Journal of Insect Physiology 47 (2001) 257–262
www.elsevier.com/locate/jinsphys
Development of teratocytes associated with
Microctonus
aethiopoides
Loan (Hymenoptera: Braconidae) in natural and novel
host species
B.I.P. Barratt
a,*, M. Sutherland
baAgResearch, Invermay Agricultural Centre, Private Bag 50034, Mosgiel, New Zealand bDepartment of Zoology, University of Otago, P.O. Box 56, Dunedin, New Zealand
Received 23 February 2000; accepted 15 June 2000
Abstract
A laboratory study investigated development of teratocytes derived from the parasitoid Microctonus aethiopoides Loan in the natural host,Sitona discoideusGyllenhal, and in three novel hosts, the introduced weed biological control agentRhinocyllus conicus
(Froehlich), and two New Zealand native speciesNicaeana cervinaBroun andIrenimus stolidusBroun. Weevils were exposed to parasitoids and then examined 6, 10 and 15 days post-parasitism for parasitoid stage and size, and teratocyte number and size. In all hosts, teratocyte numbers decreased and size increased as parasitoid development progressed, although 6 days after parasitism, fewer, larger teratocytes were found inI. stolidusthanS. discoideusorN. cervina. In weevils containing second-third instar parasitoid larvae, the most permissive hosts,S. discoideusandN. cervinacontained more teratocytes than the least permissive hostsI. stolidus
andR. conicus.Host gender influenced some aspects of parasitoid and teratocyte development. Total teratocyte volume was greater in female than male S. discoideus at all sampling times, and at 10 days post-parasitism in N. cervina. A possible relationship between host suitability and teratocyte development is discussed. 2001 Elsevier Science Ltd. All rights reserved.
Keywords:Teratocyte development; Parasitoid larval development;Microctonus aethiopoidesLoan;Sitona discoideusGyllenhal; Novel hosts
1. Introduction
Teratocytes are cells derived from the serosal mem-brane of parasitoid eggs which become dissociated soon after hatching (Dahlman, 1990). These cells then become dispersed in the haemolymph of the host, often dramati-cally increasing in size and developing microvilli on the surface (Zhang et al., 1994). Teratocytes are found in many braconid species, and their function varies between species. They may be nutritive, immunosuppressive, or secretory and can be involved in regulating host devel-opment (Dahlman and Vinson, 1993).
Most examples of teratocyte structure, function and development have been described for parasitoids of immature stage hosts, especially Lepidoptera larvae. However, research has been carried out on teratocytes
* Corresponding author. Tel.: +64-3-489 9059; fax: +64-3-489 3739.
E-mail address:[email protected] (B.I.P. Barratt).
0022-1910/01/$ - see front matter2001 Elsevier Science Ltd. All rights reserved. PII: S 0 0 2 2 - 1 9 1 0 ( 0 0 ) 0 0 1 0 8 - 6
associated with the braconid parasitoid Dinocampus coccinellae (Schrank), a parasitoid of adult Coccinelli-dae. This parasitoid is in the same subfamily as
Microctonus, the Euphorinae. Teratocytes of D. cocci-nellae are thought to be primarily nutritive in function since they are consumed by parasitoid larvae (Okuda and Kadono-Okuda, 1995). They also synthesize a terato-cyte-specific polypeptide (TSP) which accumulates in the cells (Kadono-Okuda et al., 1998). These authors considered that the nutritive role of teratocytes in D. coccinellaemay be related to differences in the way that adult and larval hosts store proteins. The latter store pro-teins in the haemolymph which are accessible to parasit-oid larvae, whereas adult hosts tend to store nutritive reserves in fat body.
(Smith, 1952). It was also noted that the number of tera-tocytes decreased as the parasitoid larva developed, and it was assumed that this occurred as a result of the para-sitoid larva feeding on teratocytes. Teratocytes have been found in the gut of D. coccinellae (Sluss, 1968).
