Host – pathogen interactions modulated by heat treatment
M. Schirra
a,*, G. D’hallewin
a, S. Ben-Yehoshua
b, E. Fallik
baC.N.R.,Istituto per la Fisiologia della Maturazione e Conser6azione del Frutto della Specie Arboree Mediterranee,
Localita` Palloni,Nuraxinieddu,09170Oristano,Italy
bDepartment of Posthar
6est Science of Fresh Produce,ARO,The Volcani Center,Bet Dagan50250,Israel
Received 13 March 2000; accepted 29 July 2000
Abstract
Prestorage heat treatment appears to be a promising method of postharvest control of decay. Heat treatments against pathogens may be applied to fresh harvested commodities by hot water dips, by vapour heat, by hot dry air or by a very short hot water rinse and brushing. Heat treatments have a direct effect slowing germ tube elongation or of inactivating or outright killing germinating spores, thus reducing the effective inoculum size and minimising rots. Heat treatment can also indirectly affect decay development via physiological responses of the fruit tissue. These responses include inducing antifungal-like substances that inhibit fungal development in the fruit tissue, or enhancing wound healing. Heat treatment can induce PR proteins such as chitinase andb-1,3 glucanase, stabilise membranes, elicit antifungal compounds, or inhibit the synthesis of cell wall hydrolytic enzymes (polygalacturonases), and delay the degradation rate of pre-formed antifungal compounds that are present in unripe fruit. Additionally, curing, as a heat treatment can cause the disappearance of wax platelets normally present in untreated fruit and make the fruit surface relatively homogeneous. Thus, cuticular fractures, microwounds and most stomata are partially or completely filled, and early-germinated spores are encapsulated and inactivated by molten wax. The occlusion of possible gaps for wound pathogens as well as the encapsulation and inactivation of early-germinated spores have been considered as additional factors in fruit protection against decay. © 2000 Elsevier Science B.V. All rights reserved.
Keywords:Heat treatment; Postharvest storage; Pathogens; Fungi; Curing; Fruit decay
www.elsevier.com/locate/postharvbio
1. Introduction
Postharvest decay is the major factor limiting the extension of storage life of many fresh har-vested commodities. All fresh fruits and vegeta-bles for domestic or export markets should be free
of dirt, dust, pathogens and chemicals before they are packed. The susceptibility of freshly harvested produce to postharvest diseases increases during prolonged storage as a result of physiological changes that enable pathogens to develop in the fruits (Eckert and Ogawa, 1988). The use of syn-thetic chemicals on harvested fresh produce is becoming more difficult to justify (How, 1991). Therefore, the interest in ‘non-conventional’ methods for postharvest decay control of horticul-* Corresponding author. Tel.: +39-783-33224; fax: +
39-783-33959.
E-mail address:M.Schirra@imfpp.ss.cnr.it (M. Schirra).
tural crops has been increasing. To minimise pre-or postharvest treatments with agrochemicals (such as e.g. fungicides, insecticides, growth regu-lators, antioxidants), research efforts are currently focused on enhancement of host resistance to pathogens through physical, chemical or biologi-cal inducers (Ben-Yehoshua et al., 1988; Wilson et al., 1994; Ben-Yehoshua et al., 1997b). However, prestorage heat treatment appears to be one of the most promising in postharvest control of de-cay (Couey, 1989; Klein and Lurie, 1991; Schirra and Ben-Yehoshua, 1999). Besides physical meth-ods, postharvest heat treatments (hot water treat-ments and curing) are currently practised and recognised as methods of controlling postharvest diseases by direct inhibition of the pathogen and by stimulating certain host-defense responses (Schirra and Ben-Yehoshua, 1999). Additionally, these treatments enhance fruit resistance to chill-ing injury in sensitive cultivars (Wild and Hood, 1989; McDonald et al., 1991; Rodov et al., 1994a; Schirra and D’hallewin, 1997; Schirra et al., 1997b).
In the first decades of the 20th century, postharvest heat treatment was used on a com-mercial scale to control fungal diseases and insect infestation of horticultural crops. However, with the development of selective synthetic fungicides, the use of heat treatment was abandoned because of the greater advantages of fungicide treatments in terms of effectiveness, lower cost and ease of application. Many factors, however, have recently contributed to the implementation of strategies for reducing dependence on agrochemicals. These include: the enhanced proliferation of resistant strains of fungus due to the improper and pro-longed use of agrochemicals, thereby diminishing their efficacy (Holmes and Eckert, 1992; Eckert, 1995); the prohibitive costs of selecting, synthesis-ing and testsynthesis-ing new active synthesis-ingredients; and difficulties in registering them, as well as the pos-sibility of deregistration of approved fungicides.
The effect of heat therapy on horticultural crops has been reviewed during the last decade (Couey, 1989; Paull, 1990; Barkai-Golan and Phillips, 1991; Klein and Lurie, 1991; Arte´s, 1995; Lurie, 1998; Schirra and Ben-Yehoshua, 1999; Ben-Yehoshua et al., 2000). This paper deals with
the relevant literature on postharvest heat ther-apy, using citrus fruit as the main example, with emphasis on host-defense reactions in fruits af-fected by heat treatment.
2. Postharvest heat treatment
Postharvest heat treatment is also known as curing or conditioning. Curing is the objective of most heat treatments, e.g. to cure wounds and injuries caused during postharvest handling. Con-ditioning is a specific application of heat so as to enable produce to withstand stronger specific stresses such as sub-optimal temperatures; sterili-sation as a quarantine treatment against fruit flies is one example. These treatments have been classified as short-term (up to 60 min in water at 45 – 60°C) or long-term (12 h to 4 days in air at 38 – 46°C). The long-term treatments will be de-noted here as curing treatments (Paull and Mc-Donald, 1994).
2.1. Curing treatment
The first curing experiments on citrus fruit were performed by Fawcett, 1922 to reduce Phytoph
-thora citroph-thora(R.E. Sm. and E.H. Sm.) infec-tions. As early as 1936, Brooks and McColloch (1936) reported that heat treatment alleviated chilling injury in grapefruit (Citrus paradisi M.) stored at 2 or 4.5°C. Ben-Yehoshua et al. (1987a,b) demonstrated that curing of seal-pack-aged fruit at 36°C and saturated humidity for 3 days effectively reduced decay without any dam-age to various citrus fruit species during stordam-age at 17°C for 35 days. This treatment did not kill the already existing and growing mycelia of Peni
-cillium digitatum Sacc., P. italicum Wehmer and
Geotrichum candidum Lk.ex Pers., but suppressed their growth from conidia.
Curing at 32°C for 3 days was found to reduce
Di Martino Aleppo, 1996). These curing condi-tions caused a delay in conidia germination of P.
digitatum but did not prevent its growth. How-ever, exposure times longer than 48 h at 36 – 37°C adversely affected taste and flavour in pigmented orange cultivars (Schirra and D’hallewin, unpub-lished results).
Investigations with ‘Star Ruby’ grapefruit (D’hallewin et al., 1997) have shown that when curing (37°C for 72 h) was carried out within 36 h post-inoculation, it effectively controlled decay during 30 days of storage at 8°C (optimal storage conditions for this cultivar in Italy, Schirra, 1992) and a subsequent 1 week of simulated shelf-life conditions at 20°C.
