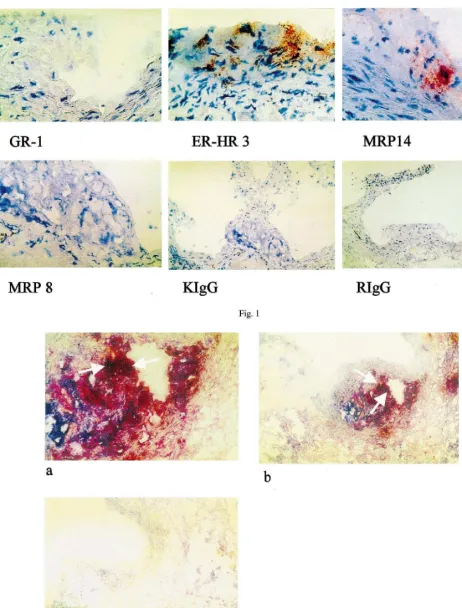
Keywords: Atherosclerosis; Apolipoprotein E null mice; S100 proteins; Macrophages; Monocytes; MRP14; S100A9
The consequences of atherosclerotic plaque forma-tion result in a number of diseases of the cardiovascular system which represent a serious health problem and major cause of death in the western world. Despite a growing research interest the pathogenesis of atherosclerosis especially regarding the initial steps of the disease and the participation of immune cells is not fully understood yet. Since these investigations are hard to manage in humans the development of animal mod-els which closely resemble human conditions has long been an important research goal. Mice deficient in production of apolipoprotein (Apo) E are a useful model since they develop advanced atherosclerotic le-sions at several locations throughout the arterial tree which show the typical features of those in man: early fatty streak formation, later calcification and develop-ment of necrotic cores and fibrous caps [1 – 3]. ApoE
− / − mice were generated by gene targeting [4 – 6] and develop advanced atherosclerotic lesions and hyper-cholesterolaemia under a normal chow diet at about 6 – 8 months age. This animal model is widely used in recent studies about the pathomechanisms of atherosclerosis [6 – 13].
The interest of this study focused on the cellular infiltration of atherosclerotic lesions at different time points and the functional characterization of these cells. The appearance of S100 protein expressing immune cells in atherosclerotic lesions has been reported in recent literature for mice, rats and humans [14 – 16] and raises the question whether these cells participate in proatherogenic mechanisms. Myeloid related proteins (MRP) 8 and 14 belong to the class of S100 calcium binding proteins (S100A8 and S100A9) and represent peripheral marker proteins for acute and chronic infl-ammation. In a number of inflammatory diseases and animal models a subpopulation of monocytes
express-plying a function in the initial steps of inflammation. Leukocytes positive for MRP8 and 14 have been found adjacent to activated endothelium [19] and, as was recently shown, the proteins are released from mono-cytes after contact to activated endothelium. MRP8 and MRP14 are cytosolic proteins occurring both in granulocytes and monocytes. They are translocated to the cell membrane after activation as it occurs under inflammatory conditions where they form a het-erodimer complex [20]. Secreted MRP proteins may modulate adhesion and transendothelial migration properties of leukocytes to activated endothelium by regulating Mac-1 (CD11b) affinity [21]. The develop-ment of atherosclerotic lesions results from an excessive response to various forms of endothelium injury and smooth muscle proliferation of the arterial wall, whereas a number of cytokines, growth factors and vasoactive molecules are involved which are also typical for early reactions during acute inflammations. From the authors’ knowledge about the migration behavior of MRP expressing cells in inflammatory models a function of these cells was proposed during atherogene-sis as well. That is why one was interested in the time course and phenotypic analysis of the infiltrating MRP expressing neutrophils.
The aorta ascendence was prepared from the animals under a dissection microscope as described earlier [22]. The tissue was thoroughly washed in phosphate buffered saline, mounted in OCT compound (Miles, Elkhart, Ind) and shock frozen in liquid nitrogen. Sec-tions (5 mm) were prepared and acetone fixed for 10 min. The sections were stained with the appropriate antibodies in a concentration of 1 mg/ml and counter stained with Mayer’s Ha¨malaun. Light microscopy was performed for morphological observations and cellular infiltration was quantified by counting using a micro-scopic grid.
