Plants respond to heavy-metal toxicity via a number of mechanisms. One such mechanism involves the chelation of heavy metals by a family of peptide ligands, the phytochelatins. Molecular genetic approaches have resulted in important advances in our understanding of phytochelatin biosynthesis. In particular, genes encoding the enzyme phytochelatin synthase have been isolated from plant and yeast species. Unexpectedly, genes with similar sequences to those encoding phytochelatin synthase have been identified in some animal species.
Addresses
Department of Genetics, University of Melbourne, Parkville, Victoria 3052, Australia; e-mail: [email protected] Current Opinion in Plant Biology2000, 3:211–216
1369-5266/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved. Abbreviations
GCS γ-glutamylcysteine synthetase GS glutathione synthetase GSH glutathione
PC phytochelatin
PCR polymerase chain reaction RT reverse transcription
Introduction
Heavy-metal toxicity can elicit a variety of adaptive responses in plants. These responses have been compre-hensively reviewed for plants exposed to cadmium [1••], the metal for which published studies are most extensive. A ubiquitous mechanism for heavy-metal detoxification is the chelation of the metal ion by a ligand. A variety of metal-binding ligands have been described in plants, and their respective roles in heavy-metal detoxification have been reviewed [2••]. Such ligands include organic acids, amino acids, peptides and polypeptides. Understanding the genetic and molecular basis of such mechanisms is an
important goal in developing plants for the phytoremedia-tion of contaminated environments [3]. My review describes recent advances in our understanding of the genetic and molecular basis of the biosynthesis and func-tion of an important class of heavy-metal-binding ligands, the phytochelatins (PCs).
The PCs form a family of peptides that consist of repeti-tions of the γ-Glu-Cys dipeptide followed by a terminal Gly — the basic structure being (γ-Glu-Cys)n–Gly [(PC)n], where n is generally in the range two to five. PCs have been identified in a wide variety of plant species and in some microorganisms. They are structurally related to glu-tathione (GSH; γ-Glu-Cys-Gly) and were presumed to be the products of a biosynthetic pathway. Numerous physio-logical, biochemical and genetic studies have confirmed that GSH is the substrate for PC biosynthesis (Figure 1). PC structure and synthesis have been reviewed previously [2••,4]. In addition, a number of structural variants of PCs, such as (γ-Glu-Cys)n–β-Ala, (γ-Glu-Cys)n–Ser and (γ -Glu-Cys)n–Glu, have been identified in some plant species.
PCs are rapidly induced in vivoby a wide range of heavy-metal ions. The enzyme PC synthase was first identified by Grill et al. [5] and has been characterised in a number of subsequent studies [6,7]. The enzyme is a γ−Glu-Cys dipeptididyl transpeptidase (EC 2.3.2.15) and it catalyses the transpeptidation of the γ−Glu-Cys moiety of GSH either onto a second GSH molecule to form PC(n=2) or onto a PC molecule to produce a PC(n+1) oligomer. PC synthase is activated by a variety of heavy-metal ions.
Genetic approaches to studying PC
biosynthesis and function
Significant recent advances in our understanding of the molecular basis of PC biosynthesis and function have come
Phytochelatin biosynthesis and function in heavy-metal
detoxification
Christopher S Cobbett
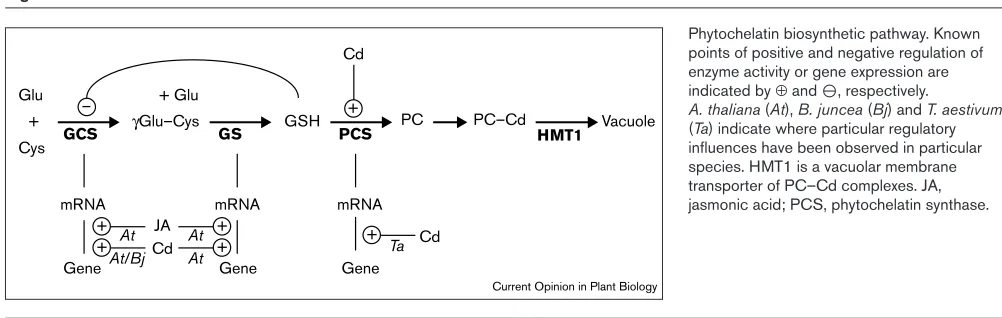
Figure 1
Phytochelatin biosynthetic pathway. Known points of positive and negative regulation of enzyme activity or gene expression are indicated by ⊕and O, respectively.
