Industrial Crops and Products 11 (2000) 249 – 257
Starch esters obtained by microwave radiation — structure
and functionality
Graz
;
yna Lewandowicz
a,*, Jo´zef Fornal
b, Aleksander Walkowski
a,
Marian Ma˛czyn´ski
a, Grzegorz Urbaniak
a, Graz
;
yna Szyman´ska
a aStarch and Potato Products Research Laboratory,ul.Zwierzyniecka18,60-814Poznan´,PolandbPolish Academy of Sciences,ul.Tuwima10,10-817Olsztyn,Poland
Accepted 8 October 1999
Abstract
The structure and functionality of starch esters obtained in rotating roasters and in microwave ovens were examined. Native potato starch was prepared for esterification according to the procedure applied in industrial processes. It was found that microwave heating is a convenient way to obtain a wide range of products of inorganic starch ester type usually produced in rotating roasters. Microwaves do not affect the kind of chemical groups substituted into the starch and the molecular mass distribution of inorganic starch esters as compared to traditional products. There are no changes in the chemical structure, microstructure and crystal structure between inorganic starch esters obtained in microwave and in conventional technology. In order to obtain a satisfactory degree of substitution by microwave radiation a significantly shorter time is required than in conventional technology. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Starch; Esters; Microwave; Structure
www.elsevier.com/locate/indcrop
1. Introduction
Microwave energy is nonionizing and causes a rise in the temperature within a penetrated medium as a result of rapid changes of the elec-tromagnetic field. When a molecule is irradiated with microwaves it rotates to align itself with the applied field. The frequency of molecular rotation is similar to the frequency of microwave radiation
and consequently the molecule continually at-tempts to realign itself with the changing field and energy is absorbed. Microwave radiation (2450 MHz) does not activate specific bonds in molecules and consequently this form of heating will not lead to any kinetic differences compared to other form of heating (Caddick, 1995). In spite of this there has been a growing interest in the use of microwave heating in organic synthesis, so called ‘MORE chemistry’, microwave oven in-duced reaction enhancement (Giguere et al., 1986; Laurent et al., 1992; Baptistella et al., 1993; Csiba et al., 1993; Loupy et al., 1994; Sowmya and
* Corresponding author. Tel.:+48-61-668-0458; fax:+ 48-61-841-7610.
E-mail address:[email protected] (G. Lewandowicz)
Balasubramanian, 1994). Significant kinetic effects for some Diels – Alder reactions have been also reported (Laurent et al., 1992), but not for ester-ification (Pollington et al., 1991). However, it is still not clear whether specific microwave effects exist, a continued development of microwave-as-sisted solid phase reactions will be particularly important (Caddick, 1995).
Starch esters, especially inorganic starch esters such as phosphates and carbamates, are usually produced in a solid state reaction with phosphate salts and/or acids, and/or urea (Solarek, 1987). They are used in many branches of industry as glues, adhesives and auxiliaries of a wide range of rheological and functional properties (Ro¨per, 1996). Depending on different recipes and time/
temperature conditions, it is possible to obtain relatively low viscosity, white-coloured products for surface sizing of paper, medium viscosity products for different fibre sizing and highly vis-cous, cream-coloured products for wall paper ad-hesives. The production process is environmental friendly because no wastewater is generated, but relatively expensive because of the high level of energy consumption due to the heat losses. The conventional process is made in rotating roasters i.e. horizontally mounted mixers, which are heated by steam jackets or coils or by direct heat. The idea of applying microwave ovens instead of rotating roasters to solid state esterification of starch seems promising, but it has not yet been examined even on laboratory scale. Most of the experiments carried out on starch-microwave ra-diation interaction have pertained to the systems of relatively high water content. There are only few papers concerning researches on the dextrini-sation and chemical modification of starches by microwave irradiation in solid state (Muzim-baranda and Tomasik, 1994; Klinger et al., 1997). In our previous investigations concerning the effect of microwave radiation on physico-chemical properties of starch we found that the rate of temperature rise depends on the moisture content of irradiated starches (Lewandowicz et al., 1997). This should be considered while designing the solid state starch modification process, because the basic parameters determining the kind of product include the temperature and moisture
content of the reaction mixture. The aim of this work was to examine the possibility of application of microwave ovens instead of rotating roasters to obtain products with a structure and functionality similar to those of industrial products.