Interestingly enough, mainly MRP14 expressing cells were found in the atherosclerotic lesions of 6 months old ApoE − / − mice, whereas MRP8 positive cells very rarely occured in the plaques (Fig. 1). Equivalent binding of both antibodies to mononuclear cells have been checked in control experiments so that the real
Fig. 1. Immunohistochemical staining of atherosclerotic lesions of 6-month-old ApoE − / − mice with Gr-1 (granolocyte marker), ERHR-3 (monocyte/macrophage marker), anti-MRP14, anti-MRP8 and isotype controls in the appropriate dilutions (1mg/ml). Magnification:×160 (IgG, Gr-1),×400 (ERHR-3, MRP8 and 14).
colocalization to MRP14 positive cells (Fig. 1). In double labeling experiments using a peroxidase labeled secondary antibody to detect MRP14 (red colour) and alkaline phosphatase for ER-HR 3 detection (blue colour) MRP14 expressing cells were identified as monocytes (Fig. 2).
In order to study the time course of cellular infiltra-tion the number of MRP14 expressing cells were deter-mined per lesion in three groups of ApoE − / − mice differing in age from 3 to 12 months. To quantify
Six-month-old mice showed several atherosclerotic plaques of massive thickness at the aorta ascendence, which were densely populated by monocytes expressing MRP14 but not MRP8. At an age of 12 months the average plaque size seemed to be diminished and so did the number of infiltrated MRP14 positive monocytes. For further studies 6 – 7 months was chosen as an optimum age.
Finally, the phenotype of infiltrating cells, especially regarding their maturation/differentiation state was
de-Table 1
Time schedule of atherosclerotic lesion formation infiltrated with myeloid related protein (MRP) 14+cellsa
No. animals per
Mice Age (months) Progession of atherosclerotic Infiltration with MRP14+cells group lesion formation (cell number per microscopic field)
− 0
3 6
C57Bl6 (controls)
ApoE−/− 3 3 c1− 0
c2+ 8
c3+/− 5
2493
c1+++
6 ApoE−/− 6
c2+++ 2894
1992
c3+++
c4+++ 2595
c5+++ 2995
c6+++ 3293
ApoE−/− 3 12 c1+ 1092
1595
c2+++
c3++ 992
aScore for estimation of plaque size:−, no visible endothelial changes;+/−, beginning of singular small lesions;+, small but frequently
appearing lesions;++, clear atherosclerotic plaque formation, numerous per aorta;+++, massive thick lesions at several locations in the vessel.
Table 2
Phenotype of infiltrating cells in atherosclerotic lesions of ApoE−/−mice (6 months)
Typical staining cells, short characteristics
Antibody Reactivity within the Reactivity outside the lesion (control) lesion
Monocytes, granulocytes
MRP8 +/− ++
MRP14 Monocytes, granulocytes ++++ +++
Circulating monocytes, mature tissue macrophages
ERHR-3 ++++ +++
GR-1 Myeloic differentiation antigen (granulocyte pathway) − ++ Peripheral monocytes, tissue macrophages +++
BM 8 ++
Mature tissue macrophages −
F4/80 (+)
ERMP-58 Monoblasts, peripheral monocytes − ++
ERMP-54 Monoblasts, monocytes − ++
− −
ERMP-12 Monoblasts, monocytes
−
Granulocytes, monocytes, NK-cells, B/T-cell-subsets (+) CD11c
Granulocytes, monocytes, NK-cells
termined using a panel of monocyte/macrophage mark-ers (Table 2). The infiltrated monocyte population was identified to be in the further advanced maturation state of a typical tissue macrophage rather than ex-pressing the monoblast/monocyte phenotype of a circu-lating premature differentiation state. Antibodies which preferentially stain macrophages of the advanced differ-entiated tissue type were shown to be positive inside the lesions (ERHR-3, BM 8), whereas a number of typical markers of the more peripheral phenotype (ERMP-58, ERMP-54, ERMP-12) were found to be negative inside the lesions although their general staining ability was ensured because of positive cells outside the vessels. CD11b was also demonstrated to be highly positive in cells infiltrating atherosclerotic lesions, but CD11c was not. This observation correlates very well with previous findings from our laboratory and others, according to which MRP14 and the MRP8/14 heterodimer complex upregulate Mac-1 (CD11b) affinity [21]. MRP8/14 posi-tive cells also express higher amounts of CD11b than MRP negative monocytes. These cells obviously possess enhanced migration abilities in atherosclerotic plaque areas because of increased adhesiveness.
The absence of MRP8 positive cells was very obvious in all investigated sections. The reason for this phe-nomenon is not quite clear, although in a number of other studies similar results were obtained. It is known that MRP8 inhibits MRP14 mediated adhesion of neu-trophils to its ligand fibrinogen. Also, in vitro monocyte transmigration was shown to be inhibited by an anti-MRP14-antibody but not by anti-MRP8, implying less importance of this molecule for migration and adhesion processes compared to MRP14.