2.2. Hot water treatment
In spite of its beneficial effects in reducing decay and chilling injury in various fruit species, curing is not widely utilised on a commercial scale. This is probably because of the high cost of heating large volumes of fruit for up to 3 days. By contrast, water-dip treatments at 50 – 53°C for 2 – 3 min have proven to be as effective as curing and are much less expensive (Rodov et al., 1993, 1994a). More recently, interest has also been fo-cused on a unique short hot water rinsing and brushing of agricultural fresh produce (Fallik et al., 1996a).
The beneficial effect of prestorage hot water dipping to prevent rot development has been shown in various fruit species world-wide (Mad-hukar and Reddy, 1990; Barkai-Golan and Phillips, 1991; Cheah et al., 1992; Jacobi and Wong, 1992; Garcı´a et al., 1995; Schirra et al., 1996a; Schirra and Ben-Yehoshua, 1999). Such an effect was thought to be partly dependent on the elimination of incipient infections by removing spores from wounds and acting directly on their viability (Couey, 1989). However, investigations at our laboratories (authors’ unpublished data) have demonstrated that spores ofPenicillium spp. cannot be easily eliminated by water dips as the number of conidia removed from wound-inocu-lated fruit following 2 min washing with water at 52°C was found to be negligible.
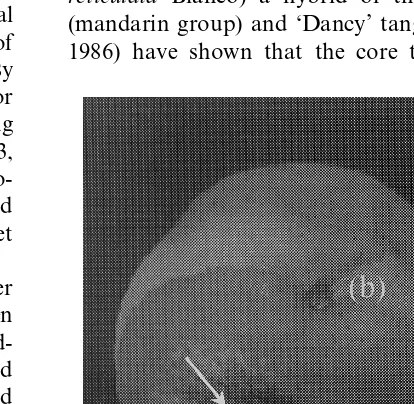
Experimental evidence on grapefruit that was wounded and inoculated with P. italicum and incubated for 4 days at 27°C has shown that dipping the infected fruit hemisphere resulted in a complete inhibition of the pathogen development in the submerged hemisphere. However, 6 days after dip treatment, mycelia outbreak and sporu-lation occurred in the area around the equatorial side of the non-heated hemisphere (Fig. 1). These results supported the evidence that heat treatment may induce fruit defense mechanisms in the outer layers of epicarp which inhibit pathogen spread (Ben-Yehoshua et al., 1988; Dettori et al., 1996; Fallik et al., 1996b; Ben-Yehoshua et al., 1997b; D’hallewin et al., 1997; Porat et al., 2000b). Al-though treatment conditions (temperatures and duration of hot water dipping) are very different from those of curing, the direct effect of heat on pathogens appears to be similar.
Investigations on ‘Fortune’ mandarin (Citrus reticulata Blanco) a hybrid of the ‘Clementine’ (mandarin group) and ‘Dancy’ tangerine (Young, 1986) have shown that the core temperature of
fruit subjected to curing at 37°C reaches room set-point temperature approximately 3 h after starting treatment (author’s unpublished data). By contrast, only the outer cell layer of the flavedo reaches equilibrium with water temperature dur-ing 3 min dippdur-ing in water at 50 – 58°C. In fact, 2 min after treatment the albedo temperature in fruits dipped at 50°C was 29.3°C and 33.0°C in those treated at 58°C (Schirra and D’hallewin, 1997). Thereafter temperatures continued to drop, rapidly reaching ambient values (about 18°C) af-ter 90 min. In the same period, the fruit core temperature rose gradually over about 20 min to a maximum of 27.5 and 29.8°C in fruit submerged at 50 – 58°C respectively, and then decreased, reaching ambient temperature approximately 2 h later.
Like curing, hot water dips at 50 – 53°C have proven to be ineffective in killing dormant spores but do cause a delay in conidia germination, growth and sporulation (Dettori et al., 1996; D’hallewin et al., 1997). These latter effects may allow fruit to build up or improve defense systems against pathogens. Studies on wounded grapefruit inoculated with P. digitatum (D’hallewin et al., 1997) have shown that a 2 min water dip at 50°C is effective against the pathogen when treatment is carried out within 36 h after inoculation. The mycelium was visibly damaged and infection was arrested. Similar results were found on ‘Star Ruby’ grapefruit inoculated withP.italicum (Det-tori et al., 1996). These findings appear to be important from a practical viewpoint as posthar-vest treatments in the packing house are usually carried out the day after harvesting. Treatment 48 – 72 h after incubation did not effectively con-trol decay development. However, inoculum po-tential was greatly affected by treatment, the incidence of fruit soiled by mold spores being reduced (Dettori et al., 1996). Water dipping at 50°C for 2 min was found to be effective only against superficial infections (3 – 4 mm deep) and ineffective on deeper infections (\5 mm). The
mycelium morphogenesis pattern of Penicillium spp. was markedly different for in vivo than for in vitro growth (Dettori et al., 1996; D’hallewin et al., 1997). In fact, in vivo morphological studies with P. italicum growing on ‘Star Ruby’
grape-fruit have shown that following water dipping at 50°C 1 h after wounding and inoculation, mycelium development was seriously affected, be-coming thinner, with reduced branching, and un-able to spread into the albedo. Pathogen growth was also hindered when dip treatments were car-ried out from 5 to 72 h after wounding and inoculation. By contrast, treatments in vitro with the pathogen growing in Petri dishes did not affect mycelium thickness and branching. Similar results were obtained when the hot water dip was applied to green lemons inoculated with P. digi
-tatum (Ben-Yehoshua et al., 1998). Mycelium of
direct heat effect on the fungus. These results suggest that host – pathogen interactions in vivo may be affected by heat-induced changes in the wounded tissue of the host.
2.3. Application of heat with other posthar6est treatments
The combination of curing over 48 – 72 h at 34 – 35°C within 48 h of harvest and individual seal-packaging of fruit in plastic film has greatly enhanced the beneficial effects of heat treatment by providing a water-saturated atmosphere and protecting the fruit from damage caused by high temperature (Ben-Yehoshua et al., 1987a,b, 1988, 1989a; Miller et al., 1990). Indeed, sealed and cured pomelo (Citrus grandis (L.) Osbeck) fruit that was not treated with any fungicides was kept for 8 weeks without any damage and decay and its appearance was judged as fresh. Fruit that was sealed but not cured had about 20% decay; fruit that was not sealed and not cured (control fruit) had about 45% decay and the non-decayed con-trol fruit was shrivelled and unmarketable (Ben-Yehoshua et al., 1987a,b). Similar results have been reported with other citrus fruit species (Yehoshua et al., 1989a,b; Kim et al., 1991; Ben-Yehoshua et al., 1992).
Mayberry and Hartz (1992) reported that the combination of polyethylene bags and water dips at 60°C for 3 min retained quality of muskmelon [Cucumis melo(L.)] for at least 28 days of storage at 3°C.
Investigations on ‘Fortune’ mandarin have shown that intermittent warming (cycles of 4 days at 2°C+3 days at 10°C) during storage improved the effectiveness of prestorage water dips at 52°C for 3 min in CI and decay control and could help reduce fungicide use in postharvest treatments (Schirra and Mulas, 1995a). Positive synergistic effects in decay control also occurred when curing (37°C for 72 h) was carried out in combination with a biocontrol agent (yeast isolate Candida famata) (D’hallewin et al., 1998). Hot water brushing has been suggested as an alternative method to SO2 fumigation for color retention of litchi fruits (Lichter et al., 2000).
