Plants are able to take up ammonium from the soil, or through symbiotic interactions with microorganisms, via the root system. Using functional complementation of yeast mutants, it has been possible to identify a new class of membrane proteins, the ammonium transporter/methylammonium permease (AMT/MEP) family, that mediate secondary active ammonium uptake in eukaryotic and prokaryotic organisms. In plants, the AMT gene family can be subdivided according to their amino-acid sequences into three subfamilies: a large subfamily of AMT1 genes and two additional subfamilies each with single members (LeAMT1;3 from tomato and AtAMT2;1 from Arabidopsis thaliana). These transporters vary especially in their kinetic properties and regulatory mechanism. High-affinity transporters are induced in nitrogen-starved roots, whereas other transporters may be considered as the ‘work horses’ that are active when conditions are conducive to ammonium assimilation. The expression of several AMTs in root hairs further supports a role in nutrient acquisition. These studies provide basic information that will be needed for the dissection of nitrogen uptake by plants at the molecular level and for determining the role of individual AMTs in nutrient uptake and potentially in nutrient efficiency.
Addresses
*Zentrum für Molekularbiologie der Pflanzen, Universität Tübingen, Morgenstelle 1, D-72076 Tübingen, Germany
†Biochimie et Physiologie Moléculaire des Plantes, Ecole Nationale
Supérieure d'Agronomie de Montpellier — Institut National de la Recherche Agronomique — Centre National de la Recherche Scientifique, Place Viala, F-34060 Montpellier, France
‡Corresponding author: e-mail: [email protected] Current Opinion in Plant Biology2000, 3:254–261 1369-5266/00/$ — see front matter
© 2000 Elsevier Science Ltd. All rights reserved. Abbreviations
AMT ammonium transporter Km Michalis–Menten constant MEP methylammonium permease SAT symbiosome ammonium transporter
Introduction: a question of the right mixture
In most soils, NH4+and NO3–are the predominant sources of N that are available for plant nutrition. Although the aver-age NH4+ concentrations of soils are often 10–1000 times lower than those of NO3–(and rarely exceed 50µM) [1], the difference in soil concentrations does not necessarily reflect the uptake ratio of each N source. Indeed, the role of NH4+ in plant nutrition has probably been underestimated, because most plants preferentially take up NH4+when both forms are present — even if NH4+is present at lower con-centrations than NO3– (see below). NH
4+ requires less energy for uptake and assimilation than NO3–, mainly because NO3– has to be reduced prior to assimilation [2]. Optimal plant growth is, however, usually achieved when N
is supplied in both forms [3]. The exclusive supply of N as NH4+is harmful to many plant species, and can cause poor root and shoot growth, and reduced mineral cation contents relative to those of plants receiving NO3–or NH4NO3 nutri-tion [1]. In part, growth depression is directly related to NH4+uptake, as the assimilation of NH
4+is accompanied by about equimolar H+production. These protons are excreted, most probably due to an increased H+-ATPase activity lead-ing to an acidification of the rhizosphere and thus to repressed cation uptake. Another reason for the restricted growth of plants receiving exclusively NH4+-N may be relat-ed to the absence of NO3–. Nitrate is not only an important osmoticum, but also an essential counter-ion for cation translocation in the xylem, and a signal that induces the expression of genes involved in N uptake, N assimilation, organic acid metabolism and starch synthesis [4•]. Moreover, changes in the N source can modify the hormonal balance in the xylem sap, thereby affecting the growth of the shoot [5,6]. Following the transfer of plants from mixed N nutrition to NH4+ alone, newly formed leaves are smaller and have fewer cells than those formed before the change in N nutri-tion; these changes coincide with a strong decline in cytokinin concentrations in the xylem sap [5]. In addition, exclusive NH4+nutrition increases the xylem concentrations of abscisic acid, a hormone that potentially contributes to the stunted growth phenotype [6]. Thus, plants benefit from mixed N nutrition, not only because NO3– usually is more readily available than NH4+but also because of the anionic character, storage and signalling properties of NO3–.
The relatively low soil concentrations of NH4+, its prefer-ential uptake compared to NO3–, and its negative effects on plant growth when supplied as the only N source empha-sise the need to understand how plant roots regulate NH4+ uptake and internal NH4+concentrations. Thus, the aim of this review is to focus on recent progress made in charac-terising the molecular basis of NH4+transport in plants.
Kinetic components in ammonium uptake by
roots
The net uptake of NH4+ by plant roots is the difference between concomitant influx and efflux of this ion [7]. NH4+ influx can be measured by short-term labelling (using either 13NH
4+or 15NH4+) because efflux of the tracer increases with time of exposure. Concentration-dependent influx of NH4+ into intact plant roots exhibits biphasic kinetics that can be separated into at least two distinct components. At <1 mM external NH4+, influx approaches Michalis–Menten kinetics, whereas at higher concentrations, uptake rates seem to increase linearly [8–10]. High-affinity and low-affinity trans-port systems are distinguished by their apparent Km values (i.e. the Michalis–Menten constant; the substrate concentra-tion that allows the reacconcentra-tion or transport process to proceed at one-half of its maximum rate) [9]. This classification implies
The molecular physiology of ammonium uptake and retrieval
that substrate affinity is the most relevant parameter in describing transport systems. High affinity is certainly an important property of a transporter that is responsible for the extraction of nutrients at low external concentrations. Low affinity, however, often correlates with high capacity, which is a crucial parameter for the maintenance of large influxes at high external availability. Thus, a distinction between high-affinity/low capacity and low affinity /high-capacity NH4+ -transport systems reflects their physiological role more pre-cisely than does a distinction based on affinity alone.