A. thaliana(At), B. juncea (Bj) and T. aestivum
(Ta) indicate where particular regulatory influences have been observed in particular species. HMT1 is a vacuolar membrane transporter of PC–Cd complexes. JA, jasmonic acid; PCS, phytochelatin synthase.
Glu
γGlu–Cys PC
Cd
PC–Cd
HMT1
GCS GS PCS
+ Glu
Cd
Gene Gene
Gene
mRNA mRNA
mRNA Cys
GSH Vacuole
+
+ + +
– +
+ +
JA Cd
At At
At/Bj At Ta
from molecular genetic studies using a number of model organisms. These approaches have centred on the identifi-cation of Cd-sensitive mutants of the plant Arabidopsis thalianaand the yeasts Schizosaccharomyces pombeand Can-dida glabrata(Table 1). In addition, the expression of plant cDNAs in strains of Escherichia coliand Saccharomyces cere-visiaehas been particularly useful in the identification and analysis of genes involved in functions related to heavy-metal detoxification. Both of these approaches have been useful in identifying the genes that encode the enzymes involved in GSH biosynthesis ([8•]; and see [9,10]) and, more recently, the genes encoding PC synthase.
PC synthase genes in plants and yeast
The cadmium-sensitive cad1 mutants of Arabidopsis have wild-type levels of GSH but are PC-deficient and lack PC synthase activity in vitro. It was predicted that CAD1 is the structural gene for PC synthase [11] (Table 1). The Arabidopsis CAD1 gene (also referred to as AtPCS1) has been isolated using a positional cloning strategy [12••]. cDNA clones of AtPCS1 [13••] and a similar gene in wheat (TaPCS1) [14••] have also been identified by their ability to confer resistance to Cd when expressed in the yeast S. cerevisiae. Both of the latter studies [13••,14••] used various yeast mutants to demonstrate that the mech-anism of Cd-resistance conferred by these cDNAs was distinct from other recognised Cd-detoxification mecha-nisms in yeast, was dependent on GSH, and mediated PC biosynthesis in vivo.
A gene, SpPCS1, that is similar to the plant PC-synthase genes was identified in the genome of the fission yeast S. pombe (Figure 2).Targeted-deletion mutants of this gene were also Cd-sensitive and PC-deficient, confirming the analogous functions of the plant and yeast genes [12••,14••] (Table 1). It is remarkable that such a Cd-sensi-tive mutant had not been isolated through various earlier
mutant screens. Biochemical confirmation of the activity of the Arabidopsisand S. pombegene products, purified as epi-tope-tagged derivatives [13••,14••] or expressed in E. coli [12••], demonstrated that each was sufficient for GSH-dependent, metal-ion-activated PC biosynthesis in vitro.
An alignment of the amino acid sequences of the Ara-bidopsis and S. pombe PC-synthase proteins shows a significant level of homology in the amino-terminal region, with little apparent conservation of the carboxy-terminal region (Figure 2). In both sequences, the latter region con-tains numerous Cys residues, many as adjacent or neighbouring pairs, although the arrangement of these pairs is not conserved between the two sequences.
