254 (2000) 143–167
www.elsevier.nl / locate / jembe
The importance of flow and settlement cues to larvae of the
abalone, Haliotis rufescens Swainson
* Anthony J. Boxshall
Department of Zoology, University of Melbourne, Parkville, Victoria 3052, Australia
Received 9 November 1999; received in revised form 28 June 2000; accepted 25 July 2000
Abstract
In flow tank experiments, I tested the relative importance of active and passive processes to larvae settling on manufactured casts that were hydrodynamically rough at a small scale (mm to
,1 cm). I predefined two distinct regions of small-scale flow that I used to manipulate larval settlement behaviour of the red abalone Haliotis rufescens Swainson. The larvae show a stringent settlement response associated with coralline red algae. Haliotis rufescens larvae settled preferentially to an inducer regardless of the flow conditions, as expected. However, the ability of H. rufescens larvae to show this stringent behaviour was altered by changing the small-scale flow. When the free-stream velocity was low, the larvae responded to a settlement cue regardless of the small-scale hydrodynamics. When free-stream velocity was higher, the larvae acted increasingly as passive particles in their deposition, but settled only in response to an inducer. The results were consistent in two flow tanks, across 2 years and between different batches of larvae. 2000 Elsevier Science B.V. All rights reserved.
Keywords: Abalone; Behaviour; Cue; Flow; Haliotis rufescens; Larvae; Settlement
1. Introduction
Increasingly, settlement by invertebrate larvae is seen as a passive delivery process to the surface (e.g. Abelson and Denny, 1997) but with an active behavioural component at the surface (e.g. Harvey and Bourget, 1997; Walters et al., 1999) and possible active components in the water column (e.g. Welch et al., 1999). As more taxa are studied, this general model is emerging but so is the understanding that there may be no
*Present address: School of Biological and Chemical Sciences, Deakin University —Rusden, 662 Blackburn Rd, Clayton, Victora 3168, Australia. Tel.: 161-3-9244-7240.
E-mail address: aboxshal@deakin.edu.au (A.J. Boxshall).
encompassing model for all larvae (Abelson and Denny, 1997) and interactions of cues must be tested (e.g. Bryan et al., 1998; Hills et al., 1998; Keough, 1998). One interaction thought to be important is the larval reaction to hydrodynamics and cues at settlement (sensu active or passive behaviour in Butman, 1987).
The larvae of many animals that live on coral and rocky reefs do not experience flows over flat substrata but encounter flows over heterogeneous surfaces. Alterations to boundary flow caused by substratum heterogeneities influence the settlement patterns of larvae (Wethey, 1986; Abelson et al., 1994; Snelgrove et al., 1999a) and larvae may encounter regions of different flow that is physically close (Harvey and Bourget, 1997, for the very small scale). Red abalone (Haliotis rufescens Swainson) larvae in this study encountered two distinct boundary flows at a scale ,1 cm. By selectively applying known settlement inducers I manipulated the behaviours of H. rufescens and studied the interaction of cues and small-scale flows on settling larvae.
1.1. Larval reactions to flow at a settlement surface
Experimental evidence suggests that larvae in near-bottom waters are essentially passive particles to within tens of centimetres of a surface (reviewed by Butman, 1987; Abelson and Denny, 1997 and see Snelgrove et al., 1993; Eckman, 1990; Harvey and Bourget, 1997). Small-scale surface topography can influence flow at the surface, and hence where, how and when larvae contact (Keen, 1987; Walters, 1992; Mullineaux and Garland, 1993; Harvey et al., 1995; Walters et al., 1997; Wright and Boxshall, 1999).
Excluding mortality at the point of initial contact with a surface, larvae can remain at the surface or return to the water column and they may, or may not, have control over either outcome. If settlement cues are hard-wired responses, a threshold dose will retain larvae at the settlement site (following Morse, 1990; Rittschof, 1993). The orientation of these larvae as they contact surfaces is important (A. Abelson, unpublished data cited in Abelson and Denny, 1997). Some larvae have control and reject the site for various reasons (e.g. an aspect of flow, Mullineaux and Butman, 1991; or lack of a cue, Walters et al., 1999). If small-scale (mm to cm) flows entrain them, larvae may have no choice but to remain (Bachelet et al., 1992; Snelgrove et al., 1993; Walters et al., 1997). There is evidence that larvae can be entrained in boundary flow (Jonsson et al., 1991) and some larvae can actively swim in the flow very close to surfaces (Grassle et al., 1992b; Pawlik and Butman, 1993; Carsen et al., 1996; Abelson, 1997; Walters et al., 1999). However, if small-scale flow is too hydrodynamically ‘harsh’ larvae may have no choice but to leave (e.g. in wave-swept environments: Denny, 1988).
Mullineaux and Garland (1993) reviewed various models for larval contact and settlement, comparing ‘passive-deposition’ and ‘passive-contact’ models. After including larval behaviours, they distinguished ‘contact and hop’ models, where larvae have a binary choice (reject or remain) and ‘contact and explore’ models, where larvae explore choices at the surface. Since then a number of authors have found evidence for these mechanisms (e.g. Harvey et al., 1995; Carsen et al., 1996; Walters et al., 1997, 1999; Snelgrove et al., 1999a,b).
Crisp, 1955; Le Tourneux and Bourget, 1988; bryozoans: Woollacott et al., 1989; coral planulae: Carlon and Olson, 1993; polychaetes: Marsden and Anderson, 1981; ascidians: Svane and Young, 1989; gastropods: Morse, 1991; opisthobranchs: Havenhand, 1991; hydrozoans: Orlov, 1997). The result is a high level of control of their movement but is only possible below critical flow values that remove the larvae from the surface (Mullineaux and Butman, 1991; Eckman and Duggins, 1998). Second, larvae may detach and tumble along close to the surface as bedload transport (modelled in Gross et al., 1992), in which case they have less control over their movement. Finally, larvae may detach and move away to re-attach later. The level of larval control over this action generally depends on boundary flow conditions (Pawlik and Butman, 1993; Snelgrove et al., 1998; for turbulent conditions: Denny, 1988; Eckman and Duggins, 1998). The conditions under which larvae can show these behaviours and the relative importance of the small-scale flow in influencing them has recently been tested (e.g. Harvey and Bourget, 1997; Hills et al., 1998; Snelgrove et al., 1999a,b; Walters et al., 1999).
1.2. Larval reactions to settlement cues
There are a number of cues known to influence larvae: chemical (reviewed in Pawlik, 1992 and see Hay, 1996), bacterial (e.g. Maki et al., 1989, 1990; Johnson et al., 1991; O’Connor and Richardson, 1998), hydrodynamic (Crisp, 1955; Keen, 1987; Mullineaux and Butman, 1991; Walters, 1992; Mullineaux and Garland, 1993; Eckman and Duggins, 1998; Welch et al., 1999) and other abiotic factors, such as tactile cues (surface contour Crisp and Barnes, 1954; Lemire and Bourget, 1996), light levels (Crisp and Ritz, 1973),
´
salinity (Vazquez and Young, 1996), pressure (Knight-Jones and Morgan, 1966), a combination of factors (Maldonado and Young, 1996) or even a process (e.g. disturbance; Woodin et al., 1995).
Some cues produce all-or-nothing effects (abalone larvae: Morse and Morse, 1984a,b) whereas some cues show high levels of behavioural variation (barnacle larvae: Rittschof et al., 1984). There has not been a recent extensive review covering the induction of larval behaviour at settlement (see Meadows and Campbell, 1972, but see Young, 1995 for a good background and Hay, 1996 for future directions). With the changes this decade in our view of inductive behaviour (see Morse, 1990; Rittschof, 1993; Clare et al., 1994; Keough and Raimondi, 1995; Bryan et al., 1997, 1998; Matsumura et al., 1998), such a review is overdue.