The kinetic properties of transport systems measured in whole plants, are highly variable and mainly dependent upon the nutritional status of the plant, which is in turn affected by environmental factors, such as light, tempera-ture and previous external substrate availability. N starvation for a few days results in an increased capacity of plants for NH4+ uptake, which corresponds to a specific stimulation of influx [7,11,12••]. In rice, a decrease in exter-nal NH4+ supply from 1 mM to 2µM during preculture resulted in both a decrease in the Kmand an increase in the Vmax (i.e. the maximum influx) of the high-affinity trans-port system [9]. This is probably an adaptative response of the uptake system to the N demand of the plant, and demonstrates the involvement of feedback regulation that involves changes in both the affinity and capacity of the uptake system. When N availability is limited, root N demand has a clear priority over shoot N demand leading to a rapid decrease in N translocation to the shoot [13].
Using Escherichia coliand yeast mutants, Soupene et al.[14] were able to show that these organisms require NH4+ -trans-port proteins for growth in low NH4+ concentrations only when the external medium has a pH of less than seven. This finding was interpreted as evidence of NH3 transport, because NH3 is the limiting species at low pH. However, this interpretation is not in agreement with reports suggest-ing that NH4+, rather than NH3, is transported in plants [9,15,16]. These studies failed to find any increase in NH4+/NH3uptake, which should be associated with higher NH3concentration in the medium as pH increases, at high pH. Moreover, electrophysiological studies [17–19] have shown that NH4+uptake is related to a strong depolarisation of the membrane potential, suggesting that the charged species (i.e. NH4+) is transported rather than NH3(which can freely diffuse across membranes).
The essentiality of NH
4+retrieval
Although NH4+ reduces growth when supplied as the sole N source for plant nutrition, it is the preferred N source when NH4NO3 is available [12••,20,21]. NH4+is also the main form of N delivered from symbiotic N2 -fix-ing bacteria [22]. Thus, NH4+is a key component of N metabolism and accumulates in mM concentrations in the cytosol, the vacuole, and even in the apoplasm (where it is highly buffered) [23,24]. High intracellular NH4+concentrations result not only from a large NH4+ -uptake capacity in roots [12••], but also from a rapid and
quantitatively important internal breakdown of amino acids, which leads to constant NH4+ leakage from roots at a rate of 11–29% that of NH4+ influx [9,25••]. In leaves, N is also lost in the form of NH3, which is main-ly released during photorespiration and to a minor extent during amino-acid transport and catabolism. The extent of NH3 emission depends on the plant species or culti-var, and increases with apoplastic pH or decreasing activities of the chloroplast glutamine synthetase [26,27]. Thus, plant cells require transport systems at the plasma membrane that mediate both the retrieval of NH3/NH4+ and the initial uptake of external NH4+.
The importance of NH3/NH+4 retrieval systems for cell via-bility has been largely underestimated, but is clearly demonstrated by two observations. First, barley and
Arabidopsismutants that lack either plastidic glutamine syn-thetase or ferredoxin-dependent glutamate synthase activity survive only under high atmospheric CO2concentrations in which photorespiration is repressed [28,29••]. Both of these enzymes are localised in the chloroplast, and so, if the large amount of photorespiratory NH3/NH4+ released from the mitochondria is to be retrieved it must be reimported into the chloroplast either directly from the cytosol or from the leaf apoplasm. This requires at least one transport step. Second, the yeast mutant 31019b that is defective in all three endogenous methylammonium permeases (MEPs), which are members of the AMT/MEP superfamily of NH4+ trans-porters, shows poor growth on any N source. This mutant constantly releases NH3/NH4+, which can be assayed by measuring the growth of other yeast strains on the same plate that require NH3/NH4+leaked from the 31019b mutant cells as a N source [30]. Thus, the 31019b mutant is unable to retrieve the NH4+that is released by amino-acid breakdown. In plants, NH4+-retrieval systems might consist of the same transporters as those mediating initial NH4+uptake from the soil solution or xylem sap. Nevertheless, retrieval systems might also be localised and regulated differently than uptake systems, for example, retrieval systems might also be induced under NO3–supply.
Whether NH4+ leakage or efflux is mediated by mem-brane transporters or largely accounted for by NH3 diffusion is still unclear. Theoretically, the relatively high pH in the cytosol, and especially in the chloroplasts and the mitochondria, should cause significant proportion of the total NH3/NH4+ to be present in the form of NH
Ammonium transport in yeast as a model for
other organisms
NH4+-uptake studies in yeast and the isolation of yeast mutants that are resistant to the toxic NH4+ analogue methylammonium have provided evidence of the exis-tence of several distinct NH4+ transport systems that differ in substrate affinity [33]. Functional complementa-tion of the yeast mutant 26972c (mep1-1 ;mep2-1), which is defective in two NH4+-uptake systems, allowed the isolation of the genes encoding the corresponding NH4+ -transporters, MEP1 and MEP2. Both of these genes
encode high-affinity transporters with Km values of 5–10µM and 1–2µM, respectively [30,34]. Although 26972c was unable to grow on 1 mM NH4+as the sole N source, the double knock-out mutant (mep1∆;mep2∆) did grow on this N concentration. This observation provided evidence of the existence of a third NH4+transporter that is differentially regulated in 26972c and the double knock-out mutant. Indeed, genome sequencing revealed the existence of a third homolog, MeP3, which encodes a transporter that has a much lower substrate affinity (Km= 1.4 – 2.1 mM) than Mep1p and Mep2. The reason for Mep3p inactivity in 26972c, was identified as an amino-acid substitution in the carboxy-terminus of Mep1p, that trans-inhibits Mep3p at the post-translational level [35••]. Moreover, substitution of the same amino acid in Mep3p also resulted in a loss of transport activity, and transformation of yeast with mutated MEP3 trans -inhibited native Mep3p [35••]. These observations are consistent with direct interaction between the Mep proteins.