3. Heat and fungicide treatments
Extensive work has been conducted in recent years to control postharvest decay of horticultural crops by using heat treatment in combination with agrochemicals. The focus of these efforts is to enhance the effectiveness of the active ingredi-ent (a.i.) in decay control and to minimise the doses of the chemicals being used with respect to conventional treatments. Positive synergistic ef-fects of combined chemical and hot water treat-ments have been corroborated on various fruit species (Wells and Harvey, 1970; Gutter, 1978; Sharma and Kaul, 1990; Barkai-Golan and Apel-baum, 1991; McDonald et al., 1991; Cohen et al., 1992; Coates et al., 1993; McGuire and Campbell, 1993; Schirra and Mulas, 1993; Conway et al., 1994; Rodov et al., 1994b; Schirra and Mulas, 1995b; Smilanick et al., 1995).
The mechanism of fungicide diffusion through the plant cuticle plays a major role in the uptake of a.i. (Riederer and Schreiber, 1995). During this process, the cuticle acts as a diffusion barrier: as the temperature increases, so does the diffusion and fungicide uptake (Cabras et al., 1999). It is thus possible to reduce the levels of chemicals usually employed in conventional postharvest treatments at ambient temperature without com-promising fruit quality and treatment efficacy in reducing decay.
Investigations on peach [Prunus persica (L.) Batsch], plum [P.salicinaLindl.] and nectarine [P.
persica (L.) Batsch var. nectarina (Alt.) Maxim.] have demonstrated that a 1.5 min dip treatment with 2,6-dichloro-4-nitroaniline (DCNA) at 51.5°C was much more effective in postharvest decay control than treatments with hot water or DCNA applied separately (Wells and Harvey, 1970). Indeed, after treatment with 225 mg/l DCNA at 51.5°C, the residue levels on fruit were found to be similar to those of 900 mg/l DCNA dips at room temperature.
50°C was as effective as 1500 mg/l at 20°C in suppressing Penicillium rots during 13 weeks of cold storage at 9°C plus 1 week of simulated shelf-life at 20°C. Given the linear relationship between the level of fungicide employed and the residue deposition on fruit, it was postulated in this study that 50 mg/l IMZ should be the mini-mum concentration at 50°C to achieve a reason-able control of Penicillium decay in lemons. This hypothesis was corroborated by later studies (Schirra et al., 1997a).
Similar results on increased activity and a.i. uptake were observed after hot thiabendazole (TBZ) treatments (Schirra et al., 1998a,b). It is worth noting that, in addition to its antifungal activity, TBZ displays physiological properties in reducing chilling injury (Schiffman-Nadel et al., 1972, 1975), and these effects were enhanced when TBZ was used in combination with hot water (see reviews: Paull and McDonald, 1994; Schirra and Ben-Yehoshua, 1999). The increased efficacy of heated compared to unheated fungicide, at the same chemical doses, was related to the increased fruit a.i. uptake (Schirra et al., 1998a), although factors other than TBZ concentration are also involved in the CI-alleviating effects. For exam-ple, it has been demonstrated that postharvest treatments with 1200 mg/l TBZ at room tempera-ture (19°C) or 200 mg/l TBZ at 50°C produced similar TBZ uptake in ‘Tarocco’ (blood) oranges, but treatment at 50°C was the most effective in reducing CI (Schirra et al., 1998b).
It is interesting to note that fruit harvested in April (late season) and treated with 1200 mg/l TBZ at room temperature contained significantly more TBZ than fruit picked earlier, probably because of the presence of gaps in the epicuticular wax of the more mature fruit (Freeman et al., 1979; El-Otmani et al., 1989; D’hallewin and Schirra, 2000). In contrast, TBZ deposition fol-lowing 200 mg/l dipping at 50°C was not signifi-cantly dependent on maturity stage due to the enhanced mobility and penetration of the a.i. through the epicuticular wax during treatment. The great persistence of TBZ in fruit treated at 50°C with respect to fruit treated at room temper-ature was related to the better encapsulation and coverage of the a.i. by epicuticular wax, thus providing better protection to the chemical.
4. Heat treatment and structural changes of epicuticular wax
Investigations on apples have shown that im-portant structural changes of epicuticular wax occurred as a result of curing (Roy et al., 1994). The epicuticular wax of non-heated fruit dis-played a number of deep surface cracks that formed an interconnected network on peel sur-face. In fruit harvested before the climacteric, these cracks became wider and deeper during long-term storage (Roy et al., 1999). A positive relationship occurred between increase in cracking and calcium uptake by fruit subjected to calcium chloride pressure infiltration (Roy et al., 1999). Following postharvest heat treatment at 38°C for 4 days, the cuticular cracks disappeared, probably as a result of the ‘melting’ of the wax platelets that had occurred in the cracks (Roy et al., 1994). Similar changes in epicuticular wax structure have been observed in various fruit species sub-jected to heating, such as 2 min water dipping at 52°C of ‘Oroblanco’ (C. grandis Osb. x C. Para
-disi, cv. Oroblanco, Syn. Sweety) grapefruit hy-brid (Rodov et al., 1996b), 2 min water dip at 50 – 54°C of ‘Fortune’ mandarins (Schirra and D’hallewin, 1996, 1997), and 3 min water dips at 52°C or curing at 37°C for 24 – 72 h cactus (prickly) pears [Opuntia ficus-indica Miller (L.)] (D’hallewin et al., 1999), as well as after hot water rinsing and brushing (HWRB) sweet pepper (Cap
-sicum anuum) (Fallik et al., 1999), melons (Cu
-cumis melo) (Fallik et al., 2000) and organically grown grapefuits (Porat et al., 2000a). Thus, fruit response to various types of heat treatments, in terms of changes of ultrastructure of epicuticular wax, appears to be quite similar.
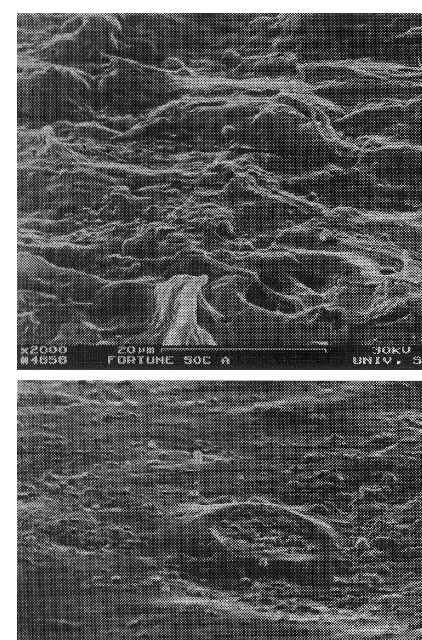
Fig. 2. SEM micrographs of ‘Fortune’ mandarin hybrid skin surface following 3 min water dips at 50°C: stomata partially (a) and completely filled by molten wax (b).
prior to penetration, presumably because of topo-graphical stimuli (Mendegen et al., 1988; Isaac, 1992).
Similar results were observed on cactus pears (Schirra et al., 1999): platelets, cracks and stomata which disappeared as a result of heat treatment at 37°C for 30 h appear again after 45 days of storage and subsequent 3 days simulated shelf-life.
