treatment could not completely restore the
[15] H.G. Kim, T. Wang, P. Olafssan, B. Lu, Neurotrophin 3 potentiates
impaired PPF, so it seems there should be other mecha- neuronal activity and inhibits GABA synaptic transmission in nisms underlying glucocorticoid-induced decrease of cortical neurons, Proc. Natl. Acad. Sci. USA 91 (1994) 12341–
12345.
synaptic plasticity.
[16] M. Korte, P. Carrol, E. Wolf, G. Brem, H. Thoenen, T. Bonhoeffer, Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor, Proc. Natl. Acad. Sci. USA 92 (1995) 8856–8860.
Acknowledgements
[17] P.W. Landfield, L.W. Campbell, S.Y. Hao, D.S. Kerr, Aging-related increases in voltage-sensitive, inactivating calcium channel in rat
We thank Mr. Hua-jin Dong for the technical assistance hippocampus: implications for mechanisms of brain and Alzheim-and Dr. Bai Lu for suggestions on this manuscript. The er’s disease, Ann. NY Acad. Sci. 568 (1989) 95–105.
study was supported by the National Natural Science [18] P.W. Landfield, D.K. Sundberg, M.S. Smith, J.C. Eldridge, M. Morris, Mammalian aging: theoretical implications of changes in
Foundation of China (No. 39570851) and ‘The Climbing
brain and endocrine systems during mid- and late-life, Peptides 1
Program–The National Key Projects for Fundamental
(Suppl.) (1980) 185–196.
Research of China’. [19] V. Lessmann, K. Gottmann, R. Heumann, BDNF and NT-4 / 5
enhance glutamatergic synaptic transmission in cultured hippocam-pal neurons, Neuroreport 6 (1994) 21–25.
[20] E.S. Levine, C.F. Dreyfus, I.B. Black, M.R. Plummer, Brain-derived
References neurotrophic factor rapidly enhances synaptic transmission in
hip-pocampal neurons via postsynaptic tyrosine kinase receptors, Proc. [1] M. Armanini, C. Hutchins, B. Stein, R.M. Sapolsky, Glucocorticoids Natl. Acad. Sci. USA 92 (1995) 8047–8077.
endangerment of hippocampal neurons is NMDA-receptor mediated, [21] J.C. Liou, R.S. Yang, W.M. Fu, Regulation of quantal secretion by Brain Res. 532 (1990) 7–12. neurotrophic factors at developing motoneurons in Xenopus cell [2] M. Beal, Does impairment of energy metabolisms result in excitoxic cultures, J. Physiol. (Lond.) 503 (1997) 129–139.
neuronal death in neurodegenerative illness?, Ann. Neurol. 31 [22] D.C. Lo, Neurotrophic factors and synaptic plasticity, Neuron 15
(1992) 119–130. (1995) 979–981.
[3] S.R. Bodnoff, A.G. Humphreys, J.C. Lehman, D.M. Diamond, G.M. [23] A.M. Lohof, N.Y. Ip, M.M. Poo, Potentiation of developing neuro-Rose, M.J. Meaney, Enduring effects of chronic corticosterone muscular synapses by the neurotrophins NT-3 and BDNF, Nature treatment on the spatial learning, synaptic plasticity, and hippocam- 363 (1993) 350–353.
pal neuropathology in young and mid-aged rats, J. Neurosci. 15 [24] B. Lu, A. Figurov, Role of neurotrophins in synapse development
(1995) 61–69. and plasticity, Rev. Neurosci. 8 (1997) 1–12.
[4] T. Bonhoeffer, Neurotrophins and activity-dependent development [25] B.S. McEwen, J.M. Weiss, Selective retention of corticosterone by of the neocortex, Curr. Opin. Neurobiol. 6 (1996) 119–126. limbic structures in rat brain, Nature 220 (1968) 911–912. [5] Y. Chou, W. Lin, R.M. Sapolsky, Glucocorticoids exacerbate [26] B. Moghaddam, M.L. Bolinao, B. Stein-Behrens, R.M. Sapolsky,
cyanide-induced aspartate accumulation in hippocampal cultures, Glucocorticoids mediate the stress-induced extracellular accumula-Brain Res. 654 (1994) 8–14. tion of glutamate, Brain Res. 655 (1994) 251–254.