2. Materials and methods
2.1. Starch esters
2.1.1. Pre-treatment
Native potato starch was prepared for esterifi-cation according to the procedure applied in in-dustrial processes. With this aim, starch was sprayed with a solution of urea, sodium phos-phates and phosphorus acid and carefully mixed to unification. The nitrogen, phosphorus and moisture contents of the prepared blends were adjusted to 3.0, 1.2 and 35%, respectively, the pH level was 2.1.
2.1.2. Heat treatment
The samples intended for traditional treatment were previously air dried to a moisture content of 16% and then heated in a rotating roaster. The samples intended for microwave processing were dried and heated with a Panasonic microwave oven emitting 2450 MHz microwave frequency, and 0.5 W g−1microwave energy. To this end 200
g dry (2% moisture content) samples were irradi-ated in 600 ml glass beakers. The temperature of the starch layer was taken periodically with a mercury thermometer after short, quick stirring. After 15 and 25 min, respectively, when the tem-perature of starch layers rose to the appropriate value (105 and 125°C) microwave radiation was interrupted and renewed if necessary to keep the temperature constant.
2.2. Analysis
2.2.1. Rheological properties
The course of gelatinisation was monitored with a Brabender viscograph under the following conditions. Measuring cartridge, 0.07 N m; heat-ing/cooling rate, 1.5°C min−1; thermostating 30
G.Lewandowicz et al./Industrial Crops and Products11 (2000) 249 – 257 251
The viscosity of starch esters was measured with a Brookfield Digital Viscometer Model DVII for the 8% solutions, prepared at 90°C for 20 min and then cooled to room temperature; the pH level was measured in solutions prepared for vis-cosity examinations.
2.2.2. Nitrogen and phosphorus content
Starch esters samples subjected to elemental analysis were previously purified by a 15-h extrac-tion process with 75% ethanol. The nitrogen and phosphorus contents were determined according to EN ISO 3188 and EN ISO 3946 standards, respectively.
2.2.3. IR spectroscopy
The FTIR measurements were performed in solid state with a FTIR Bruker IFS 113v spec-trometer, under the following conditions, KBr pellet (200/1.5 mg); resolution 2 cm−1.
2.2.4. GPC in6estigations
Starch samples were dispersed in a DMSO/ wa-ter mixture (9:1) at room temperature, then heated while shaking at 90°C for 30 min. The solution was subsequently filtered through a 5 mm Durapor filter and injected into an aqueous sys-tem consisting of four Shodex Sugar (Showa Denko K.K.) columns. The Shodex columns (KS-806, 804, 803 and 802) were linked in a series and maintained at 50°C. The model 410 Waters’ re-fractive index detector with the cell temperature maintained at 50°C was used to detect carbohy-drate peaks. Data were collected and analysed
using Millennium Chromatography Manager
PDA software. Molecular mass (up to 788 000) was calibrated with pullulan Shodex STAN-DARD P-82 (Showa Denko K.K.).
2.2.5. Microscopic examinations
The starch samples to be examined by light microscopy were prepared by the smear method. To this end starch suspensions were heated at the initial gelatinization temperature (as measured ac-cording to Brabender) at 95°C. A drop of the resulting paste was applied to a microscope slide and on cooling, the smear was stained with iodine according to Kaczyn´ska et al. (1993) and ob-served with an Olympus BX60 light microscope.
The starch samples to be examined by scanning electron microscopy were prepared according to Fornal (1985) and observed with a Jeol JSM 5200 microscope.
2.2.6. X-ray diffractometry
X-ray diffractometry was carried out with a TUR 62 Carl Zeiss X-ray diffractometer under the following conditions. X-ray tube Cu Ka (Ni filter); voltage 30 kV; current 15 mA; scanning from U=2 to 18°.