The function of MRP14 expressing monocytes in relatively early atherosclerotic lesions remains unclear at this point. Apoptosis of these cells was observed after they have moved deep inside the plaques (immun-histochemical staining with CM-1, a polyclonal anti-body staining activated caspase 3, which specifically occurs in apoptotic but not in necrotic cells, data not shown). MRP14+ cells in close contact to the arterial vessel wall were stained only by CM-1, suggesting cell death as a consequence of interaction between vessel and monocytes. Investigations are currently underway to analyse which functional consequences derive from this observation.
References
[1] Nakashima Y, Plump AS, Reines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atheroscle-rosis throughout the arterial tree. Arterioscler Thromb 1994;14:133 – 40.
[2] Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progres-sion. Arterioscler Thromb 1994;14:141 – 7.
[3] Zhang SH, Reddick RL, Piedrahita JA, Maeda N. Spontaneous hypercholesterolemia and arterial lesions in mice lacking apolipoprotein E. Science 1992;258:468 – 71.
[4] Plump AS, Smith JD, Hayek T, et al. Severe hypercholes-terolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell 1992;71:343 – 53.
[5] Piedrahita JA, Zhan SH, Hagaman JR, Oliver PM, Maeda N. Generation of mice carrying a mutant apolipoprotein E gene inactivated by gene targeting in embrionic stem cells. Proc Natl Acad Sci USA 1992;89:4471 – 5.
[6] Tsukamoto K, Tangirala R, Chun SH, Pure E, Rader DJ. Rapid regression of atherosclerosis induced by liver-directed gene trans-fer of ApoE in ApoE-deficient mice. Arterioscler Thromb Vasc Biol 1999;19:2162 – 70.
[7] Zhou X, Hansson GK. Detection of B cells and proinflamma-tory cytokines in atherosclerotic plaques of hypercholestero-laemic apolipoprotein E knockout mice. Scand J Immunol 1999;50:25 – 30.
[8] Couffinhal T, Silver M, Kearney M, et al. Impaired collateral vessel development associated with reduced expression of vascu-lar endothelial growth factor in ApoE− / − mice. Circulation 1999;22:3188 – 98.
[9] Ramos CL, Huo Y, Jung U, Ghosh S, Manka DR, Sarembock IJ, Ley K. Direct demonstration of P-selectin- and VCAM-1-de-pendent mononuclear cell of vascular endothelial growth factor in ApoE− / − mice. Circ Res 1999;84:1237 – 44.
[10] George J, Gilburd B, Levkovitz H, et al. Hyperimmunization of apo-E-deficient mice with homologous malondialdehyde low-density lipoprotein suppresses early atherogenesis. Atherosclero-sis 1998;138:147 – 52.
[11] Nakashima Y, Raines EW, Plump AS, Breslow JL, Ross R. Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arte-rioscler Thromb Vasc Biol 1998;18:842 – 51.
[12] Gupta S, Pablo AM, Jiang Xc, Wang N, Tal I. IFN-gamma potentiates atherosclerosis in ApoE knock-out mice. J Clin In-vest 1997;99:2752 – 61.
[13] Fazio S, Babaev VR, Murray AB, et al. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci USA 1997;94:4647 – 52.
[14] Bobryshev YV, Babaev VR, Iwasa S, Lord RSA, Watanabe T. Atherosclerotic lesions of apolipoprotein E deficient mice con-tain cells expressing S 100 protein. Athersosclerosis 1999;143:451 – 4.
[15] Bobryshev YV, Lord RSA. S-100 positive cells in human arterial intima and in atherosclerotic lesions. Cardiovasc Res 1995;29:689 – 96.
[16] Ozmen J, Lord RSA, Bobryshev YV, Ashwell KWS, Munro VF. S 100 protein is expressed in induced atherosclerotic lesions of hypercholesterolaemic rats. Biomed Res 1998;19:279 – 87. [17] Topoll H, Zwadlo G, Lange DE, Sorg C. Phenotypic dynamics
of macrophage subpopulations during human experimental gingivitis. J Peridontal Res 1989;24:106 – 11.
[18] Roth J, Sunderko¨tter C, Goebler M, Gutwald J, Sorg C. Expres-sion of the Ca2+binding proteins MRP8 and MRP14 by early infiltrating cells in experimental contact dermatitis. Int Arch Allergy Immunol 1992;98:140 – 8.
[19] Hogg N, Allen C, Edgeworth G. Monoclonal antibody 5.5 reacts with p8, 14, a myeloid molecule associated with some vascular endothelium. Eur J Immunol 1989;19:1053 – 60.
C. Sorga E-mail: [email protected]