The objective of this study was to investigate the com-parative developmental characteristics of teratocytes associated withMicroctonus aethiopoidesLoan larvae in the natural host, Sitona discoideus Gyllenhal, and in three other weevil species known to be permissive hosts (i.e. which allow development and emergence of the parasitoid). M. aethiopoides was introduced into New Zealand for biological control ofS. discoideusand since its release it has been found to parasitize a number of non-target species, hence the term “novel” hosts. The three novel hosts included in this study were the weed biological control agentRhinocyllus conicus(Froehlich), and two New Zealand native species Nicaeana cervina
Broun andIrenimus stolidusBroun (Barratt et al., 1997).
S. discoideus is a weevil of Mediterranean origin which has become a pest of lucerne (Medicago sativa
L.) since establishing in New Zealand. It has an annual life cycle with adult emergence in early summer and becoming reproductively mature from autumn through to the following spring (Goldson et al., 1984).R. conicus
is a European species introduced into New Zealand for biological control of nodding thistle (Carduus nutans
L.). The species has an annual life cycle (Jessep, 1975), adults emerging from thistle seed-heads in early autumn and becoming reproductively mature in spring and sum-mer. The two New Zealand native weevil species emerge as adults in winter–early spring and live for approx. 6 months (Barratt et al., 2000).
2. Materials and methods
2.1. Insects
M. aethiopoides adults were reared in the laboratory from S. discoideus collected from the field in lucerne growing at Sutton, Otago, New Zealand. The weevils were held in plastic cages (160×180×75 mm deep) with a plastic-coated mesh floor inserted into the top of another similar container with textured absorbent paper towel covering the base. This served as a substrate for pupation of emergent pre-pupal parasitoids which moved down through the mesh from the upper to the lower cage. Once they had pupated they were removed to another con-tainer until the adult wasps emerged.
Weevils were fed on bundles of Grasslands cv. Wai-rau lucerne seedlings grown to a height of about 100 mm in cell trays containing commercial seed raising mix. When required, the roots and soil were enclosed in 100×75 mm plastic bags secured at the base of the plants using a plastic cable clip. Fresh plants were supplied to
weevils every 3–4 days, and water was provided in the form of two to three saturated cotton dental wicks placed in each cage. The weevils were held for 30 days to allow parasitoids from field parasitism to emerge, so it could be ensured that experimental insects were free of parasit-ism.
R. conicus collected in the field from nodding thistle plants in autumn were held in storage at 4°C over winter and brought to 20°C for 1 week before being used in the experiment.N. cervinaandI. stoliduswere collected from pasture at Sutton, Otago and brought into the lab-oratory. Like S. discoideus, they were held for 30 days to clear them of field parasitism before being used in the experiment.
2.2. Experiment
Five replicate cages of 40 weevils of each species, cleared of field parasitism, were exposed to six 2-day old mated femaleM. aethiopoides. The wasps were removed after 24 h and the weevils maintained as above. The cages were held at 20±2°C, approx. 60% r.h. and in a 14:10 light:dark photoperiod.
After 6, 10, and 15 days, 10 weevils were removed from each cage, and preserved in 70% ethanol until dis-section. After removal of the elytra and dorsal abdominal cuticle, the abdomen was examined for teratocytes and parasitoid larvae. Teratocytes were flushed from the host, counted, and the diameter of a sub-sample of 10 teratocytes was measured. If more than one parasitoid larva was found in a weevil, the number of teratocytes was divided by the number of larvae. Total teratocyte volume (mean teratocyte diameter×p×teratocyte number
per parasitoid) was also calculated. For teratocyte diam-eter measurements, data from hosts containing only a single parasitoid were included in analyses and those from superparasitized hosts excluded.
The number, stage of development, and length of parasitoid larvae was recorded. M. aethiopoides larvae develop through four instars before they emerge from the host (Loan and Holdaway, 1961), but since second and third instar larvae are difficult to distinguish, these were combined and only three stages were recognized in the study.
The standard errors of means of proportion of hosts parasitized, number of parasitoids per hosts, and parasit-oid length (Table 1), and teratocyte number and diameter (Table 2) were calculated. For comparisons between means, SED values were calculated andt ratios used to determine levels of probability.