A second gene, AtPCS2, with significant homology to CAD1/AtPCS1 has been found in the Arabidopsis genome [12••]. This discovery was unexpected because PCs were not detected in cad1mutants after prolonged exposure to Cd, suggesting the presence of a single active PC synthase [11]. When AtPCS2 is expressed in yeast it confers Cd-resistance, indicating that its gene product is active (CS Cobbett, unpublished data). The function of this gene remains to be determined. It seems likely that, in most tis-sues, AtPCS2 is expressed at a relatively low level compared with AtPCS1. Nevertheless, for it to have been preserved throughout evolution as a functional PC syn-thase, AtPCS2 must presumably be the predominant PC synthase in some tissue(s) or environmental conditions.
Interestingly, although PC(n=2) has been described in the yeast S. cerevisiae, there is no homologue of the PC-synthase genes in the S. cerevisiaegenome. An alternative pathway for PC biosynthesis in S. pombehas been proposed [15], howev-er, and it may be that this pathway functions in S. cerevisiae. Nevertheless, the cad1-3mutant of Arabidopsisand the PC-synthase deletion mutant of S. pombeboth lack detectable Table 1
Genes involved in phytochelatin biosynthesis and function.
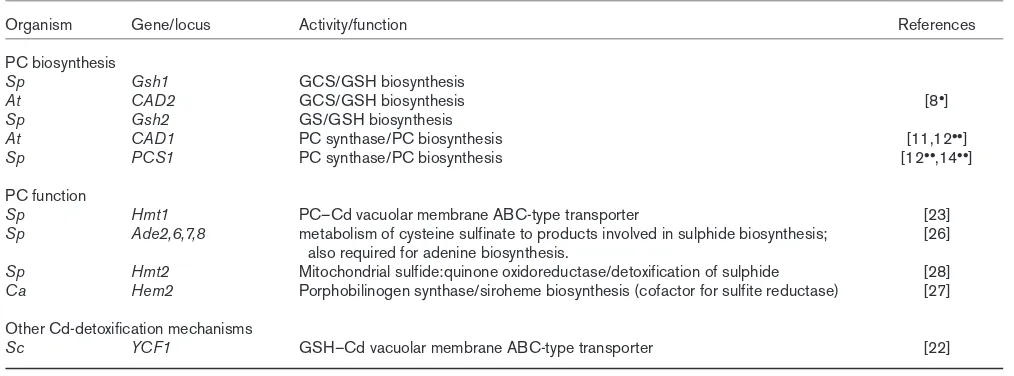
Organism Gene/locus Activity/function References
PC biosynthesis
Sp Gsh1 GCS/GSH biosynthesis
At CAD2 GCS/GSH biosynthesis [8•]
Sp Gsh2 GS/GSH biosynthesis
At CAD1 PC synthase/PC biosynthesis [11,12••]
Sp PCS1 PC synthase/PC biosynthesis [12••,14••]
PC function
Sp Hmt1 PC–Cd vacuolar membrane ABC-type transporter [23]
Sp Ade2,6,7,8 metabolism of cysteine sulfinate to products involved in sulphide biosynthesis; [26] also required for adenine biosynthesis.
Sp Hmt2 Mitochondrial sulfide:quinone oxidoreductase/detoxification of sulphide [28]
Ca Hem2 Porphobilinogen synthase/siroheme biosynthesis (cofactor for sulfite reductase) [27] Other Cd-detoxification mechanisms
Sc YCF1 GSH–Cd vacuolar membrane ABC-type transporter [22]
PCs, suggesting that such an alternative pathway is of little physiological relevance in these organisms.
Regulation of PC biosynthesis
PC biosynthesis may be regulated by a number of mecha-nisms. For example, in Brassica juncea, exposure to Cd produces a requirement for both cysteine and GSH for PC biosynthesis, that is met by coordinate transcriptional reg-ulation of genes involved in sulphur transport and assimilation [16,17] and GSH biosynthesis [18]. Similarly, exposure of Arabidopsis plants to Cd and Cu causes an increase in the transcription of genes encoding GSH reductase and enzymes involved in the GSH biosynthetic pathway: γ-glutamylcysteine synthetase (GCS) and glu-tathione synthetase (GS) [19••] (Figure 1). The signal molecule, jasmonate, mediates a similar effect in the absence of heavy-metal exposure, although it has not been demonstrated that jasmonate is directly involved in medi-ating the effects of heavy-metal stress on gene expression [19••]. There is also circumstantial evidence for the post-transcriptional regulation of GCS expression [9], in addition to the well-recognised regulation of GCS activity through GSH feedback inhibition [9,10] (Figure 1).