[6] D.M. Diamond, M.C. Bennett, M. Fleshner, G.M. Rose, Inverted-U [27] J.T. O’Brien, D. Ames, I. Schweitzer, M. Mastwyk, P. Colman, relationship between the level of peripheral corticosterone and the Enhanced adrenal sensitivity to adrenocorticotrophic hormone magnitude of hippocampal primed burst potentiation, Hippocampus (ACTH) is evidence of HPA axis hyperactivity in Alzheimer’s
2 (1992) 421–430. disease, Psychol. Med. 26 (1996) 7–14.
[29] R.M. Sapolsky, Glucocorticoids neurotoxicity in the hippocampus: [38] T. Tanaka, H. Saito, N. Matsuki, Inhibition of GABA synaptic reversal by supplementation with brain fuels, J. Neurosci. 6 (1986) responses by brain-derived neurotrophic factor (BDNF) in rat
2240–2248. hippocampus, J. Neurosci. 17 (1997) 2959–2966.
[30] M.J. Schaaf, J. de Jong, E.R. de Kloet, E. Vreugdenhil, Downregula- [39] H. Thoenen, Neurotrophins and neuronal plasticity, Science 270 tion of BDNF mRNA and protein in the rat hippocampus by (1995) 593–598.
corticosterone, Brain Res. 813 (1998) 112–120. [40] T. Wang, K.W. Xie, B. Lu, Neurotrophins promote maturation of [31] D. Schmitz, T. Gloveli, R.M. Empson, U. Heinemann, Potent developing neuromuscular synapse, J. Neurosci. 15 (1995) 4796–
depression of stimulus evoked field potential responses in the medial 4805.
enthorhinal cortex by serotonin, Br. J. Pharmacol. 128 (1999) [41] Y. Watanabe, N.G. Weiland, B.S. McEwen, Effects of adrenal steroid
248–254. manipulations and repeated restraint stress on dynorphin mRNA
[32] T.J. Shors, S. Levine, R.F. Thompson, Effect of adrenalectomy and levels and excitatory amino acid receptor binding in hippocampus, demedullation on the stress-induced impairment of long-term poten- Brain Res. 680 (1995) 217–225.
tiation, Neuroendocrinology 51 (1990) 70–75. [42] X.L. Wei, Y.X. Zhang, J.H. Zhou, Differential display and cloning of [33] M.A. Smith, S. Makino, R. Kvetnansky, R.M. Post, Stress and the hippocampal gene mRNA in senescence accelerated mouse,
glucocorticoids affect the expression of brain-derived neurotrophic Neurosci. Lett. 275 (1999) 17–20.
factor and neurotrophin-3 mRNAs in the hippocampus, J. Neurosci. [43] J.Z. Zhou, Y.X. Zhang, J.H. Zhou, Neurotoxic effect of corticos-15 (1995) 1768–1777. terone on primary cultured hippocampal neurons and its relationship [34] M. Smriga, H. Saito, N. Nishiyama, Hippocampal long- and short- with excitatory amino acids, Chin. J. Pharmacol. Toxicol. 13 (1999)
term potentiation is modulated by adrenalectomy and corticosterone, 161–167.
Neuroendocrinology 64 (1996) 35–41. [44] J.Z. Zhou, Y.X. Zhang, J.H. Zhou, Increased corticosterone levels in [35] M.N. Starkman, S.S. Gebarski, S. Berent, D. Schteingart, Hippocam- both plasma and hippocampus and their relationship with hippocam-pal formation volume, memory dysfunction and cortisol levels in pal ATP depletion in senescence accelerated mouse (SAM), Chin. J. patients with Cushing’s syndrome, Biol. Psychiatry 32 (1992) 756– Pharmacol. Toxicol. 12 (1998) 12–15.
764. [45] J.Z. Zhou, Y.X. Zhang, J.H. Zhou, Neurotoxic effect of corticos-[36] B. Stein-Behrens, E. Elliott, C. Miller, J. Schilling, R. Newcombe, terone on primary cultured hippocampal neurons and its underlying
R.M. Sapolsky, Glucocorticoids exacerbate kainic acid-induced mechanisms, Chin. J. Neurosci. 14 (1998) 11–16.
extracellular accumulation EAAs in the rat hippocampus, J. Neuro- [46] J.Z. Zhou, Y.X. Zhang, J.H. Zhou, C.G. Liu, Inhibitory effect of chem. 58 (1992) 1730–1735. corticosterone on the formation of long-term potentiation in rat [37] M. Talmi, E. Carlier, B. Soumireu-Mourat, Similar effects of aging hippocampal dentate gyrus, Chin. J. Pharmacol. Toxicol. 13 (1999)