3. Results
3.1. Parasitism
259
B.I.P. Barratt, M. Sutherland / Journal of Insect Physiology 47 (2001) 257–262
Table 1
Mean proportion of hosts parasitized, number of parasitoids per host and length of parasitoids 6, 10 and 15 days after parasitoid exposure of four host species toM. aethiopoides. Means are shown±SE
Host species Days post parasitism
6 10 15
Proportion Parasitoid Proportion Parasitoid Proportion Parasitoid
Paras/host Paras/host Paras/host
parasitized length (mm) parasitized length (mm) parasitized length (mm)
S. discoideus 0.59±0.06 1.17±0.13 0.72±0.03 0.58±0.07 1.57±0.23 1.32±0.07 0.60±0.11 1.31±0.17 2.75±0.19
N. cervina 0.37±0.08 1.53±0.15 0.71±0.03 0.45±0.12 1.80±0.21 1.15±0.09 0.44±0.11 1.70±0.30 1.47±0.24
I. stolidus 0.19±0.09 1.75±0.43 0.96±0.08 0.25±0.08 1.27±0.19 1.28±0.19 0.27±0.10 1.15±0.09 1.86±0.25
R. conicus 0.14±0.05 1.50±0.29 0.74 0.14±0.10 2.09±0.26 1.10±0.21 0.36±0.20 2.40±0.20 1.70±0.16
significantly higher than that for bothI. stolidusof which 22.8±4.4% were parasitized and R. conicus of which 23.0±7.6% were parasitized. Mean parasitism levels for each treatment are shown in Table 1.
The mean number of parasitoids per host was gener-ally higher in the novel host species than inS. discoideus
(Table 1). This was particularly evident in R. conicus
where 10 and 15 days post-parasitism, an average of over two parasitoids per weevil was recorded, and by 15 days 2.4±0.2 parasitoids were found per host. This level of superparasitism was significantly higher (P,0.05) than in any of the other three host species (Table 1).
Parasitoid larval length at 6 days post-parasitism was very similar in all host species except for those found in I. stolidus, where after 6 days, parasitoid larvae were about 25% larger than in the natural host,S. discoideus
(t=3.97; P,0.001) (Table 1). After 10 days there was no significant difference between parasitoid larval length in relation to host species, but by 15 days, parasitoid larvae in S. discoideus were significantly longer than those in the other three species (t=4.01;P,0.001) (Table 1). This concurs with faster development times inS. dis-coideus. Most parasitoid larvae in this species had reached the fourth instar by 15 days whereas only a small proportion (N. cervina) or no parasitoids (I. stol-idus and R. conicus) had developed beyond the third instar (Table 2).
3.2. Teratocyte number and size
Six days post-parasitism, about 25% of all parasitized weevils still contained parasitoid eggs (Table 2), although this may be an under-estimate because of the difficulty in detecting eggs during dissection. At this stage, 77–88% of S. discoideus, N. cervina andI. stol-idus which contained parasitoid larvae also contained teratocytes. By 10 days post-parasitism, all parasitized weevils contained teratocytes, but by 15 days, no terato-cytes remained inS. discoideus containing fourth instar parasitoids 4 mm or greater in length, or in the two N. cervina containing fourth instar parasitoids (Table 2).
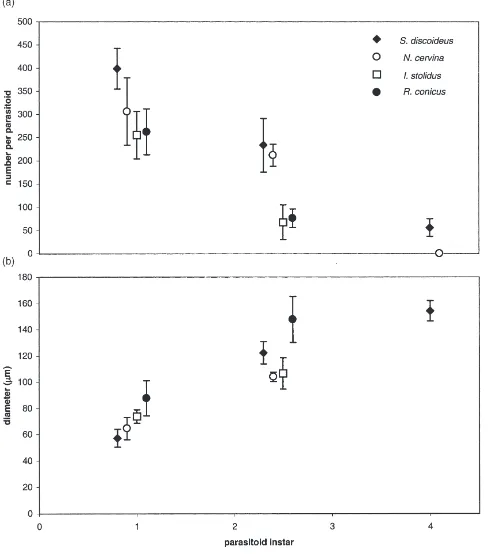
For most host species, the mean number of teratocytes per parasitoid larva decreased with time after parasitism (Table 2), and with advancing stage of parasitoid larval development [Fig. 1(a)], while the mean diameter of ter-atocytes increased with time (Table 2) and with parasit-oid instar [Fig. 1(b)]. In S. discoideus and N. cervina, weevils containing parasitoid larvae 6 days post-parasit-ism contained 440–470 teratocytes per parasitoid with a mean diameter of 22–27µm (Table 2).I. stolidus, how-ever, contained only half the number of teratocytes, but they were over twice the diameter (Table 2). Ten days post-parasitism, bothN. cervinaandI. stoliduscontained significantly fewer teratocytes than the other two species, but by 15 days, mean teratocyte numbers were in the range 137–183 for all hosts, although this varied con-siderably with the stage of parasitoid development (Table 2).