3. Results and discussion
As shown in Table 1, it is possible to obtain a wide range of products of different degree of substitution and physico-chemical properties us-ing the same starch – chemicals blend and chang-ing only the time/temperature conditions. At short reaction times (30 min) the most pro-nounced process was degradation, which resulted in a significant decrease of starch derivative solu-tions viscosity. The nitrogen and phosphorus con-tents were very low even at 135°C. Likewise, the viscosity and pH values up to 135°C were low. Only at 160°C did the pH level rise over 6.0, and the substitution degree of ester groups reached a significantly higher level. An increase in reaction time caused an increase in all the examined parameters i.e. the degree of substitution, viscos-ity and pH. This indicated that in a prolonged reaction time subsequent neutralisation and crosslinking reactions took place. All these results showed that starch esterification with nitrogen and phosphorus compounds is a very complicated process comprising a few concurrent reactions
relatively deep, controlled degradation,
slight substitution by phosphate and carbamate
groups,
neutralisation,
crosslinking,
whose mutual competitiveness depends on both reaction time and temperature.
G
Substituted nitrogen and phosphorus contents in relation to reaction conditions
Nitrogen content Phosphorus content
Reaction temperature Viscosty of 8% solutions
Reaction time pH
Rotating roaster 0.5 105 0.04 0.07 30 3.2
0.09 41 3.2
Rotating roaster 1.0 105 0.12
Rotating roaster 2.0 105 0.27 0.12 58 3.3
3.2
Rotating roaster 115 0.40
0.68 420 6.7
7.0
Rotating roaster 115 1.00
3.2
0.11 40
0.11 Rotating roaster 0.5 125
0.12 45 3.2
125
Rotating roaster 1.0 0.16
6.8
2.0 50
Rotating roaster 125 0.77 0.49
610
4.0 125 1.30 0.53 6.8
Rotating roaster
0.12 50 3.6
0.5
Rotating roaster 135 0.17
0.29 53 6.0
1.0
Rotating roaster 135 0.48
7.2
0.47 60
0.90 Rotating roaster 2.0 135
Rotating roaster 4.0 135 1.10 0.66 890 6.8
6.8
0.23 66
Rotating roaster 0.5 160 0.60
Reference industrial product 0.70 0.45 7.2
Rotating roaster 100
70
Microwave 1.0 105 0.70 0.31 7.1
heating
130 7.2
1.00
Microwave 1.0 125 0.44
G.Lewandowicz et al./Industrial Crops and Products11 (2000) 249 – 257 253
revealed similar physico-chemical properties. The most significant difference was displayed in the correlation between the degree of substitution and the reaction time. A similar nitrogen and phos-phorus content as in the microwave oven after 1 h
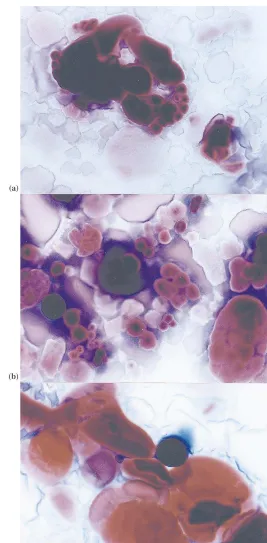
Fig. 3. GPC curves of potato starch carbamate/phosphate esters as compare to native starch. NP, native potato starch; IP, industrial product; MLT, microwave product obtained at 105°C; MHT, microwave product obtained at 125°C.