All three MEPgenes are controlled by N, being repressed in the presence of an N source that is readily metabolised, such as NH4+ [30]. In response to N starvation, MEP2 expression increases strongly; at the same time a morpho-logical adaptation, that is the filamentous outgrowth of pseudohyphae, allows the yeast cells to better forage for the substrate. This morphological response is absent in diploid mep2∆ mutant strains, suggesting that the NH4+ transporter Mep2p functions in pseudohyphal differentia-tion, probably as an NH4+ sensor [36••]. Although experimental evidence of similar sensing functions in plants is still poor they probably do exist; yeast therefore provides an attractive tool for the identification of plant transport and sensing mechanisms.
Complementation of yeast mutants that are defective in NH4+uptake was used for the first isolation of the genes encoding NH4+transporters in yeast and plants (see above) [34,37]. The sequences of these NH4+ transporters were also the basis for the first identification of NH4+ trans-porters from bacteria [38]. Moreover, the sequences of the NH4+transporters that were uncovered share significant sequence similarity with the Rhesus blood group polypep-tides. This finding raises the possibility that Rhesus proteins might themselves be NH4+ transporters, or that they might originate from NH4+transporters and perform some other related function [39].
Plant genes encoding ammonium transporters
Heterologous complementation of the double mutant yeast strain 26972c (mep1-1;mep2-1) with cDNAs from an
Arabidopsis thalianalibrary allowed the isolation of the first plant NH4+ transporter, AtAMT1;1 (Arabidopsis thaliana ammonium transporter 1;1; GenBank accession number X75879 [37]). The screening of DNA libraries and PCR-based approaches subsequently led to the isolation of further members of the AMT1 gene family in A. thaliana, namely AtAMT1;2 (accession number AF083036), Figure 1
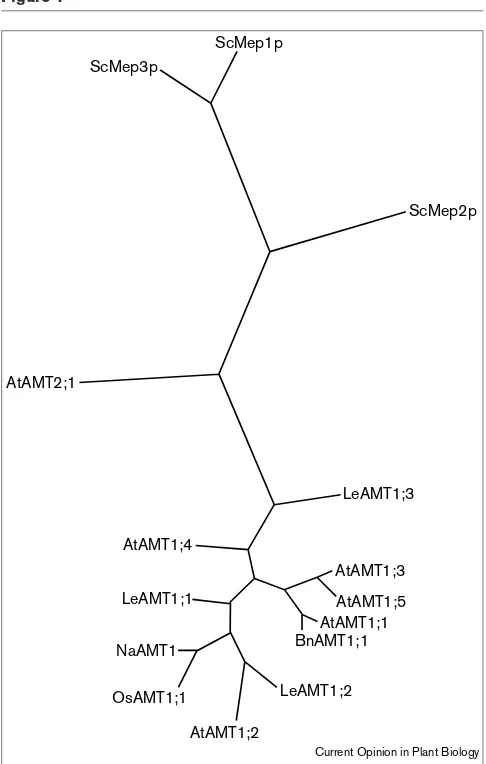
Phylogenetic tree of ammonium transporters of the AMT/MEP gene family in yeast (Saccharomyces cerevisiae [Sc]) and plants (Arabidopsis thaliana [At], Lycopersicon esculentum [Le], Oryza sativa [Os], Brassica napus [Bn] and Nepenthes alata [Na]). Parsimony analysis and heuristic tree search was performed using PAUP 4d65 (Swofford, 1997). GeneBank accession numbers are: ScMep1p, ScMep2p, ScMep 3p: 730015, X83608, T38466, respectively; AtAMT1;1, AtAMT1;2, AtAMT1;3, AtAMT1;4, AtAMT1;5: X758879, AF083036, AF083035; AL35353, AP000382,
respectively; AtAMT2;1: AC003028; BnAMT1;1: AAF01774 (partial sequence); LeAMT1;1, LeAMT1;2, LeAMT1;3: X92854, X95098, AF118858, respectively; OsAMT1;1 AF001505; and NaAMT1: 4322319 (partial sequence).
LeAMT1;3
LeAMT1;1
LeAMT1;2 AtAMT1;3 AtAMT1;4
AtAMT2;1
AtAMT1;5 AtAMT1;1
AtAMT1;2 OsAMT1;1
NaAMT1 BnAMT1;1
ScMep2p ScMep1p
ScMep3p
AtAMT1;3 (accession number AF083035), AtAMT1;4
(accession number AL35353) and AtAMT1;5 (accession number AP000382) ([12••]; S Gazzarrini, N von Wirén, unpublished data). AtAMT1;1and AtAMT1;4 map to chro-mosome IV, AtAMT1;2 maps to chromosome I and
AtAMT1;3 and AtAMT1;5 map to chromosome III (S Gazzarrini, N von Wirén, unpublished data). The AtAMT1;3 and AtAMT1;5 proteins show the greatest homology to each other with >90% similarity at the amino-acid level, whereas the sequence of AtAMT1;1 shows about 80% homology to AtAMT1;3 and AtAMT1;5. AtAMT1;2 and AtAMT1;4 are more distantly related with ~70% similarity to the other AtAMTs (Figure 1).