The importance of the regulation of GSH biosynthesis in modulating PC expression is reinforced by the observation that PC biosynthesis and Cd tolerance are increased in trans-genic B. juncea in which either GCS or GS was over-expressed [20,21], although similar effects were not observed when GCS was over-expressed in poplar (see [10]).
PC-synthase activity is expected to to be the major deter-minant of the rate of PC synthesis. Kinetic studies using plant cell cultures exposed to Cd demonstrated that PC biosynthesis occurs within minutes of exposure to the heavy metal and is independent of de novoprotein synthe-sis [2••]. PC synthase extracted from plant cells or tissues is activated by various heavy-metal ions [5–7] (Figure 1). Likewise, in in vitrostudies of PC synthase expressed in E. colior in S. cerevisiae, the enzyme was activated to vary-ing extents by Cd, Cu, Ag, Hg, Zn and Pb ions [12••–14••].
The mechanism by which PC synthase is activated appears to be relatively non-specific with respect to the activating metal ion, although some metals are more effective than others. One model for the function of PC synthase is that the conserved amino-terminal domain confers the catalytic activity of this enzyme. Activation probably arises from an interaction between residues in this domain and free metal ions or metal–GSH complex-es. This model is supported by evidence from the molecular characterisation of the cad1-5 mutant of Ara-bidopsis, which has a nonsense mutation that results in premature termination of translation after the conserved amino-terminal domain [12••]. The truncated polypep-tide is predicted to lack nine of the ten Cys residues in the carboxy-terminal domain [12••]. This mutant retains some activity (as measured by in vivoPC concentrations
and sensitivity to Cd), but this activity is expressed only in the presence of Cd [11], demonstrating that the car-boxy-terminal domain is not absolutely required for either catalysis or activation.
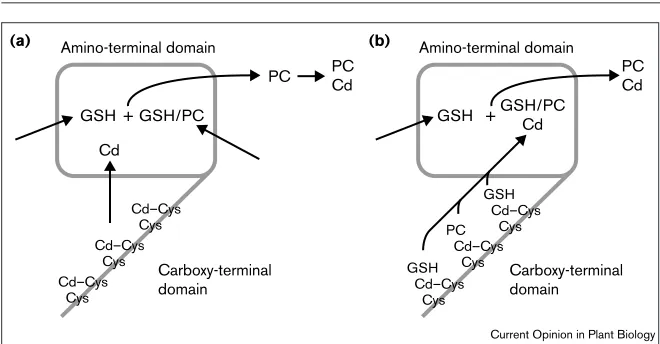
It has been suggested that the carboxy-terminal domain acts as a local sensor by binding heavy-metal ions (pre-sumably via the multiple Cys residues) and bringing them into contact with the activation site in the amino-terminal catalytic domain [12••]. This model is consistent with the findings of biochemical studies using epitope-tagged AtPCS1, which demonstrated that this enzyme binds Cd ions at high affinity (Kd = 0.54±0.20µM) and high capac-ity (stoichiometric ratio = 7.09±0.94) [13••]. Although PC synthase can bind Cd ions in the absence of GSH, an inter-esting and unresolved issue is whether Cd ions are solely involved in the activation of the enzyme or form an inte-gral component of the substrate. In the former model, Cd ions would interact with an activation site in the amino-ter-minal domain that is distinct from the GSH–PC substrate-binding sites. In the latter model, GSH/PC–Cd complexes would be the substrate for the enzyme, and individual Cd ions bound to the substrate would remain bound to the PC product. A schematic illustration of these alternative models is shown in Figure 3.