Hydrodynamic cues have not been studied a great deal as they are hard to uncouple from other cues. Mullineaux and Garland (1993) discussed the null hypotheses necessary to test for a true hydrodynamic cue (the first paragraph in this section lists all the seven studies that show evidence for true hydrodynamic cues). There is a short recent review of the effects of other abiotic cues on larvae (Young, 1995) but it does not cover settlement and there is no recent review of bacterial cues and biofilms (but see Keough and Raimondi, 1995, O’Connor and Richardson, 1996, Unabia and Hadfield, 1999 for recent discussions of the effects of biofilms on settlement).
Strathmann, 1989) or even potential food sources (e.g. Morse and Morse, 1984a) and many are secondary metabolites (Hay, 1996). Some chemical cues can be inhibitors, rather than inducers (e.g. algae: De Nys et al., 1995; Walters et al., 1996; ascidians: Davis, 1991; sponges: Young and Chia, 1981, Bingham and Young, 1991, Davis et al., 1991). The evidence for chemical cues to settlement is across invertebrate phyla (barnacles: Knight-Jones and Stevenson, 1950; polychaetes: Knight-Jones, 1951; bryozoans: Brancato and Woollacott, 1982; ascidians: Davis and Wright, 1990; corals: Morse and Morse, 1991; molluscs: Zimmer-Faust and Tamburri, 1994). Few chemical cues have actually been identified and isolated, let alone synthesized (Pawlik, 1992), however in a few cases the actual pathways of chemical action are being studied (e.g. Yamamoto et al., 1998; Hay, 1996; Matsumura et al., 1998).
Despite the lack of knowledge about the specifics of individual cues, it is clear that cues may not operate in isolation from each other. There are synergies of cues (see Dineen and Hines, 1994, Neal and Yule, 1994; Maldonado and Young, 1996; Neal et al., 1996; Keough, 1998; Wright and Boxshall, 1999; Walters et al., 1999). Note that the designation of cues into areas such as ‘bacterial’ or ‘chemical’etc. should be considered semantic only as the release of a chemical may be the active component of a bacterial cue, or the small-scale flow may determine salinity differences. Identification of specific cues and their mechanics are two important areas for future work.
1.3. The interaction of flow and cues in larval settlement
Most work on the relationship between flow and cues in settlement is done in soft-sediment environments with chemical cues to settlement that are contact-mediated (Capitella sp. I and Mercenaria mercenaria in a series: Butman et al., 1988; Bachelet et al., 1992; Grassle et al., 1992; Butman and Grassle, 1992; and more recently for different taxa: Snelgrove et al., 1998; Snelgrove et al., 1999a,b). Snelgrove et al. (1993) directly tested for an interaction between the influence of small-scale flow and larval reactions to a settlement cue in soft-sediment for Capitella sp. I and M. mercenaria. The results suggested that although the larvae of both species actively choose the cue over the control, the patterns were altered by passive entrainment of larvae into depressions regardless of cue availability.
Somewhat surprisingly, few authors have directly tested for an interaction between the influence of small-scale flow and larval reactions to a settlement cue for animals that live on hard substrata. A number of authors suggest this interaction could be important (Crisp, 1955; Wethey, 1986; Mullineaux and Garland, 1993; Minchinton and Scheibling, 1993). Recent field studies tested for an interaction between flow and cues at a scale measured in tens of centimetres (Miron et al., 1996) or smaller (Walters et al., 1997; Wright and Boxshall, 1999). At the smaller scale, settlement has been compared with patterns of initial contact of inert PVC particles and authors have inferred settlement as a result of larval behaviour after contact. Using inert particles as a predictor of delivery rates, and hence settlement, offers a useful way to infer larval behaviour (e.g. Wethey,
´
1986; Walters, 1992; Gregoire et al., 1996; Harvey and Bourget, 1997).
larvae (Phragmatopoma lapidosa californica: Pawlik et al., 1991; Pawlik and Butman, 1993; Hydroides elegans: Walters et al., 1997; Spicsula solodossima: Snelgrove et al., 1998; Bugula neritina and Balanus amphitrite: Walters et al., 1999). In an exciting departure from the focus on contact-mediated cues, Turner et al. (1994) provided good evidence that the reactions of the oyster Crassostrea virginica to a water-borne cue is modified by flow. Although the design of their experimental array was spatially confounded, they showed conclusively that C. virginica larvae can respond to a water-borne cue in flow. The study of water-borne cue in larval settlement is proving to be an interesting and expanding field of study (see Zimmer-Faust and Tamburri, 1994; Walters et al., 1996; Diaz et al. 1999).
1.4. Present study
This study explores the relative importance of small-scale hydrodynamics and chemical settlement cues on settlement in laboratory flow tanks at a scale of mm to cm. As surface roughness can affect larval settlement, and because natural substrata are heterogeneous, I chose to use physically rough surfaces on which to test this interaction. Using physically rough surfaces in flumes has draw-backs because the hydrodynamics on such surfaces are hard to characterize (see Nowell and Jumars, 1984, 1987; Muschenheim et al., 1986; Vogel, 1994), especially in turbulent flows (Denny, 1988). To circumvent these problems, I devised a method that empirically defined two distinct regions of flow (one higher velocity, one lower velocity) on a very rough substratum onto which I placed known settlement cues. The high velocity areas have higher surface contact of passing particles but low water retention while the reverse occurs in low velocity areas. By selectively applying a settlement cue (or a control) to the two regions of different small-scale flow, I could directly test whether larvae are active or passive on the small scale (mm to cm) by making a priori predictions about settlement.
As a test of this interaction, I used the red abalone Haliotis rufescens. The settlement behaviour of this species has been studied extensively and they possess a well-known suite of behaviours (Morse and Morse, 1984a; Barlow, 1990). They react stringently to a naturally occurring settlement cue associated with crustose coralline algal species (Morse and Morse, 1984a). The peptide basis of this cue has been isolated but not yet synthesised. It is a mimetic of a common neuro-transmitter, g-amino butyric acid (GABA) and hence GABA can be used to elicit settlement responses (Morse, 1990; Barlow, 1990; but see Johnson et al., 1991; Slattery, 1992; Daume et al. 1999) There are existing techniques to spawn these abalone as well as to maintain large larval cultures (Morse et al., 1979; Hooker and Morse, 1985).
H. rufescens larvae, at approximately 350 mm, are not fast swimmers (max. ,3
21
cm s : personal observations). In field studies, abalone of a different species (H. rubra)
21
proportion of actual and predicted settlement should be the same. Access to a cue will be different in areas of high and low velocity and will change with increasing free-stream velocity.
2. Materials and methods
2.1. Settlement surfaces
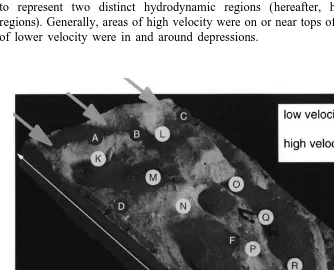
Each experimental settlement surface (Fig. 1) was a replicate cast taken from an original that was not a mould of an actual natural surface, but one that allowed me to create a flow structure within the range of flows experienced by H. rufescens larvae. To promote a fully turbulent boundary layer over the casts, they were not used flush with the channel floor. Each surface has 20 target points at which I counted settlers (Fig. 1).
2
These points were approximately 0.36 cm in area (i.e. 0.630.6 cm), and were chosen to represent two distinct hydrodynamic regions (hereafter, high- or low-velocity regions). Generally, areas of high velocity were on or near tops of projections and areas of lower velocity were in and around depressions.