Recently, a new gene, given the preliminary name
AtAMT2;1 (accession number AC003028), was identified fromA. thaliana. The protein encoded by AtAMT2;1has a sequence that shares greater similarity with those of the yeast Mep proteins than with those of the A. thaliana
AMT1 transporters. Although data on the function and regulation of AtAMT2;1are not yet available, it is speculat-ed that this gene is a member of a new subfamily exhibiting specific functions (see ‘Update’).
Additional AMT1homologs have been isolated from other plant species: one from rice (OsAMT1;1, [40]), and three from tomato (LeAMT1;1-3, [41,42••]). In contrast to LeAMT1;1 and LeAMT1;2, which share 76% amino acid sequence sim-ilarity, LeAMT1;3 is the most distantly related AMT1 member described so far (<63% similarity to any other plant AMT) and carries two unique structural characteristics. First, LeAMT1;3 has an extremely short amino-terminus of 14 amino acids, compared to 35–54 amino acids in the amino-termini of other AMT members. Second, the mRNA of LeAMT1;3 possesses two untranslated open reading frames in front of the main open reading frame. These additional untranslated open reading frames are thought to control the translational efficiency of the mRNA [43], a feature which has so far been associated with regulatory genes controlling growth and development [44]. Although the consequences of these structural differences have yet to be examined in detail, they may point to a different post-transcriptional reg-ulation of LeAMT1;3 and maybe even to a different functional role for the product of this gene.
Computer-based predictions suggest that the AMTs share a structure with 11 transmembrane helices (Figure 2). This is in agreement with the experimentally determined topol-ogy of the AMT/MEP proteins from yeast and E. colithat included an extracytosolic amino-terminus (AM Marini, B André, personal communication; [45]).
Function of ammonium transporters in plants
When expressed in the yeast mutant 26972c, AtAMT1;1 shows properties that are similar to those of Mep1p, such as a negligible permeability for di- and trimethylamine and for other monovalent cations, including K+. Furthermore, the activity of both Mep1p and AtAMT1;1 strongly
depends on a proton motive force, having a pH optimum of 7.0 [37]. A strong inhibition of high-affinity NH4+ trans-port by protonophores and ATPase inhibitors has also been observed in rice roots [19]. Whether the membrane depo-larisation upon NH4+transport and its dependence on the proton motive force result from a H+–NH
4+ symport or NH4+ uniport remains an unanswered question. Electrophysiological studies on AMT1-mediated NH4+ transport in Xenopus oocytes might answer this question but have been unsuccessful so far (A Miller, W-N Fischer, personal communication).
Heterologous expression of AtAMT1;1, AtAMT1;2 and AtAMT1;3, in the yeast triple knock-out strain 31019b (∆mep1;∆mep2;∆mep3) has been used to compare the kinetic properties of these three Arabidopsis proteins. The use of 310196 avoids a possible suppression of trans-port activity that is mediated by Mep3p as was observed in the strain 26972c [35••]. In uptake studies using 14 C-labeled methylammonium and competition by NH4+, AtAMT1;2 and AtAMT1;3 were found to have Kmvalues of 11–40µM for both methylammonium and NH4+, whereas AtAMT1;1 discriminated between the two sub-strates and had Km values of 8µM and <0.5µM for methylammonium and NH4+, respectively [12••]. Thus,
Arabidopsis roots have three NH4+transporters that vary in their substrate affinities, indicating that their biochem-ical properties regulate the high-affinity transport of NH4+ [12••]. The reduced or absent selectivity of AtAMT1;2 and AtAMT1;3 against methylammonium might explain why methylammonium can be a competent
Figure 2
Structural model of AtAMT1;1 with 11 transmembrane-spanning domains. Carboxy-terminal Amino-terminal NH4
+
NH4+
Current Opinion in Plant Biology Outside
inhibitor of NH4+ uptake, especially when methylammo-nium is present at higher external concentrations [46].
Experimental evidence of the physiological function of NH4+transporters in vivois mainly derived from studies of the correlation between AMT1 paralog expression and 15NH
4+influx [12••]. Transfer of plants to N-free nutrient solution steeply increased 15NH
4+influx into roots within two days; this increased 15NH
4+ influx coincided with an increase in AtAMT1;1 transcript levels, whereas transcrip-tion of AtAMT1;2 and AtAMT1;3 remained constant and only slightly increased, respectively. AtAMT1;1 is the only one of the three AMT transporters that has a substrate affinity in the nM range; hence, these results indicate that N deficiency induces the transporter that has the highest substrate affinity [12••]. When N-starved plants were resupplied with NH4NO3, AtAMT1;1 mRNA levels and 13NH
4+influx declined rapidly unless the assimilation of NH4+ to glutamine was inhibited by methionine sulfox-imine [47••]. Moreover, a negative correlation between 13NH
4+ influx and glutamine concentration in roots sug-gested that glutamine might be the feedback signal for repression of NH4+influx and AtAMT1;1 transcription fol-lowing N resupply. This hypothesis is also supported by studies in tomato roots with LeAMT1;1, the probable ortho-logue of AtAMT1;1 [42••]. In addition, NH
4+influx might be directly repressed by internal NH4+ concentrations because NH4+ influx responds more rapidly than AtAMT1;1 mRNA levels to N resupply [47••].