Previous studies indicated that PC synthase was expressed constitutively and that the levels of this enzyme in cell cultures or intact plants were generally unaffected by exposure to Cd [5–7]. This is supported by Northern or RT-PCR (reverse transcription polymerase chain reaction) analysis of the expression of CAD1/AtPCS1, which showed that levels of mRNA were not influenced by exposure of plants to Cd, even under conditions of severe heavy-metal induced stress ([12••,13••]; CS Cobbett, unpublished data). Interesting-ly, however, RT-PCR analysis of TaPCS1 expression in wheat roots indicated induction of mRNA on exposure to Cd [14••] (Figure 1). This evidence suggests that, in some species, PC-synthase activity may be regulated at both the transcriptional and post-translational levels. Figure 2
Schematic comparison of PC-synthase polypeptides from different organisms. The positions of Cys residues are indicated by vertical bars. The conserved amino-terminal domains exhibit at least 40% identical amino acids in pair-wise comparisons of the four sequences.
At,A. thaliana(CAD1/AtPCS1; GenBank accession numbers, AF135155 and AF085230); Ta, Triticum aestivum(TaPCS1; AF093252); Sp, S. pombe(SpPCS; Z68144); and Ce, C. elegans
(CePCS1; Z66513). At Ta Sp
Ce
Conserved Variable
Amino- terminus Carbo
xy-terminus
PC synthase genes in animals?
Although PCs have not yet been identified in an animal species there is a gene similar to AtPCS1 in the nematode, Caenorhabditis elegans (Figure 2). The amino-terminal region of the predicted gene product is equally similar to the plant and yeast proteins. In contrast, the carboxy-termi-nal domain has little obvious similarity to the plant or yeast gene-products, except that it contains multiple pairs of Cys residues. In addition, an homologous expressed sequence tag (GenBank accession number AU061531) has been iden-tified in the slime mould Dictyostelium discoideum and, using PCR, sequences similar to the conserved amino-terminal regions of PC synthase genes have been identified from the aquatic midge Chironomusand earthworm species (CS Cob-bett, unpublished data). There is, as yet, no evidence that these animal genes encode PC-synthase activity. Nonethe-less, it seems likely that they too encode PC synthase. The existence of PC-synthase-like genes in animals suggests that PCs play a wider role in heavy-metal detoxification than previously expected. A superficial view of the limited selection of species in which such sequences have been identified might suggest that organisms with an aquatic or soil habitat are more likely to express PCs.
Other aspects of cadmium detoxification
In both plant and yeast species, heavy metals (Cd in par-ticular) are sequestered to the vacuole. In S. cerevisiae, YEAST CADMIUM FACTOR 1 (YCF1) [22], and in S. pombe, HEAVY-METAL TOLERANCE 1 (Hmt1) [23], encode members of the ATP-binding cassette (ABC) fam-ily of membrane transporters that transport GSH–Cd and PC–Cd complexes, respectively, into the vacuole and play important roles in Cd detoxification (Table 1). There is also increasing evidence that vacuolar localisation ofheavy-metal ions plays an important role in naturally evolved heavy-metal-tolerant plants [24,25]. In the vac-uole, sulphide ions are an essential component of PC–Cd complexes, and a number of Cd-sensitive mutants believed to be affected in aspects of sulphide metabolism have been identified in yeast species [26] (Table 1). For example, in Candida glabrata, the Cd-sensitive hem2 mutant is deficient in porphobilinogen synthase, an enzyme involved in the biosynthesis of siroheme, which is a cofactor for sulphite reductase [27]. A different mutant in S. pombe, hmt2, appears to lack a sulphide:quinone oxidore-ductase that is believed to be important for the detoxification of excess sulphide formed in response to Cd exposure and PC biosynthesis [28]. Further studies are required to establish the pathways of sulphide metabolism, particularly in plants in which Cd-sensitive sulphide-metabolism mutants have not been identified.