By adding abalone settlement cue to the 20 points, each cast has four treatments with five replicate points: high velocity region with a settlement cue, low velocity region with a settlement cue, high velocity region without a settlement cue, low velocity region without a settlement cue. I alternated the treatment (with or without a settlement cue) between replicate runs for each group of five points and used a new, identical cast for each replicate run. The casts were made from polyester / epoxy resin mixed with a talc filler (K&H Kahfil Plastic Putty, K&H Pty. Ltd., Dandenong, Australia) and were dyed grey (‘dark grey’ pigment, RF Services, North Melbourne, Australia) to ease the counting of settlers.
The experimental design spreads any effects from toxic residues on the casts evenly across all treatments. As a precaution, I soaked all casts in freshwater for at least 2 days changing the water daily. After, I rinsed and soaked them in seawater for a minimum of 2 days, initially changing the water daily, but less frequently after 1 week of submersion. I rinsed all casts in freshwater and air dried them before applying cues. Pilot studies of larval settlement behaviour in both the laboratory and field showed no impact of residues after this treatment (unpublished data).
2.2. Flow tanks and flow conditions
I used two different flow tanks modified from the design for a recirculating laboratory flow tank by Vogel and LaBarbera (1978). Both were small which meant there was no way of avoiding secondary circulation effects due to the side walls (Nowell and Jumars, 1987). The larger flow tank used an impellor and had a channel 15 cm wide, 1 m long with a total volume of 35 l at a water depth of 14 cm. The start of the cast was 45 cm from the end of the baffle. Both flow tanks had baffles that complied with the suggested length:width ratios (Nowell and Jumars, 1987), although the impact was negligible. Calculations of the roughness Reynolds number (Re : Denny, 1988) showed the*
boundary layer above all casts was at least partially, and in most cases fully, turbulent and not at equilibrium on any cast.
The smaller flow tank was portable, powered by an in-line bilge pump (Rule 25D) and had a channel 10 cm wide, 1 m long with a total volume of 16 l at a water depth of 12 cm. The start of the cast was 50 cm from the end of the baffle. The pump conditions in the small flow tank were not ideal for larvae as flow was forced into a narrow pipe to pass an impellor. I expected increased larval mortality, although the actual level was difficult to gauge. In other experiments, coral larvae that survived the flow-tank conditions exhibited normal settlement behaviour (Boxshall, unpublished data). This simplified design resulted in bad entrance and exit conditions (Nowell and Jumars, 1987) and in both flow tanks exit conditions were almost certainly reflected upstream. The narrow channel widths and the change from circular pipes to rectangular channels made secondary circulation due to the side walls an integral part of flow over the casts. Due to the non-equilibrium turbulent flow conditions and the influence of secondary circulation in the flow tanks, measurements of the actual flow conditions are unhelpful. I measured the free-stream velocity (U ) in the large flow tank by timing dye streams. To`
based on the designs of Vogel (1981) to take ten replicate measurements from mid-column at 7 cm depth, upstream from the start of the cast.
2.3. Assigning regions of flow to the casts
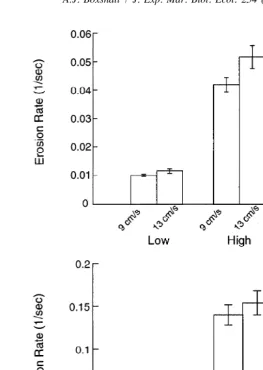
Due to secondary flow characteristics, the designation of points on the casts was specific for each flow tank. The ten high-velocity points and ten low-velocity points were taken from a larger number of points (83 points in the large flow tank, 110 points in the small flow tank). To assign flow regions, I measured the erosion of dye on the surface of test cast (sensu Wethey, 1986) and ranked points on the surface by their water residence times (four replicate casts in the large flow tank and three in the small flow tank). This method assumes dye erosion relates to hydrodynamic parameters important to larvae as they settle, however note the vertical component of flow will be more important to the retention of contacting larvae than it will be to dye. Although the mean erosion rates generally increased (i.e. water retention decreased) as the free-stream velocity increased, the difference between high and low velocity regions remained large (Fig. 2).
2.4. Specific experimental methods
Flow tank experiments were done at the Marine Biotechnology Center at the University of California, Santa Barbara (UCSB) in constant temperature rooms (15– 168C) with continual overhead lighting on two occasions: May and June, 1994 in the large flow tank, October 1995 in both flow tanks. Parallel to each flow-tank run were still-water control experiments in which I noted differences in larval responses between batches or experiments.
The 1994 experiments tested larval reactions at two free-stream velocities in the large
21
flow tank: 9 and 13 cm s . There were four replicate casts and a different batch of larvae tested at each velocity. In 1995, I tested larval reactions at one free-stream
21
velocity (11 cm s ) in the large flow tank with four replicate casts and a single batch of
21
larvae. In the small flow tank, I used two free-stream velocities (3.5 and 5 cm s ) with four replicate casts. I used larvae from one batch for both free-stream velocities in the small flow tank. To control for any effect due to the different ages of larvae between the two free-stream velocities, I alternated the velocities between replicate runs.
Fig. 2. Mean (6S.E.) dye erosion in the high and low velocity regions in the large flow tank (S.E. from four replicate plates) and small flow tank (S.E. from three replicate plates).
the force of a jet of water from a pipette is required), however some deposited particles may have shifted.
In 1994, still-water controls ran for 7 h. I used six-well tissue culture trays (Corning, 15 ml volume) with between ten and 224 larvae in each well. Each control test was a Latin-rectangle design (Mead, 1988) with three replicates of each of two treatments: 7
26
ml of seawater containing GABA at 10 M or 7 ml of unfiltered seawater. In 1995, I continued some still-water controls for up to 60 h. I used 20 ml disposable plastic beakers (Fisher) containing 10 ml of unfiltered seawater and between four and 62 larvae
2
coralline alga (Lithothamnium californicum) as the control. This alga was raised as a monoculture at UCSB along with tanks of dead and sun-bleached alga of the same species. The dead, non-inductive alga was kept underwater and developed bacterial films, but was air-dried before use as the control surface. Pilot experiments indicated no difference between settlement with or without a control chip in unfiltered seawater (unpublished data).
2.5. Larvae and settlement cue
Haliotis rufescens larvae were raised according to the method in Hooker and Morse
(1985), which uses two males and two females for each spawning and larvae are competent to settle 7 days after hatching (Morse and Morse, 1984a). In 1994, larvae ranged from day 1 competency (i.e. 7 days after hatching) to day 5 competency (i.e. 12 days since hatching) and between 20 000 and 35 000 larvae were used per run. In 1995, I used larvae from day 1 competency to day 3 competency for both experiments. I used between 31 000 and 45 000 larvae per run in the large flow tank and between 6000 and 11 000 larvae per run in the small flow tank. As an inducer, I used small (0.630.6 cm) chips of coralline algae.
2.6. Analysis of behavioural data
I counted both the settlers and deposited particles at the end of each flow tank run. Deposited particles included empty larval shells, dead veligers and inactive larvae; all of which I used as larval mimics. All larval mimics were deposited passively and did not react to a cue (see Results). I used these larval mimics to quantify accumulation rates of larvae in flow regions and, hence, potential access to a settlement cue. Short of actually measuring larval deposition or contact rates (by video: Mullineaux and Butman, 1991; using vacuum grease to trap particles: Walters, 1992), it is important to estimate contact or deposition rates by using passive particles of the same settling velocity as the larvae being studied (Wethey, 1986; Grassle et al., 1992; Pawlik and Butman, 1993). Using H.
rufescens larval mimics not only maintained the size and settling velocity but the same
shape (Pennington and Strathmann, 1990) as the settling larvae.