In tomato roots, two transporter genes (i.e. LeAMT1;1and
LeAMT1;2) are preferentially expressed in root hairs, sug-gesting that their gene products have a role in NH4+ acquisition from the rhizosphere. LeAMT1;1mRNA is most
abundant when N availability is restrictive to growth— as is AtAMT1;1 mRNA— whereas transcription of LeAMT1;2
is induced by the supply of NH4+and even more so by the supply of NO3– [41,42••]. Similar responses have not yet been observed for any of the AMT1 transporters in
A. thaliana. The opposite responses of LeAMT1;1 and
LeAMT1;2 to N supply indicates that different NH4+ trans-porters are required to meet the plant’s demand for N when growing in N-poor and N-rich soil.
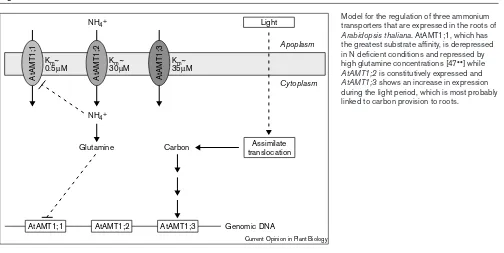
NH4+ influx also shows a diurnal pattern with maximal uptake at the end of the light period and a strong decrease after offset of light ([12••]; L Lejay, A Gojon, unpublished data). All three AtAMT1 genes showed diur-nal variation in root expression; but only AtAMT1;3
transcript levels peaked simultaneously with 15NH 4+ influx at the end of the light period, suggesting that the regulation of AtAMT1;3 may provide a link between NH4+uptake and carbon provision in roots [12••]. This is confirmed by the observation that AtAMT1;3expression is sugar-inducible (L Lejay, A Gojon, unpublished data). The fact that N starvation and light, do not affect the expression of the same AtAMT1genes, suggests that the regulation of NH4+uptake by either the N- or C-status of the plant may involve distinct sensing/signalling mechanisms (Figure 3).
Two of the tomato AMT1 genes are diurnally regulated in leaves. Expression of LeAMT1;2 and LeAMT1;3 showed reciprocal diurnal patterns with highest LeAMT1;3 tran-script levels occurring during the dark period and the highest LeAMT1;2 transcript levels occurring after the onset of light [42••]. These results might indicate that LeAMT1;2 is involved in the uptake of xylem-derived
Figure 3
Model for the regulation of three ammonium transporters that are expressed in the roots of
Arabidopsis thaliana. AtAMT1;1, which has the greatest substrate affinity, is derepressed in N deficient conditions and repressed by high glutamine concentrations [47••] while
AtAMT1;2is constitutively expressed and
AtAMT1;3shows an increase in expression during the light period, which is most probably linked to carbon provision to roots.
AtAMT1;1 AtAMT1;2 AtAMT1;3
Km~ 30µM Km~
0.5µM
Km~ 35µM
NH4+
Apoplasm
Cytoplasm
NH4+ Light
Assimilate translocation
Glutamine Carbon
Genomic DNA AtAMT1;2
AtAMT1;1 AtAMT1;3
NH4+or in the retrieval of photorespiratory NH3. In con-trast, LeAMT1;3 might be required for transport or retrieval of NH4+during the dark period when NH4+could be released from reactions involving other light-repressed enzymes, such as asparagine synthetase and glutamate dehydrogenase [48,49•].
The role of ammonium transporters in nitrogen
fixation
NH4+is probably the main form of N exported from symbi-otic N2-fixing microorganisms to their host plants and therefore contributes to the N nutrition of several plant fami-lies [22]. In root nodules, NH4+ is transported across the symbiosome membrane that separates the bacteroids from the plant cytosol. NH4+concentrations in the plant cytosol can be approximately 50-times lower than in the bacteroids [50]; hence the plant must have high-capacity transport sys-tems to ensure an efficient import of microbially fixed N [51].
Electrophysiological studies of legume symbiosome mem-branes, identified a monovalent cation channel with preference for NH4+ that most probably represents the main route of N export from the symbiosome to the plant [51]. In an attempt to isolate a corresponding NH4+ trans-porter gene from soybean, the yeast mutant 26972c (mep1-1;mep2-1) was complemented with a cDNA library prepared from soybean nodules [52••]. Western blot analy-sis localised the GmSAT1 protein in the symbiosome membrane and patch-clamp studies with yeast spherob-lasts expressing SAT1, showed an inward current of NH4+, which was inhibited by Ca2+. The unusual structure of SAT1, which includes only one transmembrane domain and sequence stretches with a high homology to basic helix–loop–helix motifs has, however, raised doubts about its function as an NH4+transporter. Indeed, recent experi-ments have shown that GmSAT1 does not complement NH4+ transport in 31019b but interferes with the inhibi-tion of Mep3p in the mutant 26972c [35••], indicating that GmSAT1 by itself is not a functional NH4+ transporter. Nevertheless, because of its ability to interact with Mep3p, GmSAT1 might play an interesting role in the post-trans-lational regulation of NH4+ transporters in soybean. Moreover, the postulated regulatory function of SAT1 is not restricted to legumes, as SAT1 homologs have also been found in Arabidopsis.
Conclusions and perspectives
In the past heterologous complementation of yeast mutants that are defective in NH4+transport provided the key methodology for the identification of NH4+ trans-porters in plants. Heterologous expression in yeast allowed researchers not only to prove functionality but also to char-acterize the biochemical properties (e.g. substrate affinity, substrate specificity and energy dependence) of isolated NH4+-transport proteins. Furthermore, detailed expres-sion analyses of NH4+-transporter genes allowed researchers to gain an idea about how transport processes are mediated by the corresponding gene products.