5. Heat treatment and host pathogen interactions
5.1. Pathogen response to heat treatment
The efficacy of heat treatment on the pathogen
Fig. 3. SEM micrographs of ‘Star Ruby’ grapefruit skin sur-face: (a) wax plates and cuticular cracks; (b) a stomata with mycelia of P.digitatumafter 6 weeks of fruit storage at 2°C and an additional week at 20°C.
is usually measured by reduced viability of the heated propagules. However, heat effects may be lethal or sublethal (Castejon-Munoz and Bollen, 1993). The response of decay-causing agent to heat can be influenced by several factors such as the moisture content of spores, age of the inocu-lum and inocuinocu-lum concentration (Barkai-Golan and Phillips, 1991), as well as the host (Klein and Lurie, 1991).
Although reports have indicated a linear rela-tionship between the logarithm of the reduction time and temperature of heat treatment (Pull-man et al., 1981; Roebroeck et al., 1991), patho-gen kill is not always proportional to the temperature-time product of the treatment. The sensitivity of fungal spores to heat treatments does not necessarily depend upon the spore size, shape or inoculum age. Fungi express consider-able species variation in sensitivity to high tem-peratures (Sommer et al., 1967; Castejon-Munoz and Bollen, 1993). In vitro experiments showed that Botrytis cinerea was found to be more sen-sitive to heat than Alternaria alternata (Fallik et al., 1996b), while A. alternata was more sensitive than Fusarium solani (Fallik et al., 2000). Col
-letotrichum gloeosporiodes was found to be more heat-sensitive than Dothiorella dominicana (Rap-pel et al., 1991), The effective time – temperature regime that killed 50% of the spores (ET50) for spore germination and germ tube elongation for
B. cinerea was significantly shorter than that for
A. alternata (Fallik et al., 1996b). Heating ger-minated spores for a very short time can also affect spore viability. Fallik et al. (2000) re-ported that the ET50 for Allernaria was 25 and 16 s at 55 and 65°C, respectively, whereas for
Fusariumthe ET50 was 18 s at 60°C.
Germinating spores were found to be more sensitive to heat treatment than was mycelial growth (Fallik et al., 1993). Penicillium expan
-sum, the main decay-causing agent on stored ap-ples in Israel, was found to be relatively resistant to high temperature. The time to re-duce spore germination of P. expansum by 50% (ET50) was found to be 12, 23 and 45% shorter than mycelial growth at 38, 42 and 46°C, respec-tively (Fallik et al., 1995). Barkai-Golan (1973) reported that germinated spores of Alternaria al
-ternata (formely A. tenuis) were more sensitive to heat than non-germinated spores. Hot water treatments were found to be ineffective in killing dormant spores (Barkai-Golan, 1973; D’hallewin et al., 1997).
The above results obtained by in vitro experi-ment could explain the direct effect of heat treatment in vivo. A certain threshold of inocu-lum level is needed to initiate decay development (Yao and Tuite, 1989; Trapero-Casas and Kaiser, 1992). As a result of heat treatments, which reduce fungal viability, the effective inocu-lum concentration which causes decay develop-ment is reduced, thus reducing rot developdevelop-ment. Hot water rinsing and brushing (HWRB) was reported to reduce significantly decay develop-ment on several fresh harvested commodities (Ben-Yehoshua et al., 1997a; Fallik et al., 1999; Prusky et al., 1999; Fallik et al., 2000; Porat et al., 2000a). Employing HWRB resulted in a 3 – 4 log reduction of the total microbial counts (CFU) of the epiphytic microorganism popula-tion, compared to untreated fruit as was re-vealed by scanning electron microscopy analysis (Fallik et al., 2000; Porat et al., 2000a). Another explanation for the reduction in rot development could be a reduction in spore survival of various decay causing agents such as with Penicillia in ‘Valencia’ oranges (Williams et al., 1994). Heat may also cause changes in nuclei and cell walls, denature proteins, destroy mitochondria and outer membranes, disrupt vacuolar membranes and formation of gaps in the spore cytoplasm (Barkai-Golan and Phillips, 1991).
5.2. Fruit responses affecting pathogen defense
parallel the breakdown of antifungal compounds that are constitutively present in the exocarp. Heat treatment is known to inhibit ripening in climacteric fruit such as tomato (Lurie, 1998), but to accelerate the ripening in mango fruit (Prusky, 1996). Plumbley et al. (1993) reported that levels of an antifungal diene, constitutively present in avocado epicarp, decreased and disappeared as the fruit ripened; at the same time quiescent infec-tions of Colletotrichum gloeosporioides Penz. oc-curred. Dry heat treatment delayed decay development in the avocado (Lurie et al. 1997b) whereas water dipping at 55°C for 10 min acceler-ated both the appearance of disease symptoms and antifungal diene breakdown (Plumbley et al., 1993). Mature green tomatoes are strongly resis-tant to infections. The increased susceptibility of tomatoes to decay during ripening has been asso-ciated with the decrease and disappearance of mRNA encoding an anionic peroxidase (Sherf and Kolattukudy, 1993). Heat treatment of ma-ture green tomatoes was proven to delay the degradation rate of such mRNA and to maintain antifungal resistance in the fruit tissue to decay (Lurie et al. 1997a).
Oil cavities of citrus flavedo (exocarp) constitu-tively contain certain compounds (such as citral in lemons), having antifungal activity (Kim et al., 1991; Ben-Yehoshua et al., 1992; Rodov et al., 1995). Mechanical wounding releases the content of the cavities, which may come into contact with such penetrating wound pathogens as P. digi
-tatum. In young mature-green citrus fruit, consti-tutive antifungal compounds act as a first line of defense against pathogens. Ben-Yehoshua et al. (1995) reported that curing lemon fruit (3 days at 36°C, 97% R.H.) inhibited the decline of antifun-gal activity in the flavedo tissue, reduced the loss of citral and suppressed decay development. Such effect of heat has been described as modulation of the ageing-associated decline of naturally occur-ring antifungal compounds (Ben-Yehoshua et al., 1995).
The presence of lignin in plant tissue is recog-nised as increasing its resistance to infections as most plant pathogens cannot degrade lignin and it serves as a strong mechanical barrier against pathogen invasion (Friend 1976). Accumulation
of lignin-like material and/or antifungal com-pounds may be induced in response to injuries or infections (Friend, 1976; Stange et al., 1993). Heating wounded pear fruits at 37°C reduced
Mucor rot and Phialophora side rot even when fruits were inoculated after heating, demonstrat-ing a wound healdemonstrat-ing response (Spotts and Chen, 1987). Curing at 32°C and 90% RH for 2 days enhanced fruit resistance to infection by induction of the healing process involving the biosynthesis of lignin-like compounds, which are catalysed by phenyl ammonialyase (PAL), a key enzyme in the phenylpropanoid pathway (Ismail and Brown, 1975; Brown et al., 1978; Ismail and Brown, 1979; Ben-Yehoshua et al., 1987a,b). This lignin-like production creates a physical barrier in the wound, which hinders pathogen penetration. Mu-las et al. (1996) reported that various factors such as wounding, chilling injury and senescence in-creased the accumulation of ethanol-extractable phloroglucinol/HCl positive compounds in flavedo tissue of ‘Oroval’ clementines, suggesting that wound gum deposition rather than lignin was more likely related to the healing process. Investi-gations with green lemons inoculated with P.
hyphae (Fallik et al., 1995). The partial recovery that was obtained, the lag in initial germination, and the decreased rate of growth all indicate that there is a residual ‘shock effect’ of heating well after application of the treatment. The physiologi-cal phenomenon is strengthened by the finding that adding crude extract from the peel of heated fruit to PDA inhibits fungal growth but also results in distorted mycelia (Fallik et al., 1995). The effect of crude extract on fungal growth supports the hypothesis that antifungal-like sub-stances are also involved in the resistance of heated apple fruit to P. expansum.