On the issue of the independence of larval contacts, in each run I used many thousands of larvae. Each larva passed by each point many times over the duration of the experiments. It is plausible that a given larva could contact the same point more than once, however each contact is independent of each other. The contact made by one larva to the surface could not affect the contact made by any of the other thousands of larvae in the same run.
I estimated the contact rate of larvae on the cast surface by using the mean dye erosion from high- and low-velocity regions as a proxy (Fig. 2). It is not a measure of actual larval contacts (Walters, 1992) and will tend to overestimate larval retention but should do so in both flow regions. I calculated a predicted settlement from the proportional erosion in the two flow regions and compared it to the proportion of actual settlement in both regions.
at each target point and standardized the counts to a density based on the total settlement or deposition divided by the total area. The total area comprised the planar, two-dimensional surface area of the chip plus the rest of the area that constituted the targeted point predefined by flow. Although the target point was a standard size the chips could not be cut to the same size.
I log-transformed the data [Log (densitye 11)] to improve normality and stabilize
21
variances. The analysis of data at 9 cm s did not need transformation but for consistency I transformed the data (Sokal and Rohlf, 1981). The factors were: Replicate Run (‘Run’; random; four replicate casts per free-stream velocity), Flow Region (‘Flow’: high, low; fixed), Inducer Type (‘Inducer’: cue, control; fixed; very little settlement to points without an inducer allowed analysis of settlement only on points with an inducer and so at times this factor was not used in analyses), Free-stream Velocity (‘Velocity’: 2 levels; fixed factor; only for analyses of data from the small flow tank). In the large flow tank, I analysed each free-stream velocity separately, with a two-factor, mixed-model ANOVA (Run, Flow). For the analysis of experiments in the small flow tank I used a partially nested ANOVA, with run nested within velocity and crossed with flow. To test whether the larval mimics were truly inactive, I used a three-way, mixed-model ANOVA (Run, Flow, Inducer) for all analyses at all velocities in both flow tanks.
3. Results
In still-water controls, larvae almost always settled only in the presence of a cue, whether the cue was GABA or chips of coralline algae. The rate of settlement was faster in GABA (Table 1). There were no differences between batches.
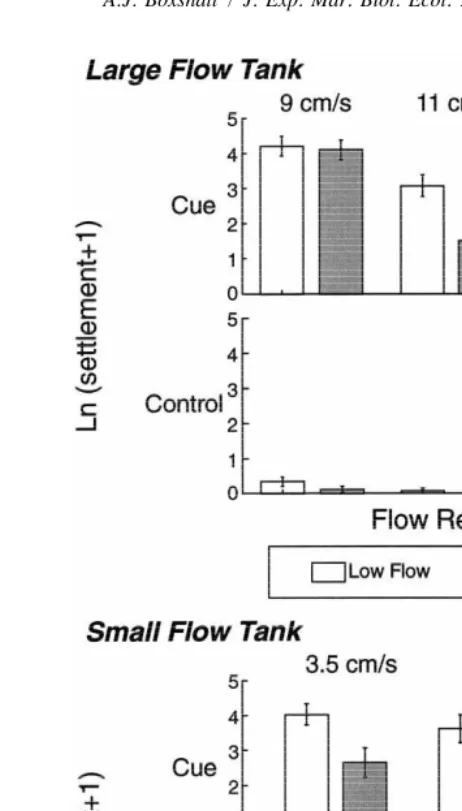
In flow tank experiments, larvae settled almost exclusively into points with an inducer
21
(Fig. 3). In all experiments, except at 9 cm s in the large flow tank, significantly less
Table 1 Parallel to 11 cm s or both runs in the small flow tank*
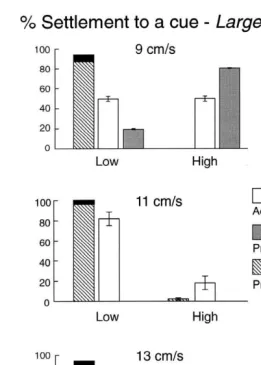
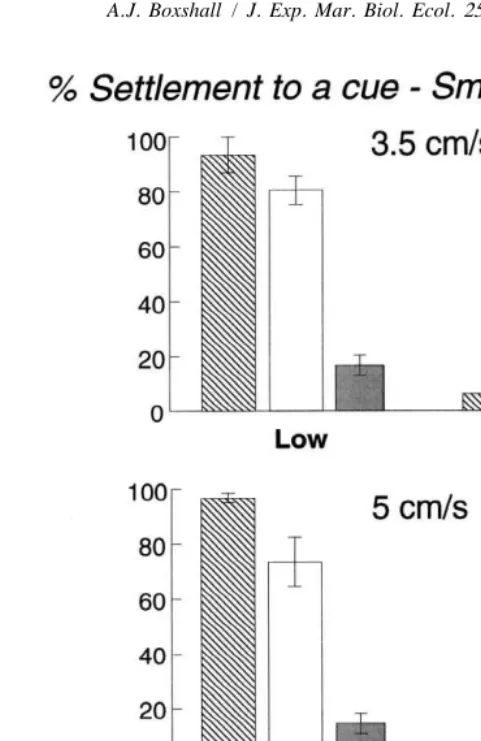
|6 h* (n522,24) 39620 0
Fig. 3. The mean Ln(settlement11) (6S.E. from four replicate casts) of abalone larvae onto cued and control surfaces in high- and low-velocity regions from all flow tank experiments.
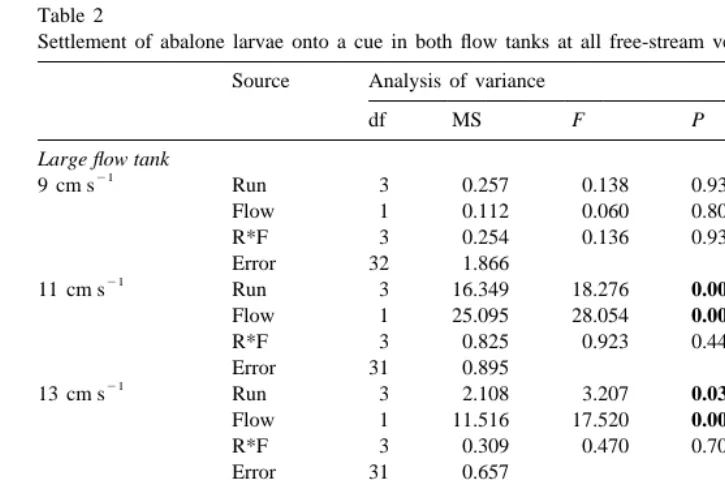
21
larvae settled in high-velocity regions (Table 2). At 9 cm s the larvae settled regardless of small-scale flow conditions. By increasing the free-stream velocity to 11 or
21
13 cm s , there was a large decrease in the numbers settling into the high velocity regions. In some experiments, the effect of ‘Run’, indicates differing amounts of larvae were used, and therefore settled, between experimental runs.
Table 2
a
Settlement of abalone larvae onto a cue in both flow tanks at all free-stream velocities Source Analysis of variance
Both U` Velocity 1 0.657 0.397 0.531
Flow 1 32.952 28.055 0.002
Settlement onto control surfaces were not included in these analyses, as larvae settled almost exclusively to an inducer (see Fig. 3). Significant results are in bold. P50.000 where P,0.0005.