Bearing in mind the extended number of homologous genes within the AMT gene family, however, these meth-ods are not sufficient to assign physiological functions to the individual transporters. In future, therefore, key tech-nologies will be the generation of antisense plants and the isolation of insertional mutants that are defective in the expression of individual transporter genes. Growth tests on different media and NH4+-influx studies with antisense lines, insertional mutants and AMT-transformed mutants are then expected to allow the dissection of the physiolog-ical functions of the individual transporter genes. In addition, the crossing of mutants will be important in investigating the extent to which homologous genes can compensate for lost transporter functions. Furthermore, methodological progress, for example constructing new yeast mutants or developing more refined screening proce-dures for complemented yeast mutants, is required to identify new NH4+ transporters that mediate low affini-ty/high capacity transport of NH4+or that are involved in NH4+ sensing. These physiologically-oriented studies should be accompanied by a more profound molecular knowledge, in particular on structure–function relations and possible interactions between individual NH4+ trans-porters or with other regulatory proteins. A promising start in this direction has already been made.
Update
Most recently, AtAMT2 has been functionally expressed in a yeast triple Mep mutant (mep1∆; mep2∆; mep3∆) and shown to complement NH4+ uptake at 1 mM external NH4+. Interestingly, transformation of the yeast mutant with
AtAMT2 did not confer methylammonium toxicity, while transformation with AtAMT1;1 did. The authors concluded that AtAMT2 cannot transport methylammonium [53].
Acknowledgements
A large part of our work was supported by the European Community BIOTECH4 program EURATINE, the Bundesministerium für Bildung und Forschung, Bonn; and the KWS Saat AG, Einbeck, Germany.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
••of outstanding interest
1. Marschner HL: Mineral Nutrition in Higher Plants. London: Academic Press; 1995.
2. Bloom AJ, Sukrapanna SS, Warner RL: Root respiration associated with ammonium and nitrate absorption and assimilation by barley.
Plant Physiol 1992, 99:1294-1301.
3. Bloom AJ, Jackson LE, Smart DR: Root growth as a function of ammonium and nitrate in the root zone. Plant Cell Environ1993,
16:199-206.
4. Stitt M: Nitrate regulation of metabolism and growth.Curr Opin
• Plant Biol 1999, 2:178-186.
An excellent review on the signalling role of nitrate in nitrogen and carbon metabolism, assimilate allocation and root architecture.
5. Walch-Liu P, Neumann G, Bangerth F, Engels C: Rapid effects of nitrogen form on leaf morphogenesis in tobacco. J Exp Botany
2000, in press.
The flows of cations, chloride and abscisic acid. New Phytol 1998,
140:625-636.
7. Morgan MA, Jackson WA: Inward and outward movement of ammonium in root systems: transient responses during recovery from nitrogen deprivation in presence of ammonium.J Exp Botany
1988, 39:179-191.
8. Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A: Ammonium uptake in Lemna gibbaG1, related membrane potential changes, and inhibition of anion uptake.Physiol Plant 1984, 61:369-376. 9. Wang MY, Siddiqi MY, Ruth TJ, Glass ADM: Ammonium uptake by
rice roots. II. Kinetics of 13NH
4+influx across the plasmalemma.
Plant Physiol 1993, 103:1259-1267.
10. Kronzucker HJ, Siddiqi MY, Glass ADM: Kinetics of NH4+influx in spruce.Plant Physiol 1996, 110:773-779.
11. Glass ADM, Siddiqi MY: Nitrogen absorption by plant roots.In
Nitrogen Nutrition of Higher Plants. Edited by Srivastava HS, Singh RP. New Delhi: Associated Publishing Co.; 1995:21-56. 12. Gazzarrini S, Lejay L, Gojon A, Ninnemann O, Frommer WB,
•• von Wirén N: Three functional transporters for constitutive, diurnally regulated and starvation-induced uptake of ammonium into Arabidopsisroots. Plant Cell1999, 11:937-948.
The authors report the isolation of two new AMT1 genes that are homolo-gous to AtAMT1;1. Uptake of 14C-methylammonium in AMT-transformed
yeast cells and inhibition by NH4+reveal that AtAMT1;1 possesses a
sub-strate affinity in the nM range, whereas AtAMT1;2 and AtAMT1;3 have a mM Km. Expression of AtAMT1;1positively correlates with 15NH4+influx into
roots growing in N-deficient soils: whereas AtAMT1;3 transcript levels vary diurnally and increase with increasing 15NH
4+influx during daylight hours.
Thus, NH4+uptake in Arabidopsis is regulated both at the transcriptional
level and by the biochemical properties of the transporters. 13. Kronzucker HJ, Schjoerring JK, Erner Y, Kirk GJD, Siddiqi MY,
Glass ADM: Dynamic interactions between root NH4+influx and long-distance N translocation in rice: insights into feed-back processes. Plant Cell Physiol1998, 39:1287-1293.
14. Soupene E, He L, Yan D, Kustu S: Ammonia acquisition in enteric bacteria: physiological role of the ammonium/methylammonium transport B (AmtB) protein.Proc Natl Acad Sci USA1998,
95:7030-7034.
15. MacFarlane JJ, Smith FA: Uptake of methylamine by Ulva rigida: transport of cations and diffusion of free base. J Exp Botany1982,
33:195-207.
16. Dyr-Jensen K, Brix H: Effects of pH on ammonium uptake by Typha latifoliaL.Plant Cell Environ 1996, 19:1431-1436.
17. Walker NA, Smith FA, Beibly MJ: Amine uniport at the plasmalemma of charophyte cells. II. Ratio of matter to charge transported and permeability of free base.J Membr Biol 1979, 49:283-296. 18. Ayling SM: The effect of ammonium ions on membrane potential
and anion flux in roots of barley and tomato.Plant Cell Environ
1993, 16:297-303.