Studies on various citrus fruit cultivars (Kim et al., 1991) have shown that curing of Penicillium spp.-inoculated fruit prevented the development of the pathogen and promoted biosynthesis of the phytoalexin scoparone by cells adjacent to the wound. Hot water dipping induces the occurrence of scoparone and scopoletin in wounds of grape-fruit approximately 12 – 14 h after treatment (D’hallewin et al., 1997). However, their appear-ance proved not to depend only on pathogenic infections. Ultra-violet illumination, gamma-irra-diation, and biological antagonists also induce the production of phytoalexins (Ben-Yehoshua et al., 1992; Rodov et al., 1992; Wilson et al., 1994; D’hallewin et al., 1999). When heat treatment was carried out before wounding, neither scoparone nor scopoletin was detected in post-treated inocu-lated wounds. Hot water dip applied to green lemons 2 days after inoculation withP.digitatum
induced scoparone production as soon as 24 h after treatment, and scoparone and scopoletin rose to an effective dose to inhibit the pathogen within 2 days of the treatment. Wounding, either followed by a hot water dip or not, induced scoparone production to a much smaller extent than the combined inoculation and heat treatment (Ben-Yehoshua et al., 1998).
Recently, Porat et al. (2000b) reported that hot water drench of 62°C for 20 s with additional brushing applied to ‘Star Ruby’ grapefruit before the inoculation with the pathogen, induced resis-tance against decay. This resisresis-tance was most effective when the inoculation was carried out 1 day after the brushing and was less effective when the fruit were inoculated at the same day or 7
days after the brushing. The heat treatment was essential to decay control as brushing with cold water did not enhance any resistance.
It has been demonstrated that pathogen-related proteins such as chitinases, which are known to play an important role in hyphae degradation (Bucheli et al., 1990), are constitutively present in orange peel and that their levels increased follow-ing pathogen inoculation and subsequent heat treatment (Rodov et al., 1996a). Porat et al. (per-sonal communication) recently found that both b-1,3-glucanase and chitinase were induced by a hot water drench with additional brushing of ‘Star Ruby’ grapefruit applied before the inocula-tion with P. digitatum. They suggested also that various heat shock proteins may be involved in the hot water brushing-induced resistance responses.
6. Concluding remarks
Heat treatments have been developed as a non-chemical method of disinfection of fresh harvested fruits and vegetables. Postharvest heat treatments to control decay are often applied for a relatively short time (seconds to minutes) because the target pathogens are found on the surface or in the first few layers under the skin of the fruit or vegetable. However, in quiescent infection where fungi are located within the tissue fruit, decay development is not necessarily controlled by heat treatment (Johnson et al., 1992). Heat treatments against decay-causing agents may be applied to fruits and vegetables in several ways: by hot water dips, by vapour heat, by hot dry air (Lurie, 1998; Schirra and Ben-Yehoshua, 1999) or by a very short hot water rinse and brushing (Fallik et al., 1996a).
Fruit dipping for 2 min in water at 53°C ar-rested the growth of P.digitatum and P.italicum
for at least 24 h, allowing the infected fruit to build up resistance mechanisms against the patho-gen. The following protective mechanisms were elicited in inoculated and subsequently hot-dipped fruit: production of lignin-like material within a few hours and on the inoculation site, followed by accumulation of the phytoalexins scoparone and scopoletin and the production of pathogen-related proteins such as chitinase and heat shock proteins. Hot water dip, by itself, did not elicit lignification or phytoalexin production in lemons and grapefruits unless the fruit was pathogen-challenged or wounded.
Hot drench with such fungicides as imazalil or thiabendazole appears to be an attractive practical solution and is already being implemented in many packing houses. The possibility of drasti-cally reducing the use of fungicides in postharvest treatments of fruit is of considerable practical importance. Indeed, given the enormous amount of these chemicals being employed today through-out the world, fungicide residues may well repre-sent a major threat to human health, with unpredictable consequences for the economy and the environment. The higher costs incurred by the heating of these mixtures with respect to ‘conven-tional’ treatments should be more than offset by the marked savings on chemicals and the lower costs involved in treatment facilities for packing house wastewater.
Acknowledgements
This research was partially funded by European Union FAIR CT-4096 and by the Agricultural Research Organisation, The Volcani Centre, Bet Dagan, Israel, Serial number 414/00.
References
Arte´s, F., 1995. Review: Innovaciones en los tratamientos fisicos para preservar la calidad de los productos hortofru-tı´colas en la po´strecoleccion. I. Pretratamientos te´rmicos. Rev. Esp. Cienc. Tecnol. Aliment. 35, 45 – 64.
Barkai-Golan, R., 1973. Postharvest heat treatment to control Alternaria tenuisAuct. rot in tomato. Phytopathol. Medit. 12, 108 – 111.
Barkai-Golan, R., Apelbaum, A., 1991. Synergistic effects of heat and sodium o-phenylphenate treatments to inactivate Penicillium spores and suppress decay in citrus fruits. Trop. Sci. 31, 229 – 230.
Barkai-Golan, R., Phillips, D.S., 1991. Postharvest heat treat-ment of fresh fruits and vegetables for decay control. Plant Dis. 75, 1085 – 1089.
Bell, A.A., 1981. Biochemical mechanism of disease resistance. Ann. Rev. Plant Physiol. 32, 21 – 81.
Ben-Yehoshua, S., Barak, S., Shapiro, B., 1987a. Postharvest curing at high temperature reduces decay of individual sealed lemons, pomelos, and other citrus fruits. J. Am. Soc. Hort. Sci. 112, 658 – 663.
Ben-Yehoshua, S., Kim, J.J., Shapiro, B., 1989a. Curing of citrus fruit. Application and mode of action. Fifth Proc. Cont. Atmosphere Research Conference. Vol 2. Other commodities and Storage Recommendations. June 14 – 16, 1989. Wenatchee, Washington, USA. 179 – 196.
Ben-Yehoshua, S., Kim, J.J., Shapiro, B., 1989b. Elicitation of resistance to the development of decay in sealed citrus fruit by curing. Acta Hortic. 258, 623 – 630.
Ben-Yehoshua, S., Nafussi, B., Peretz, J., Rodov, V., 1998. Mode of action of heat treatments of citrus fruits in reducing decay. COST 98 Meeting, Madrid, Spain. In press.
Ben-Yehoshua, S., Peretz, J., Rodov, V., Nafussi, B., Yeku-tieli, O., Wiseblum, A., Regev, R., 2000. Postharvest appli-cation of hot water treatment in citrus fruits: The road from the laboratory to the packing-house. Acta Hortic. 518, 19 – 28.
Ben-Yehoshua, S., Peretz, J., Rodov, V., Nafussi, B., Yeku-tieli, O., Regev, R., Weiseblum, A., 1997a. Commercial application of hot water treatments to reduce decay inci-dence in Kumquat fruit. Alon haNotea 52, 348 – 352. Ben-Yehoshua, S., Rodov, V., Fang, D.Q., Kim, J.J., 1995.
Preformed antifungal compounds of citrus fruit: effects of postharvest treatments with heat and growth regulators. J. Agric. Food Chem. 43, 1062 – 1066.