Inducer, nor three-factor interactions nor interactions with Flow. In most cases, the probabilities associated with these effects were .0.5, so I am very confident these
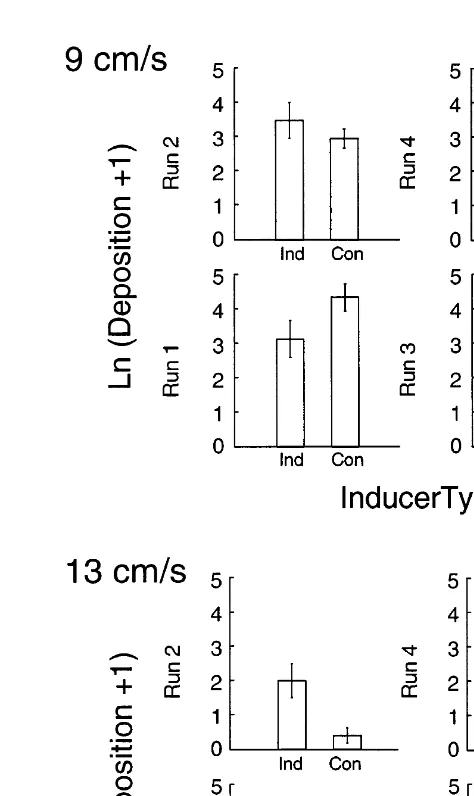
21
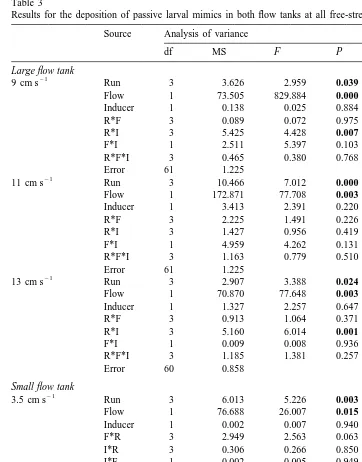
effects did not occur (Table 3). At 9 and 13 cm s in the large flow tank there was an interaction between Run and Inducer (Table 3, Fig. 4). This is an artefact of my experimental design. I alternated the points on to which I placed the inducer or control to avoid accidental random assignment of points into blocks influenced by secondary flow. Runs 1 and 3 and 2 and 4 used the same block of points. The result does not indicate a preference for or against the inducer by deposited particles.
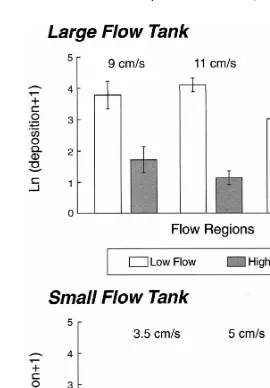
The dominant effect was increased deposition of mimics into points in low-velocity regions, regardless of free-stream velocity (Table 3, Fig. 5). Between 88 and 98% of all mimics were deposited in low-velocity regions in the large flow tank. Hence, the full range of deposition of mimics into the high-velocity regions was between 2 and 12% in
¯
the larger flow tank. In the small flow tank, between 77 and 100% (x6S.E.59465%)
21
of mimics were deposited in the low velocity regions at 3.5 cm s and between 94 and
21 ¯
100% (x6S.E.59762%) at 5 cm s , again showing little deposition in the high velocity regions.
Neither the estimated contact rate of larvae, using dye-erosion as a proxy, nor the patterns of deposition by mimics predicted settlement in either flow tank (Figs. 6 and 7).
21
At 9 and 13 cm s , the proportion of settlement predicted by estimated larval contact
21
Table 3
a
Results for the deposition of passive larval mimics in both flow tanks at all free-stream velocities Source Analysis of variance
21
Fig. 4. The Ln(deposition11) (6S.E. from four replicate casts) of larval mimics at 9 and 13 cm s in the large flow tank illustrating a significant Inducer by Run interaction.
21
flow region whereas at 13 cm s the proportion of settlement increased in low-velocity regions, which was the opposite pattern to that predicted by estimated larval contact.
21
There was no measurement at 11 cm s .
21
The settlement of real larvae at 9 cm s was as different from patterns predicted by contact as it was from patterns predicted by deposition of larval mimics. In the large
21
Fig. 5. The Ln(deposition11) (6S.E. from four replicate casts) of larval mimics into high- and low-velocity regions at all free-stream velocities in both flow tanks.
tank (Fig. 7). Real larvae always settled on a cue in the high-velocity regions far more than mimics were deposited and retained. The difference between real larvae and mimics decreased with higher free-stream velocity and in the small flow tank. In the small flow
21
Fig. 6. The % settlement into high- and low-velocity regions predicted by deposition and estimates of larval contact versus the actual % settlement of abalone larvae. These data are for settlement onto an inducer in the large flow tank. The error bars are S.E. of the mean from four replicate runs. Note the range (not S.E.) is displayed in the low velocity regions for settlement predicted by deposition. The % data are only presented for visual ease (see text for actual analyses).
4. Discussion
Fig. 7. The % settlement into high- and low-velocity regions predicted by deposition and estimates of larval contact versus the actual % settlement of abalone larvae. These data are for settlement onto an inducer in the small flow tank. The error bars are S.E. of the mean from four replicate runs. The % data are only presented for visual ease (see text for actual analyses).
influence the behaviour of Haliotis rufescens larvae at the scale of settling larvae. By defining two distinct, small-scale flow regimes to make a priori predictions of larval settlement, I found that H. rufescens larvae were influenced by both small-scale flow and chemical cues. Larvae were more likely to settle in response to chemical cues where small-scale flows were less harsh. However, in harsher small-scale flows, patterns of settlement began to resemble the deposition of larval mimics. Only when the overall
21
Following Butman (1987), the larvae were active at all velocities tested as they responded to and settled on an inducer. However as the small-scale flow changed, usually with the free-stream velocity, the settlement patterns indicated larvae behaved more as a passive particle in the flow. Given the deposition and retention patterns of the larval mimics, the diminished settlement in high-velocity regions was most likely due to the inability of H. rufescens larvae to maintain access to an inducer. Likewise, the increased settlement in regions of lower velocity was most likely a result of increased deposition, retention and, hence, access to an inducer. Although untested in this experiment, there is no evidence that H. rufescens larvae actively cue to a component of flow (unlike barnacles: Crisp, 1955, Mullineaux and Garland, 1993).
The fact that actual settlement was different from both the estimates of larval contact and deposition indicated H. rufescens larvae ‘behaved’ on the surface (see also Pawlik and Butman, 1993; Harvey and Bourget, 1997; Snelgrove et al., 1999b). There should be a point where flow conditions allow no influence of the behaviour of larvae; an absolute threshold flow above which larvae can not settle (sensu Eckman, 1990; Eckman et al., 1990; Pawlik and Butman, 1993). The flow regions I defined were below such a threshold.
As the velocities increased, why did the larvae become increasingly passive particles? Larvae were either not induced or unable to settle. Assuming that all larvae were equally competent, there are two possible mechanisms. First, the larvae were passive and therefore removed from regions of higher velocity and accumulated in regions of lower velocity. Second, it’s possible they actively avoided regions of higher velocity or cued to an aspect of the flow in low-velocity regions. I did not directly test, and hence falsify, if
H. rufescens larvae actively cue to certain aspects of flow (see Mullineaux and Butman, 1991; Mullineaux and Garland, 1993). If larvae are passive and are removed from regions of high velocity, the most likely mechanism is a mixture of flow and physiology. The larvae may not have the time needed to receive a dose of inducer (e.g. Morse, 1990; Rittschof, 1993) or they may have landed on the surface in the wrong orientation (A. Abelson cited in Abelson and Denny, 1997). If this mechanism is correct, then larvae will continue to be removed and contact the surface until conditions allow them to settle. Although the question of a cue to flow by H. rufescens larvae was untested, the most likely scenario in regions of low velocity is that larvae passively accumulated and once there were induced to settle (see also Snelgrove et al., 1993; Walters et al., 1997). In these areas of higher water retention, the larvae should have enough time (and / or swimming ability) to gain the correct orientation and access the necessary inducer.