The diameter of teratocytes in hosts containing single first instar parasitoid larvae was similar forS. discoideus
and N. cervina but those in I. stolidus and R. conicus
were significantly larger, although fewer in number than those in S. discoideus [Fig. 1(a) and (b)]. In hosts con-taining second-third instar parasitoids teratocyte size was still largest in R. conicus but no longer significantly larger than those in S. discoideus. Teratocytes inS. dis-coideuscontaining fourth instar parasitoids were reduced in numbers and reached a mean diameter of 154 µm (Table 2).
3.3. Host gender and size
Data on the effect of host gender on parasitoid and teratocyte development were analysed only forS. disco-ideusandN. cervinabecause of insufficient numbers of parasitized weevils of each sex in each treatment for the other species. Mean parasitoid larval length inS. disco-ideuswas significantly greater in female (2.95±0.23 mm) than male (2.37±0.27 mm) hosts at 15 days (t=2.31;
B.I.P.
Barratt,
M.
Sutherland
/
Journal
of
Insect
Physiology
47
(2001)
257–262
Table 2
Percentage of hosts containing teratocytes, and mean number and diameter of teratocytes found in different developmental stages of host species 6, 10 and 15 days after exposure toM. aethiopoides. Means are shown±SE
Para Days post parasitism
Host species
instar
6 10 15
% with T no.±SE (per T diam.±SE % with T no.±SE (per T diam.±SE % with T no.±SE (per T diam.±SE
No. No. No.
Ta parasit) (µm) T parasit) (µm) T parasit) (µm)
S. discoideus Egg 7 0 0 – 0 – – – 0 – – –
1 23 78.2 441.7±58.75 22.2±2.4 26 100 395.5±30.2 80.8±6.6 6 100 283.8±37.4 83.7±43.2
2/3 0 – – – 4 100 362.6±96.7 105.3±2.4 5 100 130.0±18.2 139.3±8.2
4 0 – – – 0 – – – 19 73.7 56.0±19.2 154.2±7.7
PEb 0 – – – 0 – – – 0 – – –
N. cervina Egg 2 0 – – 0 – – – 0 – – –
1 17 76.5 469.1±77.5 27.0±3.9 15 100 201.6±30.3 79.9±4.6 9 78 204.8±95.0 127.5±8.5
2/3 0 – – – 4 100 282.3±32.7 101.0±3.0 3 100 117.3±7.0 110
4 0 – – – 0 – – – 2 0 0 –
PE 0 – – – 0 – – – 5 – – –
I. stolidus Egg 0 – – – 0 – – – 0 – – –
1 8 87.5 203.5±31.1 57.7±10.3 8 100 299.4±67.2 84.2±6.9 6 83.3 258.1±48.8 70.3±7.9
2/3 0 – – – 3 100 125.0±50.8 102.2±6.2 4 100 24.5±16.9 110.9±25.6
4 0 – – – 0 – – – 0 0 0 –
PE 0 – – – 0 – – – 0 0 – –
R. conicus Egg 6 0 – – 0 – – – 0 – – –
1 1 100 106.5 25.6 5 100 385.1±60.9 100.0±7.0 7 100 197.0±60.9 62.8
2/3 0 – – – 0 – – – 8 88.9 76.4±20.2 147.7±17.5
4 0 – – – 0 – – – 0 – – –
PE 0 – – – 0 – – – 0 – – –
261
B.I.P. Barratt, M. Sutherland / Journal of Insect Physiology 47 (2001) 257–262
Fig. 1. Mean teratocyte number per parasitoid (a) and mean diameter (b) in four host species containingM. aethiopoideslarvae in the first, second-third and fourth instars. Bars indicate standard errors of means.
days post-parasitism (1.36±0.11 and 0.92±.0.09 mm, respectively; t=4.38; P,0.001), where female weevils also tend to be larger than males. In both sexes at all three sampling times, parasitoid length in S. discoideus
was significantly greater (approx. 20%) than that in the smaller host, N. cervina.