Ben-Yehoshua, S., Rodov, V., Kim, J.J., Carmeli, S., 1992. Preformed and induced antifungal materials of citrus fruits in relation to the enhancement of decay resistance by heat and ultraviolet treatments. J. Agric. Food Chem. 40, 1217 – 1221.
Ben-Yehoshua, S., Rodov, V., Peretz, J., 1997b. The constitu-tive and induced resistance of citrus fruit against patho-gens. In: Johnson, G.I., Highly, E., Joyce, D.C. (Eds.), Disease Resistance in Fruit, ACIAR Proc. No. 80, Can-berra, Australia, pp. 78 – 92
Ben-Yehoshua, S., Shapiro, B., Moran, R., 1987b. Individual seal packaging enables the use of curing at high tempera-tures to reduce decay and heal injury of citrus fruits. HortScience 22, 777 – 783.
patho-gens and its enhancement by curing. In: Goren, R., Mendel, K. (Eds.), Proc 6th Int. Citrus Congr. Balaban Publishing, Rehovot, Israel, pp. 1371 – 1374.
Brooks, C., McColloch, C., 1936. Some storage diseases of grapefruit. J. Agric. Res. 52, 319 – 351.
Brown, G.E., Ismail, M.A., Barmore, C.R., 1978. Lignification of injuries to citrus fruit and susceptibility to green mold. Proc. Fla. State Hort. Soc. 91, 124 – 126.
Bucheli, P., Doares, S.H., Albersheim, P., Darvill, A., 1990. Host pathogen interactions XXXVI: Partial purification and characterization of heat-labile molecules secreted by the rice blast pathogen that solubilizes plant cell wall fragments that kill plant cells. Physiol. Molec. Plant Pathol. 36, 159 – 173.
Cabras, P., Schirra, M., Pirisi, F.M., Garau, V.L., Angioni, A., 1999. Factors affecting imazalil and thiabendazole uptake and persistence in citrus fruits following dip treat-ments. J. Agric. Food Chem. 47, 3352 – 3354.
Castejon-Munoz, M., Bollen, G.J., 1993. Induction of heat resistance in Fusarium oxysporum and Verticillium Dahliae caused by exposure to sublethal heat treatments. Netherland J. Plant Pathol. 99, 77 – 84.
Cheah, L.H., Irving, D.E., Hunt, A.W., Corrigan, V.K., 1992. Effect of hot water dips on Botrytis storage rot and quality of kiwi fruit. Postharvest Biol. Technol. 2, 1 – 6.
Coates, L.M., Johnson, G.I., Cooke, A.W., 1993. Postharvest disease control in mangoes using high humidity hot air and fungicide treatments. Ann. Appl. Biol. 123, 441 – 448. Cohen, E., Chalutz, E., Shalom, Y., 1992. Reduced chemical
treatment for postharvest control of citrus fruit decay. Proc. Int. Soc. Citriculture 3, 1064 – 1065.
Conway, W.S., Sams, C.E., Wang, C.Yi., Abbott, J.A., 1994. Additive effects of postharvest calcium and heat treatment on reducing decay and maintaining quality in apples. J. Amer. Soc. Hort. Sci. 119, 49 – 53.
Couey, H.M., 1989. Heat treatment for control of postharvest diseases and insect pests of fruits. HortScience 24, 198 – 202.
Dettori, A., D’hallewin, G., Agabbio M., Marceddu, S., Schirra, M., 1996. S.E.M. studies on Penicillium italicum — ‘Star Ruby’ grapefruit interactions as affected by fruit hot water dipping. Proc. VIII Int. Citrus Congress, 12 – 17 May 1996, Sun City Resort, South Africa. Vol. 2, pp. 1158 – 1163.
D’hallewin, G., Dettori, A., Marceddu, S., Schirra, M., 1997. Evoluzione dei processi infettivi di Penicillium digitatum Sacc. in vivo e in vitro dopo immersione in acqua calda. Italus Hortus 4, 23 – 26.
D’hallewin, G., Schirra, M., 2000. Structural changes of epicu-ticular wax and storage response of ‘Marsh’ grapefruits after ethanol dips at 21 and 50°C. Proc. Int. Symp. Postharvest 2000. March 26 – 31, Jerusalem, Israel. (In press).
D’hallewin, G., Schirra, M., Manueddu, E., 1999. Effect of heat on epicuticular wax of cactus pear fruit. Trop. Sci. 39, 244 – 247.
D’hallewin, G., Arras, G., Dessı`, R., Dettori, A., Schirra, M., 1998. Citrus green mould control in stored ‘Star Ruby’ grapefruit by the use of a bio-control yeast under curing conditions. Acta Hortic. 495, 111 – 115.
Eckert, J.W., 1995. Postharvest disease control: experience with citrus fruit. Tree Fruit Postharvest J. 6, 9 – 12. Eckert, J.W., Eaks, I.L., 1988. Postharvest disorders and
diseases of citrus fruit. In: Reuther, W, Calavan, E.C., Carman, G.E. (Eds). The Citrus Industry. University of California Press, pp.179-260.
Eckert, J.W., Ogawa, J.M., 1988. The chemical control of postharvest diseases: Deciduous fruits, berries, vegetables and root/tuber crops. Ann. Rev. Phytopathol. 26, 433 – 469.
El-Otmani, M., Arpaia, M.L., Coggins, Jr. Ch. W., Pehrson, J.E. O’Connell, N.V., 1989. Developmental changes in ‘Valencia’ orange fruit, epicuticular wax in relation to position on the tree. Sci. Hortic. 41, 69 – 81.
Fallik, E., Klein, J., Grinberg, S., Lomaniec, E., Lurie, S., Lalazar, E., 1993. Effect of postharvest heat treatment of tomatoes on fruit ripening and decay caused by Botrytis cinerea. Plant Dis. 77, 985 – 988.
Fallik, E., Grinberg, S., Gambourg, M., Klein, J.D., Lurie, S., 1995. Prestorage heat treatment reduces pathogenicity of Penicillium expansumin apple fruit. Plant Pathol. 45, 92 – 97.
Fallik, E., Aharoni, Y., Yekutieli, O., Wiseblum, A., Regev, R., Beres, H., and Bar-Lev, E. 1996a. A method for simultaneously cleaning and disinfecting agricultural pro-duce. Israel patent No. 116965.
Fallik, E., Grinberg, S., Alkalai, S., Lurie, S., 1996b. The effectiveness of postharvest hot water dips on the control of gray and black moulds in sweet red pepper (Capsicum annuum). Plant Pathol. 45, 644 – 649.
Fallik, E., Grinberg, S., Alkalai, S., Yekutieli, O., Wiseblum, A., Regev, R., Beres, H., Bar-Lev, E., 1999. A unique rapid hot water treatment to improve storage quality of sweet pepper. Postharv. Biol. Technol. 15, 25 – 32. Fallik, E., Aharoni, Y., Copel, A., Rodov, R., Tuvia-Alkalai,
S., Horev, B., Yekutieli, O., Wiseblum, A., Regev, R., 2000. A short hot water rinse reduces postharvest losses of Galia melon. Plant Pathol. 49, 333 – 338.
Fawcett, H.S., 1922. Packing house control of brown rot. Citrograph 7, 232 – 234.