Roles for PCs in metal detoxification or
metabolism?
The PC-deficient mutants of Arabidopsis and S. pombeare most sensitive to Cd and the arsenate anion, and are less sen-sitive to other heavy metals such as Cu and Hg [12••,14••]. Although this clearly demonstrates that PCs can have an important role in detoxification, it remains to be determined whether this is the function for which they evolved or an incidental role. Other proposed roles for PCs have included their involvement in essential heavy-metal homeostasis, Fe metabolism and sulphur metabolism [2••,4]. Although the evidence of a role for PCs in detoxification is strong, there is only circumstantial evidence in support of these alternatives.
Can PCs have a role in Cd detoxification at levels of Cd exposure relevant to plants in a natural environment? It has Figure 3
Schematic models for the function of PC synthase. The carboxy-terminal domain acts as a local sensor of heavy-metal ions, such as Cd. The Cys residues bind Cd ions, bringing them into closer proximity and transferring them to the amino-terminal, catalytic domain. The activated amino-terminal domain catalyses the transfer of the γ-Glu-Cys moiety of a molecule of GSH onto a second molecule of GSH (or an existing PC molecule) to form a PC product. In model (a), Cd alone interacts with the Cys residues and subsequently with an activation site in the amino-terminal domain that is distinct from the GSH/PC-substrate-binding sites. The PC product is formed and binds Cd subsequently. In model (b), cytoplasmic Cd ions bound to GSH or a previously formed PC interact first with the Cys residues in the carboxy-terminal domain and subsequently with the catalytic site in the amino-terminal domain where Cd remains an integral component of the substrate and, consequently, the product of the reaction. A variation of this model could involve GSH–Cd complexes binding at both the donor and acceptor sites.
been estimated that solutions of non-polluted soils contain Cd concentrations of less than 0.3µM [29]. In contrast, most experimental studies use Cd concentrations more than 1µM [1••]. Wagner [29] has argued that at low levels of Cd nor-mally present in most soils, Cd would largely be complexed with vacuolar citrate, and only at high Cd concentrations (not generally found in natural environments) would PCs play a role. Nonetheless, the sensitivity of the Arabidopsis cad1-3 mutant to concentrations of Cd as low as 0.6µM [11] at least suggests that PCs may have a role in heavy-metal detoxifica-tion in an unpolluted environment. Even at lower concentrations of Cd, at which sensitive plants may not be easily distinguished from tolerant genotypes by obvious phe-notypic differences, a plant expressing PCs may have a selective advantage. It would be of interest to test the fitness of the cad1-3mutant compared to wild-type plants on a range of soils from natural environments.
Conclusions
The identification of PC-synthase genes from plants and other organisms is a significant breakthrough that will lead to a better understanding of the regulation of a critical step in PC biosynthesis. Nonetheless, we must keep in mind the numerous other aspects of PC biosynthesis and func-tion, and the ways in which they, too, are regulated at a cellular and physiological level in response to heavy-metal exposure. These include aspects of sulphur assimilation, GSH and sulphide biosynthesis, PC compartmentalisation and the signal pathways through which metal toxicity leads to gene regulation. In the long term, we aim to understand heavy-metal detoxification at the whole plant level and to exploit such knowledge. The concept of phytoremediation of contaminated soils has been increasingly supported by research in recent years. The understanding of heavy-metal detoxification processes afforded by investigations in model systems such as ArabidopsisandS. pombewill, in the near future, allow us to explore the mechanisms by which some species are capable of hyper-accumulation of metals such as Cd and how they may be best used for phytoreme-diation. Ultimately, the genetic manipulation of such plants may further enhance their usefulness for this purpose.