Total teratocyte volume was significantly greater in female than maleS. discoideusat all three sampling per-iods (Fig. 2), a function of both larger mean teratocyte diameter and number. InN. cervina, however, total tera-tocyte volume was similar in males and females at 6 days, but significantly greater in females than males at 10 days post-parasitism. There were no femaleN. cerv-ina present at 15 days which were not superparasitized.
Fig. 2. Total teratocyte volume in males and females of two host species 6, 10 and 15 days after exposure to M. aethiopoides. Bars indicate standard errors of means.
4. Discussion
The rates of parasitism achieved by M. aethiopoides
in this experiment varied between host species as found previously (Barratt et al., 1997), but for S. discoideus
was a little lower than the 66% parasitism which would have been predicted from a 20:6 host:parasitoid ratio and 24 h exposure time (Barratt et al., 1996). However, superparasitism was frequent in the caged conditions of the experiment, probably attributable to the relatively high parasitoid:host ratio. The mean number of parasit-oids per host was often higher in the novel host species than inS. discoideus, especially in the case ofR. conicus. The relatively low incidence of parasitism inR. conicus
in this experiment coupled with a high incidence of superparasitism suggests that parasitoid attack was con-centrated on fewer susceptible individuals.M. aethiopo-idesis thought to require a mobile host to facilitate ovi-positor penetration (Phillips, 1996), and the observation was made that theR. conicusweevils used in the experi-ment were relatively inactive in the cages.
Differences recorded between host species in parasit-oid larval growth rates possibly reflects faster develop-ment times that might be expected in a “natural” host species with which M. aethiopoidesevolved, compared with recently established novel hosts. In S. discoideus, parasitoid larval growth between 10 and 15 days was greater than between 6–10 days, which is consistent with parasitoid larval growth in D. coccinellae, where body length increased gradually up to 9 days after parasitism, and more rapidly after moulting into the second instar up to 15 days post-parasitism (Kadono-Okuda et al., 1995). For the other three species, larval growth rates were generally slower, reflecting slower progression to second-third and fourth instars. It has been demonstrated that M. aethiopoides reared from smaller hosts tend to be smaller in size (Phillips et al., 1993). However, of the weevils used in this experiment, onlyN. cervinawas smaller in body size than S. discoideus. I. stolidus is similar in size, and R. conicus is larger than S. disco-ideus.
The general pattern of increase in teratocyte size and decrease in numbers as parasitoids developed in this study is consistent with similar observations in adult
indicates that they may have been consumed by the para-sitoid larvae.
M. aethiopoidesteratocyte “phenology” was found to be variable in the host species included in this study, and possibly there were associated differences in the capacity for teratocytes to deliver nutrients to developing parasitoid larvae. The most permissive host afterS. dis-coideus was N. cervina, in which the most similar characteristics of teratocyte development were observed. Teratocyte development in I. stolidus and R. conicus
(less permissive hosts) deviated more from that observed in S. discoideus. Whether variable teratocyte function is instrumental in reducing the success of parasitoid devel-opment, or alternatively, other poorly matched physio-logical factors in less suitable hosts reduces teratocyte function or efficiency is yet to be determined.
Aspects of parasitoid and teratocyte development tended to progress more rapidly in femaleS. discoideus
and N. cervina compared with males. Although this might simply reflect the generally larger weevil size, and hence resource availability in females, further exper-imentation is required to determine the relative impor-tance of the clearly interrelated factors, host size, gender and nutrient quality in influencing teratocyte develop-ment in different host species. Examination of the data available from this experiment detected no significant relationship between host size and teratocyte number, size or volume within genders in eitherS. discoideusor
N. cervina at any stage post-parasitism. This suggests that either the size range was not large enough within a gender to detect any size effect, or that nutrient quality (e.g. vitellogenin in females) and availability in males and females influences teratocyte development to a greater extent than host size.