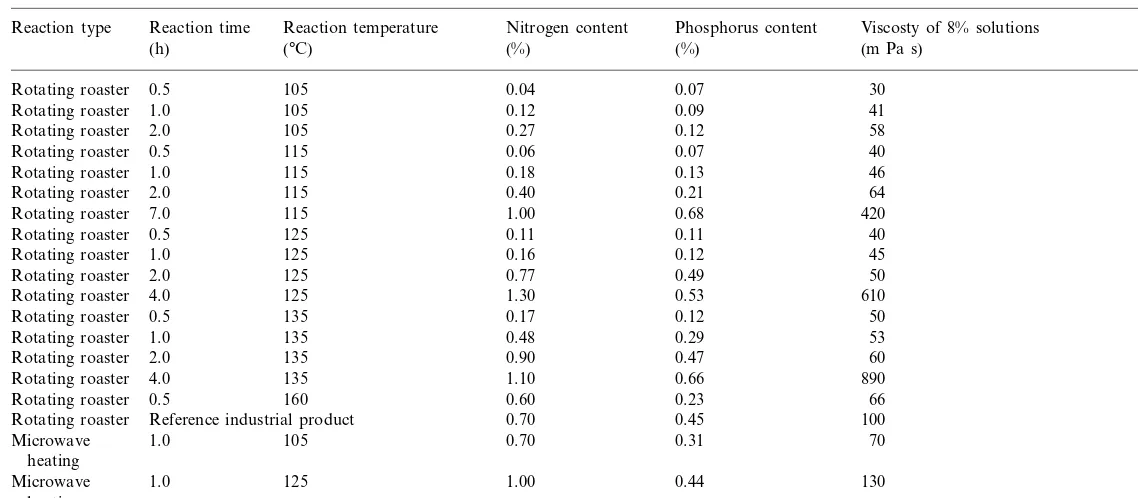
Fig. 1. Brabender viscosity curves (c=12%) of potato starch carbamate/phosphate esters. IP, industrial product; MLT, mi-crowave product obtained at 105°C; MHT, mimi-crowave product obtained at 125°C.
was reached in a laboratory-rotating roaster at the same temperature after 2 – 4 h.
Brabender viscosity curves of all carbamate/
phosphate starch esters (Fig. 1) revealed a high type of swelling characteristic typical of native potato starch. The most significant property dis-criminating modified starches from the native one was the pasting temperature. Both microwave as-sisted and traditional technology esters gelatinised at lower temperatures than native potato starch. The pasting temperature increased with an in-crease in esterification temperature. Microwave assisted carbamate/phosphate esters obtained at 105 and 125°C gelatinised at 55°C and room temperatures, respectively, whereas the industrial product gelatinised at 40°C. This showed a possi-bility to obtain a wide range of products of differ-ent functional properties by microwave heating.
IR investigations (Fig. 2) proved that mi-crowave heating did not influence the kind of chemical groups substituted into starch. It is be-lieved that as a result of the reaction of starch with urea the carbamate groups are formed (So-larek, 1987; Hebeish et al., 1991; Khail et al., 1994). In fact a strong band at 1717 cm−1
, identi-fying carbonyl, absent in a native starch spectrum, was observed in all carbamate/phosphate starch esters spectra. The intensity of this band increased with an increase in the nitrogen degree of substi-tution. The qualification of the kind of phosphate bonds is more difficult. The hydrogen bonded PO band, typically located at 1150 – 1250 cm−1
G.Lewandowicz et al./Industrial Crops and Products11 (2000) 249 – 257 255
(Bellamy 1958; Marusza and Tomasik 1991), could be observed only on the slope of strong 1169 cm−1 band.
A GPC analysis showed that microwaves also did not influence the molecular mass distribution as well (Fig. 3). All products obtained both in a traditional rotating roaster technology and by microwave heating revealed similar GPC profiles. A native potato starch GPC curve comprised two amylose and amylopectin peaks. After a modifica-tion process two peaks could no more be ob-served. Carbamate/phosphate esters GPC curves consist of only one peak situated between the amylose and amylopectin of native starch. This proved that the applied modification method comprised not only degradation and substitution reactions, but was also a more complicated system including crosslinking processes.