Freeman, B., Albrigo, L.G., Biggs, R.H., 1979. Ultrastructure and chemistry of epicuticular waxes of developingCitrus leaves and fruits. J. Amer. Soc. Hort. Sci. 104, 801 – 808. Friend, J., 1976. Lignification of infected tissue. In: Friend, J.,
Threlfall, D.R. (Eds.), Aspects of Plant Parasite Relation-ships. Ann. Proc. Phytochemical. Soc, vol. 13. Academic Press, New York, pp. 291 – 303.
Holmes, G.H., Eckert, J.W., 1992. Reduced sensitivity of Penicillium digitatum to imazalil, thiabendazole and o-phenylphenol. Phytopathol. 82, 1069 Abstr.
How, R.B., 1991. Marketing Fresh Fruits and Vegetables. New-York: AVI Book by Van Nostrand Reinhold. 336 p. Isaac, S., 1992. Fungal-Plant interactions. Chapman & Hall,
London, p. 418.
Ismail, M.A., Brown, G.E., 1975. Phenolic content during healing of ‘Valencia’ orange peel under high humidity. J. Amer. Soc. Hort. Sci. 100, 249 – 251.
Ismail, M.A., Brown, G.E., 1979. Postharvest wound healing in citrus fruit: induction of phenylalanine ammonia-lyase in injuried ‘Valencia’ orange flavedo. J. Amer. Soc. Hort. Sci. 104, 126 – 129.
Jacobi, K.K., Wong, L.S., 1992. Quality of ‘Kensington’ mango (Mangifera indicaLinn.) following hot water and vapor heat treatments. Postharvest Biol. Technol. 1, 349 – 359.
Johnson, G.I., Mead, A.J., Cooke, A.W., Dean, J.R., 1992. Mango stem end rot pathogen-fruit infection by endo-phytic colonization of the inflorescence and pedicel. Ann. Appl. Biol. 120, 225 – 234.
Kim, J.J., Ben-Yehoshua, S., Shapiro, B., Henis, Y., Carmeli, S., 1991. Accumulation of scoparone in heat-treated lemon fruit inoculated with Penicillium digitatum Sacc. Plant Physiol. 97, 880 – 885.
Klein, J.D., Lurie, S., 1991. Postharvest heat treatment and fruit quality. Postharvest News Inf. 2, 15 – 19.
Lanza, G., Di Martino Aleppo, E., 1996. Control of green mould of oranges and lemons by curing at high tempera-tures, Proc. VIII Int. Citrus Congress, 12 – 17 May, 1996. Sun City Resort, South Africa. Vol. 2, pp. 1187 – 1191. Lichter, A., Dvir, O., Rot, I., Akerman, M., Regev, R.,
Wiesblum, A., Fallik, E., Zauberman, G., Fuchs, Y., 2000. Hot water brushing: an alternative method to SO2
fumiga-tion for color retenfumiga-tion of litchi fruits. Postharvest. Biol. Technol. 18, 235 – 244.
Lurie, S., 1998. Postharvest heat treatments of horticultural crops. Hort. Rev. 22, 91 – 121.
Lurie, S., Fallik, E., Handros, A., Shapira, R., 1997a. The involvement of peroxidase in resistance ofBotrytis cinerea in heat treated fruit. Physiol. Molec. Plant Pathol. 50, 141 – 149.
Lurie, S., Handros, A., Ackman, M., Shapira, R., Zauberman, G., 1997b. Prestorage heat treatments of avocados. In: Gazit, S. (Ed.), Proc of Avocado Congress. Tel Aviv, Israel, pp. 31 – 37.
Madhukar, J., Reddy, S.M., 1990. Control of fruit rot of guava by hot water treatment. Indian Phytopathol. 43, 234 – 236.
Mayberry, K.S., Hartz, T.K., 1992. Extention of muskmelon storage life through the use of hot water treatment and polyethylene wraps. HortScience 27, 324 – 326.
McDonald, R.E., Miller, W.R., McCollum, T.G., Brown, G.E., 1991. Thiabendazole and Imazalil applied at 53°C reduce chilling injury and decay of grapefruits. HortScience 26, 397 – 399.
McGuire, R.G., Campbell, C.A., 1993. Imazalil for posthar-vest control of anthracnose on mango fruits. Acta Hortic. 341, 371 – 376.
Mendegen, K., Schneider, A., Sterk, M., Fink, W., 1988. The differentiation of infection structures as a result of recogni-tion events between some biotrophic parasites and their hosts. J. Phytopatol. 123, 259 – 272.
Miller, W.R., Chun, D., Risse, L.A., Hatton, T.T., Hinch, R.T., 1990. Conditioning of Florida grapefruit to reduce peel stress during low temperature storage. HortScience 25, 209 – 211.
Mulas, M., Lafuente, M.T., Zacarias, L., 1996. Lignin and gum deposition in wounded ‘Oroval’ clementines as af-fected by chilling and peel water content. Postharvest Biol. Technol. 7, 243 – 251.
Nafussi, B., Ben-Yehoshua, S., Rodov, V., Peretz, J., Ozer, B.K., D’hallewin, G., 2000. Mode of action of hot water dip in reducing decay in lemon fruit. Proc. Int. Symp. Postharvest 2000. March 26 – 31, Jerusalem, Israel. In press.
Paull, R.E., 1990. Postharvest heat treatments and fruit ripen-ing. Postharvest News & Inf. 1, 355 – 363.
Paull, R.E., McDonald, R.E., 1994. Heat and cold treatments. In Insect Pests and Fresh Horticultural Products: Treat-ments and Responses. in: R.E. Paull and J.W. Armstrong (Eds.), CAB Intl. Wallingford, UK, pp. 191 – 222. Plumbley, E.A, Prusky, D., Kobiler, I., 1993. The effect of hot
water treatment on the levels of antifungal diene and quiescence of Colleotrichum gloeosporioides in avocado fruit. Plant Pathol. 42, 116 – 120.
Porat, R., Daus, A., Weiss, B., Cohen, L., Fallik, E., Droby, S., 2000a. Reduction of postharvest decay in organic citrus fruit by a short hot water brushing treatment. Postharvest Biol. Technol. 18, 151 – 157.
Porat, R., Pavoncello, D., Peretz, J., Weiss, B., Daus, A., Cohen, L., Ben-Yehoshua, S., Fallik, E., Droby, S., Lurie, S., 2000b. Induction of resistance toPenicillium digitatum and chilling injury in ‘Star Ruby’ grapefruit by a short hot water rinse and brushing treatment. J. Hort. Sci. Biotech. 75, 428 – 432.
Prusky, D., 1996. Pathogen quiescence in postharvest diseases. Ann. Rev. Phytopathol. 34, 413 – 434.
Prusky, D., Fuchs, Y., Kobiler, I., Roth, I., Weksler, A., Shalom, Y., Fallik, E., Zaurberman, G., Pesis, E., Aker-man, M., Yekutieli, O., Wiseblum, A., Regev, R., Arte´s, L., 1999. Effect of hot water brushing, prochloraz treat-ment and waxing on the incidence of black spot decay caused byAlternaria alternatain mango fruit. Postharvest Biol. Technol. 15, 165 – 174.
Pullman, G.S., DeVay, J.E., Garber, R.H., 1981. Soil solariza-tion and thermal death: a logarithmic relasolariza-tionship between time and temperature for four soilborne plant pathogens. Phytopathol. 71, 959 – 964.