The overall settlement pattern was consistent with the ‘contact and hop’ model of Mullineaux and Garland (1993) where any question of active choice was exercised at the surface with an inducer. Larvae will settle when they have the ability to commit to a settlement site and are induced to metamorphose. Surface behaviour may be expressed as larval ability to attach and / or detach from the substratum. Settlement will occur if attachment is physically possible and an inducer present.
larvae were being induced to metamorphose by contact at a target point with an inducer and then moving off to find another suitable site, larvae would be expected to settle all over the cast. This did not happen. A series of pilot studies with video observations and small, silicone grease fences to trap crawling larvae also failed to detect any movement between the predefined flow points (unpublished data). Differential post-settlement mortality also can not explain the results as I measured settlement (Keough and Downes, 1982). This outcome was achieved by having short duration tests, a localisation of settlers into target points where they were easily visible and the lack of severe post-settlement processes (e.g. predation).
It was never the intention of this study to test which components of flow were important to larvae (Butman, 1987; Mullineaux and Garland, 1993; Abelson et al., 1994; Eckman et al., 1994 are examples which do). The free-stream velocities, but more importantly estimates of the turbulence intensities and shear velocities, were within the range of natural flows experienced by H. rufescens larvae (based on information from Hooker and Morse, 1985; Raimondi and Schmitt, 1992). I did not simulate a naturally occurring flow and the free-stream velocities that I used in these experiments do not translate directly to the ocean. As many authors have noted (e.g. Gambi et al., 1990; Mullineaux and Garland, 1993, but especially Nowell and Jumars, 1984, 1987), there are many important parameters of flow that must be replicated or measured for a laboratory flow to simulate a natural flow. I only intended to create regions where the flow ranked, on average, differently and presented settling H. rufescens larvae with two defined and repeatable flow conditions. Within these two regions I followed the behaviour of larvae reacting to settlement cues. Any study aiming to determine the actual flow parameters, or their magnitude, that are important settling larvae should never follow my protocol. Characteristics of larvae life-cycles may interact with behaviour or flow during settlement. For example, larvae with short planktonic periods may never leave the influence of boundary flow. For such larvae, an interaction between small-scale flow and their behavioural reactions to a surface cue will dominate their dispersal, far more than for a larva that disperses through the plankton for weeks or months. From an ecological perspective, the next step is to ask whether the differential settlement due to behavioural reactions and small-scale flow translates into differences in recruitment on a small-scale (sensu Walters and Wethey, 1996). If not, then such fine-scale biases by larvae, although interesting in a mechanistic sense, are without much ecological relevance.
Acknowledgements
comments on drafts. Final thanks to Sonya Mulholland. This paper is dedicated to the memory of Margaret Lohan. [AU]
References
Abelson, A., Weihs, D., Loya, Y., 1994. Hydrodynamic impediments to settlement of marine propagules, and adhesive-filament solutions. Limnol. Oceanogr. 39, 164–169.
Abelson, A., 1997. Settlement in flow — upstream exploration of substrata by weakly swimming larvae. Ecology 78 (1), 160–166.
Abelson, A., Denny, M., 1997. Settlement of marine organisms in flow. Annu. Rev. Ecol. Syst. 28, 317–339. Bachelet, G., Butman, C.A., Webb, C.M., Starczak, V.R., Snelgrove, P.V.R., 1992. Non-selective settlement of Mercenaria mercenaria (L.) larvae in short-term, still-water, laboratory experiments. J. Exp. Mar. Biol. Ecol. 161, 241–280.
Brancato, M.S., Woollacott, R.M., 1982. Effect of microbial films on settlement of bryozoan larvae (Bugula simplex, B. stolonifera and B. turrita). Mar. Biol. 71, 51–56.
Bryan, P.J., Qian, P.-Y., Kreider, J.L., Chia, F.-S., 1997. Induction of larval settlement and metamorphosis by pharmacological and conspecific associated compounds in the serpulid polychaete Hydroides elegans. Mar. Ecol. Prog. Ser. 146 (1-3), 81–90.
Bryan, P.J., Kreider, J.L., Qian, P.-Y., 1998. Settlement of the serpulid polychaete Hydroides elegans (Haswell) on the arborescent bryozoan Bugula neritina (L.): evidence of a chemically mediated relationship. J. Exp. Mar. Biol. Ecol. 220 (2), 171–190.
Butman, C.A., 1987. Larval settlement of soft sediment invertebrates: The spatial scales of pattern explained by active habitat selection and the emerging role of hydrodynamical processes. Oceanogr. Mar. Biol. Annu. Rev. 25, 113–165.
Butman, C.A., Grassle, J.P., Webb, C.M., 1988. Substrate choices made by marine larvae settling in still water and in a flume flow. Nature 333, 771–773.
Carlon, D.B., Olson, R.R., 1993. Larval dispersal distance as an explanation for adult spatial pattern in two Caribbean reef corals. J. Exp. Mar. Biol. Ecol. 173, 247–263.
Carsen, A.E., Hatcher, B.G., Scheibling, R.E., 1996. Effect of flow velocity and body size on swimming trajectories of sea scallops, Placopecten magellanicus (Gemlin): A comparison of laboratory and field measurements. J. Exp. Mar. Biol. Ecol. 203 (2), 223–243.
Clare, A.S., Freet, R.K., McClary, M.J., 1994. On the antennular secretion of the cyprid of Balanus amphitrite amphitrite, and its role as a settlement pheromone. J. Mar. Biol. Assoc. UK 74, 243–250.
Crisp, D.J., 1955. The behaviour of barnacle cyprids in relation to water movement over a surface. J. Exp. Biol. 32, 569–590.
Crisp, D.J., Barnes, H., 1954. The orientation and distribution of barnacles at settlement with particular reference to surface contour. J. Anim. Ecol. 23, 142–162.
Crisp, D.J., Ritz, D.A., 1973. Responses of cirrepede larvae to light. I. Experiments with white light. Mar. Biol. 23, 327–335.
Davis, A.R., 1991. Alkaloids and ascidian chemical defense: evidence for the ecological role of natural products from Eudistoma olivaceum. Mar. Biol. 111, 375–379.
Davis, A.R., Butler, A.J., van Altena, I., 1991. Settlement behaviour of ascidian larvae: preliminary evidence for inhibition by sponge allelochemicals. Mar. Ecol. Prog. Ser. 72, 117–123.
Denny, M., 1988. Biology and the Mechanics of the Wave-swept Environment, 1st Edition. Princeton University Press, Princeton, NJ.
De Nys, R., Steinberg, P.D., Willemsen, P., Dworjanyn, S.A., Gabelish, C.L., King, R.J., 1995. Broad spectrum effects of secondary metabolites from the red alga Delisea pulchra in antifouling assays. Biofouling 8, 259–271.
Dineen, J.F.J., Hines, A.H., 1994. Larval settlement of the polyhaline barnacle Balanus eburneus (Gould): cue interactions and comparisons with two estuarine congeners. J. Exp. Mar. Biol. Ecol. 179, 223–234. Daume, S., Brand-Gardner, S., Woelkerling, W.J., 1999. Settlement of abalone larvae (Haliotis laevigata
Donovan) in response to non-geniculate coralline red algae (Corallinales, Rhodophyta). J. Exp. Mar. Biol. Ecol. 234 (1), 125–143.
Eckman, J.E., 1990. A model of passive settlement by planktonic larvae onto bottoms of differing roughness. Limnol. Oceanogr. 35, 887–901.
Eckman, J.E., Duggins, D.O., 1998. Larval settlement in turbulent pipe flows. J. Mar. Res. 56 (6), 1285–1312. Eckman, J.E., Savidge, W.B., Gross, T.F., 1990. Relationship between duration of cyprid attachment and drag
forces associated with detachment of Balanus amphitrite cyprids. Mar. Biol. 107, 111–118.