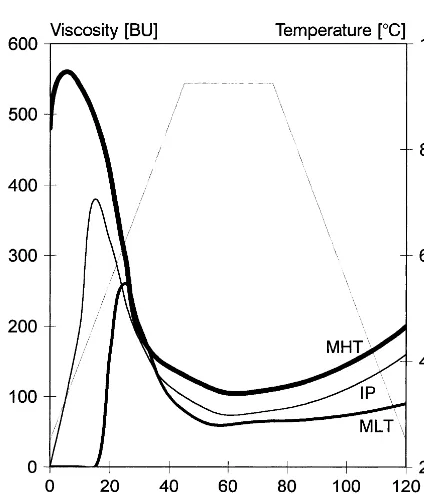
Although the molecular mass distribution curve consists of only one peak, light microscopy pic-tures (Fig. 4) of inorganic starch esters made at a gelatinisation temperature showed two colour phases, blue, which could be ascribed to amylose and red – violet, ascribed to amylopectin. A typical image characteristic of the early stage of gelatini-sation of the native potato starch is amylose leakage from starch granules (Lewandowicz et al.,
1997). The first stage of the carbamate/phosphate starch esters gelatinisation was to some extent similar to that of native starch. A blue coloured substance leaked out of the red – violet granules. However, a significant difference could be ob-served, apart the amylose leakage, the disintegra-tion of starch granules into smaller subunits took place. Similar pattern of gelatinisation with strong correlation to degree of substitution is revealed by starch acetate (Lewandowicz et al., 1998).
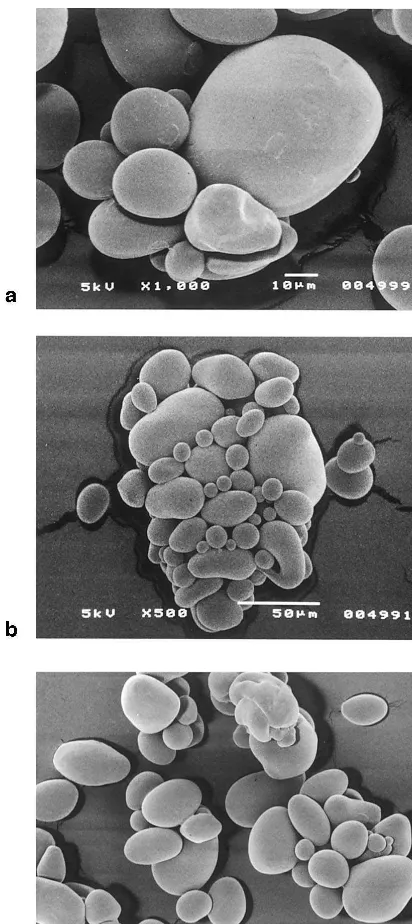
An X-ray investigation (Fig. 5) indicated a deep decrease of crystallinity as a result of the modifi-cation processes. The microwave assisted carba-mate/phosphate esters obtained at 105°C revealed a slightly higher crystallinity than the industrial product and microwave assisted carbamate/ phos-phate ester obtained at 125°C, but the difference was not significant. These deep changes in the crystal structure were only slightly reflected in the granular structure. Scanning electron microscopy investigations (Fig. 6) showed that the treatment applied, caused no significant changes in the structure of starch granules as compared to the image of the native starch (Kawabata et al., 1994). Only a small part of starch ester granules underwent damage. The susceptibility to damage increased with the increase in the intensity of heat treatment.
Fig. 6. SEM microphotographs of inorganic starch esters. (a) Industrial product; (b) microwave product obtained at 105°C; (c) microwave product obtained at 125°C.
starch ester type usually produced in rotating roasters.
Microwave radiation does not affect the kind
of chemical groups substituted into starch as compared to the traditional esterification process.
Microwave radiation does not affect molecular
weight distribution and granular structure of modified starches as compared to products of traditional technology.
Microwave as well as traditional heating causes
a decrease in crystallinity of inorganic starch esters to the same level.
Microwave as well as traditional heating causes
changes in microstructure of inorganic starch esters to the same level.
A significantly shorter time is necessary to
ob-tain satisfactory degree of substitution by mi-crowave radiation than in conventional technology.
Acknowledgements
This research was supported by Grant No. 5 P06 G 017 13 from the State Committee for Scientific Research (KBN).
References
Baptistella, L.H.B., Neto, A.Z., Onaga, H., Godoi, E.A.M., 1993. An improved synthesis of 2,3- and 3,4-unsaturated pyranosides: the use of microwave energy. Tetrahedron Lett. 34, 8407 – 8410.