19. Wang MY, Glass ADM, Shaff JE, Kochian LV: Ammonium uptake by rice roots. III. Electrophysiology.Plant Physiol1994, 104:899-906. 20. Gojon A, Soussana JF, Passama L, Robin P: Nitrate reduction in
roots and shoots of barley (Hordeum vulgareL.) and corn (Zea maysL.) seedlings. I. 15N study.Plant Physiol 1986, 82:254-260.
21. Geßler A, Schneider S, von Sengbusch D, Weber P, Hanemann U, Huber C, Rothe A, Kreutzer K, Rennenberg H: Field and laboratory experiments on net uptake of nitrate and ammonium by the roots of spruce (Picea abies) and beech (Fagus sylvatica) trees. New Phytol1998, 138:275-285.
22. Udvardi MK, Day DA: Metabolite transport across symbiotic membranes of legume nodules.Annu Rev Plant Physiol Plant Mol Biol1997, 48:493-523.
23. Wells D, Miller AJ: Intracellular measurement of ammonium in
Chara corallinausing ion-selective microelectrodes.Plant Soil
2000, in press.
24. Nielsen KH, Schjoerring JK: Regulation of apoplastic NH4+ concentration in leaves of oilseed rape. Plant Physiol 1998,
118:1361-1368.
25. Feng J, Volk RJ, Jackson WA: Source and magnitude of ammonium •• generation in maize roots. Plant Physiol1998, 118:835-841. Exposing maize roots to 15NH4+, and subsequent analysis of the 14N and 15N
fractions in the roots, showed that 14NH4+efflux over a 3 d period coincided
with a decline in soluble organic 14N in roots while insoluble 14N remained
constant. Thus, catabolism of soluble organic N, rather than protein N, is the primary source of endogenous NH4+generation in roots.
26. Mattsson M, Häusler RE, Leegood RC, Lea PJ, Schjoerring JK:
Leaf-atmosphere NH3exchange in barley mutants with reduced activities of glutamine synthetase. Plant Physiol1997, 114:1307-1312. 27. Pearson J, Clough ECM, Woodall J, Havill DC, Zhang XH: Ammonia
emission to the atmosphere from leaves of wild plants and
Hordeum vulgaretreated with methionine sulfoximine. New Phytol
1998, 138:37-48.
28. Wallsgrove RM, Turner JC, Hall NP, Kendally AC, Bright SWJ: Barley mutants lacking chloroplast glutamine synthetase — biochemical and genetic analysis. Plant Physiol 1987, 83:155-158.
29. Coschigano KT, Melo-Oliveira R, Lim J, Coruzzi GM: Arabidopsisgls •• mutants and distinct Fd-GOGAT genes: implications for
photorespiration and primary nitrogen assimilation.Plant Cell
1998, 10:741-752.
The authors report the isolation of two genes, GLU1 and GLU2, encoding ferredoxin-dependent glutamate synthases in Arabidopsis. These genes are distinctly regulated and their contrasting expression patterns suggest that GLU1 plays a major role in photorespiration and primary nitrogen assimilation in leaves, whereas GLU2 may be responsible for primary nitrogen assimilation in roots. This work is an excellent example of a successful combination of genetic approaches and molecular tools to elucidate gene function in plants. 30. Marini AM, Soussi-Boudekou S, Vissers S, André B: A family of
ammonium transporters in Saccharomyces cerevisiae. Mol Cell Biol1997, 17:4282-4293.
31. Asman WAH, Sutton MA, Schloerring JK: Ammonia: emission, atmospheric transport and deposition. New Phytol 1998, 139:27-48. 32. Kronzucker HJ, Siddiqi MY, Glass ADM, Kirk JD: Nitrate-ammonium • synergism in rice. A subcellular flux analysis.Plant Physiol 1999,
119:1041-1045.
The authors show that NH4+plasma membrane fluxes and NH4+metabolism
in rice are enhanced by the presence of NO3–, whereas NO3–fluxes and
metabolism are strongly repressed by NH4+.
33. Dubois E, Grenson M: Methylamine/ammonia uptake systems in
Saccharomyces cerevisiae: multiplicity and regulation. Mol Gen Genet1979, 175:67-76.
34. Marini AM, Vissers S, Urrestarazu A, André B: Cloning and
expression of the MEP1gene encoding an ammonium transporter in Saccharomyces cerevisiae. EMBO J 1994, 13:3456-3463. 35. Marini AM, Springael J-Y, Frommer WB, André B: Cross-talk •• between ammonium transporters in yeast and interference by the
soybean SAT1 protein.Mol Microbiol2000, 35:378-385.
The authors demonstrate a trans-inhibitory effect of a mutated NH4+
trans-porter Mep1p on the transport activity of Mep3p in yeast. GmSAT1, which has been proposed to be an NH4+transporter from soybean [52••], does not
complement a yeast mutant that is defective in all three Mep proteins, but does complement a yeast mutant in which only Mep3p is inactive. Thus, GmSAT1 is probably not an NH4+transporter itself, but interferes with the
inhibition of Mep3p.
36. Lorenz MC, Heitman J:The MEP2 ammonium permease regulates •• pseudohyphal differentiation in Saccharomyces cerevisiae.
EMBO J1998, 17:1236-1247.
The presence of the yeast NH4+transporter Mep2p in yeast is essential for
the development of pseudohyphae as part of the nitrogen starvation stress response. Thus, Mep2p can be regarded as an NH4+sensor that links
exter-nal substrate availability to a morphological response.