Acknowledgements
I wish to acknowledge the contributions of members of my laboratory and valuable collaborations with colleagues. The work of my laboratory is supported by the Australian Research Council.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Sanita di Toppi L, Gabbrielli R: Response to cadmium in higher •• plants.Environ Exp Bot1999, 41:105-130.
An excellent overview of the adaptive responses of plants to Cd toxicity. The need for an integrated approach to understanding the complex physiological mechanisms is emphasised.
2. Rauser WE: Structure and function of metal chelators produced •• by plants; the case for organic acids, amino acids, phytin and
metallothioneins.Cell Biochem Biophys1999, 31:19-48.
A timely review examining the contributions of various heavy-metal-binding ligands to detoxification processes in plants.
3. Salt DE, Smith RD, Raskin I: Phytoremediation.Annu Rev Plant Physiol Plant Mol Biol1998, 49:643-668.
4. Zenk MH: Heavy metal detoxification in higher plants – a review. Gene1996, 179:21-30.
5. Grill E, Loffler S, Winnacker E-L, Zenk MH: Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γγ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase).Proc Natl Acad Sci USA1989, 86:6838-6842.
6. Klapheck S, Schlunz S, Bergmann L: Synthesis of phytochelatins and homo-phytochelatins in Pisum sativumL..Plant Physiol1995, 107:515-521.
7. Chen J, Zhou J, Goldsbrough PB: Characterization of phytochelatin synthase from tomato.Physiol Plant1997, 101:165-172.
8. Cobbett CS, May MJ, Howden R, Rolls B: The glutathione-deficient, • cadmium-sensitive mutant, cad2-1, of Arabidopsis thalianais
deficient in γγ-glutamylcysteine synthetase.Plant J1998, 16:73-78. This paper describes the molecular characterisation of the first glutathione-deficient mutant identified in plants and provides an important genetic con-firmation of the role of glutathione as the substrate for PC biosynthesis. 9. May MJ, Vernoux T, Leaver C, Van Montague M, Inze D: Glutathione
homeostasis in plants: implications for environmental sensing and plant development.J Exp Botany1998, 49:649-667.
10. Noctor G, Arisi A-CM, Jouanin L, Kunert K, Rennenberg H, Foyer CH: Glutathione: biosynthesis, metabolism and relationship to stress tolerance explored in transgenic plants.J Exp Botany1998, 49:623-647.
11. Howden R, Goldsbrough PB, Andersen CR, Cobbett CS: Cadmium-sensitive, cad1, mutants of Arabidopsis thalianaare phytochelatin deficient.Plant Physiol1995, 107:1059-1066.
12. Ha S-B, Smith AP, Howden R, Dietrich WM, Bugg S, O’Connell MJ, •• Goldsbrough PB, Cobbett CS: Phytochelatin synthase genes from
Arabidopsisand the yeast, Schizosaccharomyces pombe.Plant Cell1999, 11:1153-1164.
One of a trio of papers describing the isolation of PC-synthase genes (see also [13••,14••]). Positional cloning of the CAD1 gene of Arabidopsis
and subsequent expression in E. coli confirmed that this gene, and an homologous gene in the yeast S. pombe,encodes PC synthase.
13. Vatamaniuk OK, Mari S, Lu Y-P, Rea PA: AtPCS1, a phytochelatin •• synthase from Arabidopsis: isolation and in vitroreconstitution.
Proc Natl Acad Sci USA1999, 96:7110-7115.
One of a trio of papers describing the isolation of PC-synthase genes (see also [12••,14••]). An Arabidopsis PC synthase cDNA was identified through
its expression in yeast conferring increased Cd-tolerance. Importantly, using a purified, epitope-tagged derivative of AtPCS1, these authors demonstrat-ed this enzyme is necessary and sufficient for PC biosynthesis and that the enzyme itself binds Cd ions.
14. Clemens S, Kim EJ, Neumann D, Schroeder JI: Tolerance to toxic •• metals by a gene family of phytochelatin synthases from plants
and yeast.EMBO J1999, 18:3325-3333.