Bellamy, L.J., 1958. The Infra-red Spectra of Complex Molecules. Wiley, New York, p. 1958.
Caddick, S., 1995. Microwave assisted organic reactions. Te-trahedron 51, 10403 – 10432.
Csiba, M., Cleophax, J., Loupy, A., Malthete, J., Gero, S.D., 1993. Liquid crystalline 5,6-O-acetals ofL -galactono-1,4-lactone prepared by a microwave irradiation on montmo-rillonite. Tetrahedron Lett. 34, 1787 – 1790.
Fornal, J., 1985. Influence of hydrothermal treatment on the starch of corn grain. Acta Alim. Polonica 11, 141 – 151. Giguere, R.J., Bray, T.L., Duncan, S.M., 1986. Application of
commercial microwave ovens to organic synthesis. Tetra-hedron Lett. 41, 4945 – 4948.
Hebeish, A., Refai, R., Ragheb, A., Abd-El-Thalouth, I., 1991. Factors affecting the technological properties of starch carbamate. Starch/Sta¨rke 43, 273 – 280.
4. Conclusions
Microwave heating is a convenient way to
G.Lewandowicz et al./Industrial Crops and Products11 (2000) 249 – 257 257
Kaczyn´ska, B., Autio, K., Fornal, J., 1993. Heat-induced structural changes in faba bean starch paste: the effect of steaming of faba bean seeds. Food Struct. 12, 217 – 224. Kawabata, A., Takase, N., Miyoshi, E., Sawayama, T.S.,
Saitama, T.K., Kudo, K., 1994. Microscopic observation and X-ray diffractometry of heat/moisture-treated starch granules. Starch/Sta¨rke 46, 463 – 469.
Khail, M.I., Farag, S., Mostafa, K.M., Hebeish, A., 1994. Some studies on starch carbamate. Starch/Sta¨rke 46, 312 – 316.
Klinger, R., Bush, K.G., Uahedi, B., 1997. Acid modification of starch in a semi-dry process. Starch/Sta¨rke 49, 391 – 395. Laurent, R., Laportie, A., Dubac, J., Berlan, J., Lefeuvre, S., Audhuy, M., 1992. Specific activation by microwaves: myth or reality? J. Org. Chem. 57, 7099 – 7102.
Lewandowicz, G., Fornal, J., Walkowski, A., 1997. Effect of microwave radiation on potato and tapioca starches. Car-bohydr. Polym. 34, 213.
Lewandowicz, G., Blaszczak, W., Fornal, J., 1998. Effect of acetylation on microstructure of potato starch. In: Struc-ture and Functionality of Food Products, Proceedings of a Conference, Mragowo 18 – 20 May, 1998. Polish J. Food Nutr. Sci. 7/48, 78 – 84.
Loupy, A., Pigeon, P., Ramdani, M., Jaquault, P., 1994. Solid – liquid phase transfer catalysis without solvent cou-pled with microwave irradiation: a quick and efficient method for saponification of esters. Synth. Commun. 24, 159 – 165.
Marusza, K., Tomasik, P., 1991. Highly phosphorylated starch. Starch/Sta¨rke 43, 66 – 69.
Muzimbaranda, C., Tomasik, P., 1994. Microwaves in physi-cal and chemiphysi-cal modifications of starch. Starch/Sta¨rke 46, 469 – 474.
Pollington, S.D., Bond, G., Moyes, R.B., Whan, D.A., Can-dlin, J.P., Jennings, J.R., 1991. The influence of mi-crowaves on the rate of reaction of propan-1-ol with ethanoic acid. J. Org. Chem. 56, 1313 – 1314.
Ro¨per, H., 1996. Application of starch and its derivatives. Carbohydr. Eur. 15, 22 – 30.
Solarek, D.B., 1987. Phosphorylated starches and miscella-neous inorganic starch esters. In: Wurzburg, O.B. (Ed.), Modified Starches Properties and Uses. CRC Press, Boca Raton, FL, pp. 97 – 112.
Sowmya, S., Balasubramanian, K.K., 1994. Microwave in-duced ferrier rearrangement. Synth. Commun. 24, 2097 – 2101.