Gambi, M.C., Nowell, A.R.M., Jumars, P.A., 1990. Flume observations on flow dynamics in Zostera marina (eelgrass) beds. Mar. Ecol. Prog. Ser. 61, 159–169.
Grassle, J.P., Snelgrove, P.V.R., Butman, C.A., 1992. Larval habitat choice in still water and flume flows by the opportunistic bivalve Mulinia lateralis. Neth. J. Sea Res. 30, 33–44.
´
Gregoire, Y., Bourget, E., Verrette, J.-L., 1996. Deposition of mimics of planktonic invertebrate larvae on simple and complex substrata in flume flows. Mar. Ecol. Prog. Ser. 135, 89–100.
Harvey, M., Bourget, E., Ingram, R.G., 1995. Experimental evidence of passive accumulation of marine bivalve larvae on filamentous epibenthic structures. Limnol. Oceanogr. 40, 94–104.
Harvey, M., Bourget, E., 1997. Recruitment of marine invertebrates onto arborescent epibenthic structures — active and passive processes acting at different spatial scales. Mar. Ecol. Prog. Ser. 153, 203–215. Havenhand, J.N., 1991. On the behaviour of opisthpbranch larvae. J. Moll. Stud. 57, 119–131.
Hay, M.E., 1996. Marine chemical ecology — what’s known and what’s next? J. Exp. Mar. Biol. Ecol. 200 (1-2), 103–134.
Hills, J.M., Thomason, J.C., Milligan, J.L., Richardson, T., 1998. Do barnacle larvae respond to multiple settlement cues over a range of spatial scales? Hydrobiology 376, 101–111.
Hooker, N., Morse, D.E., 1985. Abalone: the emerging development of commercial cultivation in the United States. In: Huner, J.V., Brown, E.E. (Eds.), Crustacean and Mollusk Cultivation in the United States. AVI Publishing, Westport, CT, pp. 365–413.
Johnson, L.E., Strathmann, R.R., 1989. Settling barnacle larvae avoid substrata previously occupied by a mobile predator. J. Exp. Mar. Biol. Ecol. 128, 87–103.
Johnson, C.R., Muir, D.G., Reysenbach, A.L., 1991. Characteristic bacteria associated with surfaces of coralline algae: a hypothesis for bacterial induction of marine invertebrate larvae. Mar. Ecol. Prog. Ser. 74, 281–294.
´
Jonsson, P.R., Andre, C., Lindegarth, M., 1991. Swimming behaviour of marine bivalve larvae in a flume boundary-layer flow: evidence for near-bottom confinement. Mar. Ecol. Prog. Ser. 79, 67–76.
Keen, S.L., 1987. Recruitment of Aurelia aurita (Cnidaria, Scyphozoa) larvae is position dependent, and independent of conspecific density, within a settling surface. Mar. Ecol. Prog. Ser. 38, 151–160. Keough, M.J., 1998. Responses of settling invertebrate larvae to the presence of established recruits. J. Exp.
Mar. Biol. Ecol. 231 (1), 1–20.
Keough, M.J., Downes, B.J., 1982. Recruitment of marine invertebrates: the role of active larval choices and early mortality. Oecologia 54, 348–352.
Keough, M.J., Raimondi, P.T., 1995. Responses of settling invertebrates larvae to bioorganic films: effects of different types of films. J. Exp. Mar. Biol. Ecol. 185, 235–253.
Knight-Jones, E.W., 1951. Gregariousness and other aspects of the setting behaviour of Spirorbis. J. Mar. Biol. Assoc. UK 30, 201–222.
Knight-Jones, E.W., Stevenson, J.P., 1950. Gregariousness during settlement in the barnacle Elminius modestus Darwin. J. Mar. Biol. Assoc. UK 29, 281–297.
Knight-Jones, E.W., Morgan, E., 1966. Responses of marine animals to changes in hydrostatic pressure. Oceanogr. Mar. Biol. Annu. Rev. 4, 267–299.
Lemire, M., Bourget, E., 1996. Substratum heterogeneity and complexity influence micro-habitat selection of Balanus sp. and Tubularia crocea larvae. Mar. Ecol. Prog. Ser. 135, 77–87.
Maki, J.S., Rittschof, D., Schmidt, A.R., Snyder, A.G., Mitchell, R., 1989. Factors controlling attachment of bryozoan larvae: a comparison of filmed and unfilmed surfaces. Biol. Bull. 177, 295–302.
Maki, J.S., Rittschof, D., Samuelsson, M.-O., Szewyk, U., Yule, A.B., Kjelleberg, S., Costlow, J.D., Mitchell, R., 1990. Effect of marine bacteria and their exoploymers on the attachment of barnacle cypris larvae. Bull. Mar. Sci. 46, 499–511.
Maldonado, M., Young, C.M., 1996. Effects of physical factors on larval behavior, settlement and recruitment of four tropical demosponges. Mar. Ecol. Prog. Ser. 138 (1-3), 169–180.
Marsden, J.R., Anderson, D.T., 1981. Larval development and metamorphosis of the serpulid polychaete Galeolaria caepitosa Lamarck. Aust. J. Mar. Freshwater Res. 32, 667–680.
Matsumura, K., Nagano, M., Fusetani, N., 1998. Purification of a larval settlement-inducing protein complex (SIPC) of the barnacle Balanus amphitrite. J. Exp. Zool. 281 (1), 12–20.
McShane, P.E., Black, K.P., Smith, M.G., 1988. Recruitment processes in Haliotis rubra (Mollusca: Gastropoda) and regional hydrodynamics in South Eastern Australia imply localized dispersal of larvae. J. Exp. Mar. Biol. Ecol. 124, 175–203.
Mead, R., 1988. The Design of Experiments. Cambridge University Press, Cambridge.
Meadows, P.S., Campbell, J.I., 1972. Habitat selection by aquatic invertebrates. Adv. Mar. Biol. 10, 271–382. Miron, G., Bourget, E., Archambault, P., 1996. Scale of observation and distribution of adult conspecifics: their influence in assessing passive and active settlement mechanisms in the barnacle Balanus crenatus
`
(Brugiere). J. Exp. Mar. Biol. Ecol. 201, 137–158.
Morse, A.N.C., 1991. How do planktonic larvae know where to settle? Am. Sci. 79, 154–167.
Morse, A.N.C., Morse, D.E., 1984a. Recruitment and metamorphosis of Haliotis larvae induced by molecules uniquely available at the surfaces of crustose red algae. J. Exp. Mar. Biol. Ecol. 75, 191–215.
Morse, A.N.C., Morse, D.E., 1984b. GABA-mimetic molecules from Porphyra (Rhodophyta) induce metamorphosis of Haliotis (Gastropoda) larvae. Hydrobiologia 16, 155–158.
Morse, D.E., 1990. Recent progress in larval settlement and metamorphosis: closing the gaps between molecular biology and ecology. Bull. Mar. Sci. 46, 465–483.
Morse, D.E., Morse, A.N.C., 1991. Enzymatic characterization of the morphogen recognized by Agaricia humilis (scleractinian coral) larvae. Biol. Bull. (Woods Hole) 181, 104–122.
Mullineaux, L.S., Butman, C.A., 1991. Initial contact, exporation and attachment of barnacle (Balanus amphitrite) cyrids settling in flow. Mar. Biol. 110, 93–103.
Mullineaux, L.S., Garland, E.D., 1993. Larval recruitment in response to manipulated field flows. Mar. Biol. 116, 667–683.
Muschenheim, D.K., Grant, J., Mills, E.L., 1986. Flumes for benthic ecologists: theory, construction and practice. Mar. Ecol. Prog. Ser. 28, 185–196.