One of a trio of papers describing the isolation of PC-synthase genes (see also [12••,13••]). A wheat cDNA encoding PC synthase was identified
through its expression in yeast conferring increased Cd-resistance. Subsequent analysis of this gene, and homologous genes in Arabidopsis and S. pombe, confirmed its function.
15. Hayashi Y, Nakagawa CW, Mutoh N, Isobe M, Goto T: Two pathways in the biosynthesis of cadystins (γγ-EC)nG in the cell-free system of the fission yeast.Biochem Cell Biol1991, 69:115-121.
16. Heiss S, Schafer HJ, Haag-Kerwer A, Rausch T: Cloning sulfur assimilation genes of Brassica junceaL.: cadmium differentially affects the expression of a putative low-affinity sulfate transporter and isoforms of ATP sulfurylase and APS reductase.Plant Mol Biol1999, 39:847-857.
17. Lee SM, Leustek T: The effect of cadmium on sulfate assimilation enzymes in Brassica juncea.Plant Sci1999, 141:201-207.
18. Schafer HJ, Haag-Kerwer A, Rausch T: cDNA cloning and expression analysis of genes encoding GSH synthesis in roots of the heavy metal accumulator Brassica junceaL.: evidence of Cd-induction of a putative mitochondrial gamma-glutamylcysteine synthetase isoform.Plant Mol Biol1998, 37:87-97.
19. Xiang C, Oliver DJ: Glutathione metabolic genes coordinately •• respond to heavy metals and jasmonic acid in Arabidopsis.Plant
Cell1998, 10:1539-1550.
authors demonstrate that the transcription of the GSH biosynthetic genes is induced both in the presence of Cd and the signal molecule, jasmonate, sug-gesting a possible role for jasmonate in the signal transduction pathway for Cd stress.
20. Zhu YL, Pilon-Smits EAH, Jouanin L, Terry N: Overexpression of glutathione synthetase in Indian Mustard enhances cadmium accumulation and tolerance.Plant Physiol1999, 119:73-79.
21. Yong LZ, Pilon-Smits EAH, Tarun AS, Weber SU, Jouanin L, Terry N: Cadmium tolerance and accumulation in Indian Mustard is enhanced by overexpressing γγ-glutamylcysteine synthetase. Plant Physiol 1999, 121:1169-1177.
22. Li Z-S, Lu Y-P, Zhen R-G, Szczypka M, Thiele DJ, Rea PA: A new pathway for vacuolar cadmium sequestration in Saccharomyces cerevisiae: YCF1-catalyzed transport of bis(glutathionato)-cadmium.Proc Natl Acad Sci USA1997, 94:42-47.
23. Ortiz DF, Ruscitti T, McCue KF, Ow DW: Transport of metal-binding peptides by HMT1, a fission yeast ABC-type vacuolar membrane protein.J Biol Chem1995, 270:4721-4728.
24. Chardonnens AN, Tenbookum WM, Kuijper LDJ, Verkleij JAC, Ernst WHO: Distribution of cadmium in leaves of cadmium tolerant and sensitive ecotypes of Silene vulgaris.Physiol Plant 1998, 104:75-80.
25. Chardonnens AN, Koevoets PLM, van Zanten A, Schat H, Verkleij JAC: Properties of enhanced tonoplast transport in naturally selected zinc-tolerant Silene vulgaris.Plant Physiol1999, 120:779-785.
26. Juang R-H, MacCue KF, Ow DW: Two purine biosynthetic enzymes that are required for cadmium tolerance in Schizosaccharomyces pombeutilize cysteine sulfinate in vitro.Arch Biochem Biophys 1993, 304:392-401.
27. Hunter TC, Mehra RK: A role for HEM2in cadmium tolerance. J Inorg Biochem1998, 69:293-303.
28. Vande Weghe JG, Ow DW: A fission yeast gene for mitochondrial sulfide oxidation.J Biol Chem1999, 274:13250-13257.