Acknowledgements
We thank Rory Logan for technical assistance, Taka-shi Okuda (National Institute of Sericultural and Ento-mological Science, Tsukuba, Japan) and Craig Phillips (AgResearch) for valuable discussion and comments on an early draft of this paper. The research was funded partly by the Foundation for Research, Science and Technology, and partly by an AgResearch student schol-arship awarded to Merinda Sutherland.
References
Barratt, B.I.P., Evans, A.A., Ferguson, C.M., Barker, G.M., McNeill, M.R., Phillips, C.B., 1997. Laboratory nontarget host range of the
introduced parasitoids Microctonus aethiopoidesandMicroctonus hyperodae(Hymenoptera: Braconidae) compared with field parasit-ism in New Zealand. Environmental Entomology 26, 694–702. Barratt, B.I.P., Evans, A.A., Ferguson, C.M., McNeill, M.R., Addison,
P., 2000. Comparative phenology of five spp. of broad nosed weev-ils (Coleoptera: Curculionidae) in Otago, Canterbury and Waikato. New Zealand Journal of Zoology 27 (2), 93–110.
Barratt, B.I.P., Evans, A.A., Johnstone, P.D., 1996. Effect of the ratios of Listronotus bonariensis and Sitona discoideus (Coleoptera: Curculionidae) to their respective parasitoidsMicroctonus hypero-daeandMicroctonus aethiopoides(Hymenoptera: Braconidae), on parasitism, host oviposition and feeding in the laboratory. Bulletin of Entomological Research 86, 101–108.
Dahlman, D.L., 1990. Evaluation of teratocyte functions: an overview. Archives of Insect Biochemistry and Physiology 13, 159–166. Dahlman, D.L., Vinson, S.B., 1993. Teratocytes: developmental and
biochemical characteristics. Parasites and Pathogens of Insects 1, 145–165.
Goldson, S.L., Frampton, E.R., Barratt, B.I.P., Ferguson, C.M., 1984. The seasonal biology ofSitona discoideus Gyllenhal (Coleoptera: Curculionidae), an introduced pest of New Zealand lucerne. Bull-etin of Entomological Research 74, 249–259.
Jessep, C.T., 1975. Introduction of a weevil for biological control of nodding thistle. In: Proceedings of the 28th New Zealand Weed and Pest Control Conference 28, 205–206.
Kadono-Okuda, K., Sakurai, H., Takeda, S., Okuda, T., 1995. Synchronous growth of a parasitoid,Perilitus coccinellae, and tera-tocytes with the development of the host Coccinella septem-punctata. Entomologia Experimentalis et Applicata 75, 145–149. Kadono-Okuda, K., Weyda, F., Okuda, T., 1998. Dinocampus
(=Perilitus)coccinellaeteratocyte-specific polypeptide: its accumu-lative property, localization and characterization. Journal of Insect Physiology 44, 1073–1080.
Loan, C., Holdaway, F.G., 1961.Microctonus aethiops(Nees) auctt. andPerilitis rutilis(Nees) auctt. (Hymenoptera: Braconidae), Euro-pean parasites of Sitona weevils (Coleoptera: Curculionidae). Can-adian Entomologist 93, 1057–1079.
Okuda, T., Kadono-Okuda, K., 1995.Perilitus coccinellaeteratocyte polypeptide: evidence for production of a teratocyte-specific 540 kDa protein. Journal of Insect Physiology 41, 819–825.
Phillips, C.B., 1996. Intraspecific variation inMicroctonus hyperodae
andM. aethiopoides(Hymenoptera: Braconidae); significance for their use as biological control agents. Ph.D. thesis, Department of Entomology, Lincoln University, New Zealand.
Phillips, C.B., Goldson, S.L., Emberson, R.M., 1993. Host-associated morphological variation in Canterbury (New Zealand) populations of the parasitoid Microctonus aethiopoides Loan (Hymenoptera: Braconidae, Euphorinae). In: Proceedings of the Sixth Australasian Grassland Invertebrate Ecology Conference 6, 405–414.
Sluss, R.R., 1968. Behavioural and anatomical responses of the conver-gent lady beetle to parasitism byPerilitus coccinellae (Schrank). Journal of Invertebrate Pathology 10, 9–27.
Smith, O.J., 1952. Biology and behaviour of Microctonus vittatae
Muesebeck. University of California Berkeley Publications in Ento-mology 9, 315–344.