Neal, A.L., Yule, A.B., 1994. The tenacity of Elminius modestus and Balanus perforatus cyprids to bacterial films grown under different shear regimes. J. Mar. Biol. Assoc. UK 74, 251–257.
Neal, A.L., Simoes, F.N., Yule, A.B., 1996. Interactions between shear rates and biofilms affecting exploratory behavior by cyprids of Elminius modestus (Cirripedia). Mar. Biol. 127 (2), 241–246.
Nowell, A.R.M., Jumars, P.A., 1984. Flow environments of aquatic benthos. Annu. Rev. Ecol. Syst. 15, 303–328.
Nowell, A.R.M., Jumars, P.A., 1987. Flumes: theoretical and experimental considerations for simulation of benthic environments. Oceanogr. Mar. Biol. Annu. Rev. 25, 91–112.
O’Connor, N.J., Richardson, D.L., 1998. Attachment of barnacle (Balanus amphitrite Darwin) larvae: responses to bacterial films and extracellular materials. J. Exp. Mar. Biol. Ecol. 226 (1), 115–129. Orlov, D.V., 1997. The role of larval settling behaviour in determination of the specific habitat of the hydrozoan
Dynamena pumila (L.). Larval settlement in Dynamena pumila (L.). J. Exp. Mar. Biol. Ecol. 208 (1-2), 73–85.
Pawlik, J.P., 1992. Chemical ecology of the settlement of benthic marine invertebrates. Oceanogr. Mar. Biol. Annu. Rev. 30, 273–335.
Pawlik, J.R., Butman, C.A., 1993. Settlement of a marine tube worm as a function of current velocity: Interaction effects of hydrodynamics and behavior. Limnol. Oceanogr. 38, 1730–1740.
Raimondi, P.T., Schmitt, R.J., 1992. Effects of produced water on settlement of larvae: field tests using red abalone. In: Ray, J.P., Engelhardt, F.R. (Eds.), Produced Water: Technological / environmental Issues and Solutions. Plenum Press, New York, pp. 415–430.
Rittschof, D., Branscomb, E.S., Costlow, J.D., 1984. Settlement and behaviour in relation to flow and surface in larval barnacle Balanus amphitrite Darwin. J. Exp. Mar. Biol. Ecol. 82, 131–146.
Rittschof, D., 1993. Body odors and neutral-basic peptide mimics: a review of responses by marine organisms. Am. Zool. 33, 487–493.
Rodriguez, S.R., Ojeda, F.P., Inestrosa, N.C., 1993. Settlement of benthic marine invertebrates. Mar. Ecol. Prog. Ser. 97, 193–207.
Slattery, M., 1992. Larval settlement and juvenile survival in the red abalone (Haliotis rufescens): an examination of inductive cues and substrate selection. Aquaculture 102, 143–153.
Snelgrove, P.V.R., Butman, C.A., Grassle, J.P., 1993. Hydrodynamic enhancement of larval settlement in the bivalve Mulinia lateralis (Say) and the polychaete Capitella sp. I. in microdepositional environments. J. Exp. Mar. Biol. Ecol. 168, 71–109.
Snelgrove, P.V.R., Grassle, J.P., Butman, C.A., 1998. Sediment choice by settling larvae of the bivalve, Spisula solidissima (Dillwyn), in flow and still water. J. Exp. Mar. Biol. Ecol. 231 (2), 171–190.
Snelgrove, P.V.R., Grant, J., Pilditch, C.A., 1999a. Habitat selection and adult–larvae interactions in settling larvae of soft-shell clam Mya arenaria. Mar. Ecol. Prog. Ser. 182, 149–159.
Snelgrove, P.V.R., Grassle, J.P., Grassle, J.F., Petrecca, R.F., Ma, H.G., 1999b. In situ habitat selection by settling larvae of marine soft-sediment invertebrates. Limnol. Oceanog. 44, 1341–1347.
Sokal, R.R., Rohlf, F.J., 1981. Biometry, 2nd Edition. W.H. Freeman, San Fransisco.
Svane, I., Young, C.M., 1989. The ecology and behaviour of ascidian larvae. Oceanogr. Mar. Biol. Annu. Rev. 1989, 45–90.
Turner, E.J., Zimmer-Faust, M.A., Palmer, M.A., Luckenbach, M., Pentcheff, N.D., 1994. Settlement of oyster Crassostrea virginica larvae: effects of water flow and a water-soluble chemical cue. Limnol. Oceanogr. 39, 1579–1593.
Unabia, C.R.C., Hadfield, M.G., 1999. Role of bacteria in larval settlement and metamorphosis of the polychaete Hydriodes elegans. Mar. Biol. 133 (1), 55–64.
´
Vazquez, E., Young, C.M., 1996. Responses of compound ascidian larvae to haloclines. Mar. Ecol. Prog. Ser. 133, 179–190.
Vogel, S., LaBarbera, M., 1978. Simple flow tanks for research and teaching. Bioscience 28, 638–643. Vogel, S., 1994. Life in Moving Fluids, 2nd Edition. Princeton University Press, Princeton, NJ. Vogel, S., 1981. Life in Moving Fluids, 1st Edition. Willard Grant Press, Boston, MA.
Walters, L., 1992. Field settlement locations on subtidal marine hard substrata: Is active larval exploration involved? Limnol. Oceanogr. 37, 1101–1107.
Walters, L.J., Wethey, D.S., 1996. Settlement and early post-settlement survival of sessile marine invertebrates on topographically complex surfaces: the importance of refuge dimensions and adult morphology. Mar. Ecol. Prog. Ser. 137, 161–171.
Walters, L.J., Hadfield, M.G., Smith, C.M., 1996. Waterborne chemical compounds in tropical macroalgae: Positive and negative cues for larval settlement. Mar. Biol. 126 (3), 383–393.
Walters, L.J., Hadfield, M.G., Del Carmen, K.A., 1997. The importance of larval choice and hydrodynamics in creating aggregations of Hydroides elegans (Polychaeta: Serpulidae). Invertebr. Biol. 116 (2), 102–114. Walters, L.J., Miron, G., Bourget, E., 1999. Endoscopic observations of invertebrate larval substratum
exploration and settlement. Mar. Ecol. Prog. Ser. 182, 95–108.
Welch, J.M., Forward, Jr. R.B., How, P.A., 1999. Behavioral responses of blue crab Callinectes sapidus postlarvae to turbulence: implications for selective tidal stream transport. Mar. Ecol. Prog. Ser. 179, 135–143.
Wethey, D.S., 1986. Ranking of settlement cues by barnacle larvae: influence of surface contour. Bull. Mar. Sci. 39, 393–400.
Woodin, S.A., Lindsay, .M. S.M., Wethey, D.S., 1995. Process-specific recruitment cues in marine sedimentary systems. Biol. Bull. 189, 49–58.
Wright, J.R., Boxshall, A.J., 1999. The influence of small-scale flow and chemical cues on the settlement of two congeneric barnacle species. Mar. Ecol. Prog. Ser. 183, 179–187.
Yamamoto, H., Tachibana, A., Saikawa, W., Nagano, M., Matsumura, K., Fusetani, N., 1998. Effects of calmodulin inhibitors on cyprid larvae of the barnacle, Balanus amphitrite. J. Exp. Zool. 280 (1), 8–17. Young, C.M., 1995. Behavior and locomotion during the dispersal phase of larval life. In: McEdwards, L.
(Ed.), Ecology of Marine Invertebrate Larvae. CRC Press, Boca Raton, FL, pp. 249–277.
Young, C.M., Chia, F.-S., 1981. Laboratory evidence for delay of larval settlement in response to a dominant competitor. Int. J. Invertebr. Reprod. 3, 221–226.