37. Ninnemann O, Jauniaux JC, Frommer WB: Identification of a high affinity ammonium transporter from plants. EMBO J 1994,
13:3464-3471.
38. Siewe RM, Weil B, Burkovski A, Eikmanns BJ, Eikmanns M, Krämer R:
Functional and genetic characterisation of the (methyl)ammonium uptake carrier of Corynebacterium glutamicum.J Biol Chem1996,
271:5398-5403.
39. Marini AM, Urrestarazu A, Beauwens R, André B: The Rh (Rhesus) blood group polypeptides are related to NH4+transporters. TIBS
1997, 22:460-461.
40. von Wirén N, Bergfeld A, Ninnemann O, Frommer WB: OsAMT1-1: a high-affinity ammonium transporter from rice (Oryza sativacv. Nipponbare).Plant Mol Biol1997, 3:681.
41. Lauter FR, Ninnemann O, Bucher M, Riesmeier JW, Frommer WB:
putative nitrate transporters in root hairs of tomato. Proc Natl Acad Sci USA 1996, 93:8139-8144.
42. von Wirén N, Lauter FR, Ninnemann O, Gillisen B, Walch-Liu P,
•• Engels C, Jost W, Frommer WB: Differential regulation of three functional ammonium transporter genes by nitrogen in root hairs and by light in leaves of tomato. Plant J 2000, 21:167-175. The authors describe the isolation of LeAMT1;2and LeAMT1;3from toma-to. LeAMT1;3is a new AMT member with exceptional structural features. Expression analysis of all three AMT1 genes reveals that LeAMT1;1and
LeAMT1;2are oppositely regulated by N supply in roots with LeAMT1;2
being induced by NH4+and NO
3–. In leaves, LeAMT1;2 and LeAMT1;3 are
reciprocally regulated by daylight with LeAMT1;3 showing its highest expression levels during dark periods.
43. Wang L, Wessler SR: Inefficient reinitiation is responsible for upstream open reading frame-mediated translational repression of the maize Rgene.Plant Cell 1998, 10:1733-1745.
44. Kozak M: An analysis of 5’-noncoding sequences from 699 vertebrate messenger RNAs.Nucleic Acids Res1987, 15:8125-8148. 45. Thomas G, Coutts G, Merrick M: The glnKamtBoperon: a
conserved gene pair in prokaryotes. Trends Genet 2000, 16:11-14. 46. Kosola KR, Bloom AJ: Methylammonium as a transport analog for
ammonium in tomato (Lycopersicon esculentumL.).Plant Physiol
1994, 105:435-442.
47. Rawat SR, Silim SN, Kronzucker HJ, Siddiqi MY, Glass ADM: AtAMT1
•• gene expression and NH4+uptake in roots of Arabidopsis
thaliana: evidence for regulation by root glutamine levels. Plant J
1999, 19:143-152. Correlation studies relating 13NH
4+influx into Arabidopsis roots to AtAMT1;1
gene expression show a downregulation of AtAMT1;1and NH4+influx after
NH4+resupply to N-starved plants. A similar effect can also be achieved by
provision of glutamine, or NH4NO3plus a glutamine synthetase inhibitor. Both AtAMT1;1transcript levels and NH4+influx are negatively correlated with root
glutamine concentrations suggesting that glutamine via AtAMT1;1 is the sig-nal for transcriptiosig-nal downregulation of NH4+influx after N resupply.
48. Lam HM, Coschigano K, Schultz C, Melo-Olivira R, Tjaden G, Oliveira I, Ngai N, Hsieh MH, Coruzzi G: Use of Arabidopsis
mutants and genes to study amide amino acid biosynthesis. Plant Cell 1995, 7: 887-898.
49. Lam HM, Hsieh MH, Coruzzi G: Reciprocal regulation of distinct • asparagine synthetase genes by light and metabolites in
Arabidopsis thaliana.Plant J1998, 16:345-353.
Expression analysis of two asparagine synthase genes (ASN1and ASN2) shows that ASN1 transcript levels are reduced by light and sugar but induced by amino acids, whereas the reverse is true for ASN2. This is the first example of a reciprocal regulation by light and metabolites of two homol-ogous genes involved in N and C metabolism.
50. Streeter J: Estimation of ammonium concentration in the cytosol of soybean nodules. Plant Physiol 1989, 90:779-782.
51. Tyerman SD, Whitehead LF, Day DA: A channel-like transporter for NH4+on the symbiotic interface of N2-fixing plants. Nature 1995, 378:629-632.
52. Kaiser BN, Finnegan PN, Tyerman SD, Whitehead LF, Bergersen FJ,
•• Day DA, Udvardi MK: Characterisation of an ammonium transport protein from the peribacteroid membrane of soybean nodules.
Science 1998, 281:1202-1206.
The authors isolated a novel gene GmSAT1 from nodulated soybean roots.
GmSAT1 is preferentially transcribed in nodules and Western analysis shows that it is localised to the peribacteroid membrane. GmSAT1 comple-ments NH4+uptake in a yeast mutant that is defective in NH4+transport and
mediates NH4+-induced currents when expressed in oocytes. This evidence
suggests that GmSAT1 functions as an NH4+transporter. A deeper analysis
of GmSAT1, however, indicates that it may not act as an NH4+transporter
but rather interferes with endogenous yeast NH4+transporters (AM Marini,
B André, personal communication).
53. Sohlenkamp C, Shelden M, Howitt S, Udvardi M: Characterization of
Arabidopsis AtAMT2, a novel ammonium transporter in plants.