Riederer, M., Schreiber, L., 1995. Waxes — The transport barriers of plant cuticles. In: Hamilton, R.J. (Ed.), Waxes: Chemistry, molecular biology and functions, vol. 6. The Oily Press, Dundee, Scotland, pp. 131 – 156.
Rodov, V., Ben-Yehoshua, S., Albagli, R., Fang, D.Q., 1994a. Reducing chilling injury and decay of stored citrus fruit by hot water dips. Postharvest Biol. Technol. 5, 119 – 127. Rodov, V., Ben-Yehoshua, S., Fang, D.Q., Kim, J.J.,
Ashke-nazi, R., 1995. Preformed antifungal compounds of lemon fruit: Citral and its relation to disease resistance. J. Agric. Food Chem. 43, 1057 – 1061.
Rodov, V., Ben-Yehoshua, S., Fang, D., D’hallewin, D., Cas-tia, T., 1994b. Accumulation of phytoalexins scoparone in citrus fruit subjected to various postharvest treatment. Acta Hortic. 381, 517 – 523.
Rodov, V., Ben-Yehoshua, S., Albagli, R., Fang, D.Q., 1993. Postharvest heat treatments of citrus fruits: curing vs. hot water application. In: Yupera, E.P., Calero, F.A., Sanchez, J.A. (Eds.), 2nd Int. Congr. Food Technology and Devel-opment, Murcia. Spain PPU Publ, Barcelona, Spain, pp. 176 – 203.
Rodov, V., Ben-Yehoshua, S., Kim, J.J., Shapiro, B., Ittah, Y., 1992. Ultraviolet illumination induces scoparone pro-duction in kumquat and orange fruit and improves decay resistance. J. Amer. Soc. Hort. Sci. 117, 788 – 792. Rodov, V., Burns, P., Ben-Yehoshua, S., Fluhr, R., Ben
Shalom, N., 1996a. Induced local disease resistance in citrus mesocarp (albedo): accumulation of phytoalexins and PR proteins. Proc. VIII Int. Citrus Congress, 12 – 17 May, 1996. Sun City Resort, South Africa. Vol. 2, pp. 1101 – 1104.
Rodov, V., Peretz, J., Agar, T., D’hallewin, G., Ben-Ye-hoshua, S., 1996b. Heat applications as complete or partial substitute of postharvest fungicide treatments of grapefruit and Oroblanco fruits. Proc. VIII Int. Citrus Congress, 12 – 17 May, 1996. Sun City Resort, South Affica. Vol. 2, pp. 1187 – 1191.
Roebroeck, E.J.A, Jansen, M.J.W., Mes, J.J., 1991. A mathe-matical model describing the combined effect of exposure time and tempertaure of hot-water treatments on survival of gladiolous corms. Ann. Appl. Biol. 119, 89 – 96. Roy, S., Conway, W.S., Watada, A.E., Sams, C.I., Erbe, E.F.,
Wergin, W.P., 1994. Heat treatment affects epicuticular wax structure and postharvest calcium uptake in ‘Golden delicious’ apples. HortScience 29, 1056 – 1058.
Roy, S., Conway, W.S., Watada, A.E., Sams, C.I., Erbe, E.F., Wergin, W.P., 1999. Changes in ultrastructure of the epicu-ticular wax and postharvest calcium uptake in apples. HortScience 34, 121 – 124.
Schiffman-Nadel, M., Chalutz, E., Waks, J., Dagan, M., 1975. Reduction of chilling injury in grapefruit by thiabendazole and benomyl during long-term storage. J. Am. Soc. Hortic. Sci. 100, 270 – 272.
Schiffman-Nadel, M., Chalutz, E., Waks, J., Lattar, F.S., 1972. Reduction of pitting of grapefruit by thiabendazole during long-term cold storage. HortScience 4, 394 – 395.
Schirra, M., Angioni, A., Ruggiu, R., Minelli, E.V., Cabras, P., 1998a. Thiabendazole uptake and persistence in lemons following postharvest dips at 50°C. It. J. Food Sci. 10, 165 – 170.
Schirra, M., 1992. Behaviour of ‘Star Ruby’ grapefruits under chilling and non-chilling storage temperatures. Postharvest Biol. Technol 2, 315 – 327.
Schirra, M., Agabbio, M., D’hallewin, G., Pala, M., Ruggiu, R., 1997b. Response of Tarocco oranges to picking date, postharvest hot water dips, and chilling storage tempera-ture. J. Agric. Food Chem. 45, 3216 – 3220.
Schirra, M., Barbera, G., D’Aquino, S., La Mantia, T., Mc-Donald, R.E., 1996a. Hot dips and air temperature condi-tioning to improve shelf quality of late-crop cactus pear fruit. Trop. Sci. 36, 159 – 165.
Schirra, M., Ben-Yehoshua, S., 1999. Heat treatments: a possi-ble new technology in citrus handling — Challenges and prospects. In: Schirra, M. (Ed.), Advances in Postharvest Diseases and Disorders Control of Citrus Fruit. Research Signpost Publisher, Trivandrum, India, pp. 133 – 147. Schirra, M., Cabras, P., Angioni, A., Melis, M., 1996b.
Residue level of imazalil fungicide in lemons following prestorage dip treatment at 20 and 50°C. J. Agric. Food Chem. 44, 2865 – 2869.
Schirra, M., Cabras, P., D’hallewin, G., Angioni, A., Garau, V.L., 1998b. Seasonal susceptibility to chilling injury of ‘Tarocco’ oranges as affected by hot water and thiabenda-zole postharvest dip treatments. J. Agric. Food Chem. 46, 1177 – 1180.
Schirra, M., D’hallewin, G., 1997. Storage performance of Fortune mandarins following hot water dips. Postharvest Biol. Technol. 10, 229 – 237.
Schirra, M., D’hallewin, G., Inglese, P., La Mantia, T., 1999. Epicuticular changes and storage potential of cactus pear [Opuntia ficus-indicaMiller (L.)] fruit following gibberellic acid preharvest sprays and postharvest heat treatment. Postharvest Biol. Technol. 17, 79 – 88.
Schirra, M., D’hallewin, G., 1996. Storage of Fortune man-darin following postharvest dips in hot water and coating with an edible sucrose polyester. Proc. VIII International Citrus Congress. Sun City Resort, South Africa 12 – 17 May, 1996. Vol. 2, 1209 – 1214.
Schirra, M., Mulas, M., 1993. Keeping quality of ‘Oroblanco’ grapefruit-type as affected by hot dip treatments. Adv. Hortic. Sci. 7, 73 – 76.
Schirra, M., Mulas, M., 1995a. ‘Fortune’ mandarin quality following prestorage water dips and intermittent warming during cold storage. HortScience 30, 560 – 561.
Schirra, M., Mulas, M., 1995b. Improving storability of ‘Tarocco’ oranges by postharvest hot-dip fungicide treat-ments. Postharvest Biol. Technol. 6, 129 – 138.
Schirra, M., Cabras, P., Angioni, A., D’hallewin, G., Ruggiu, R., Minelli, E.V., 1997a. Effect of heated solutions on decay control and residues of imazalil in lemons. J. Agric. Food Chem. 45, 4127 – 4130.
Sherf, B.A., Kolattukudy, P.E., 1993. Developmentally regu-lated expression of the wound and pathogen-responsive tomato anionic peroxidase in green fruits. Plant J. 3, 829 – 833.
Smilanick, J.L., Margosan, D.A., Jenson, D.J., 1995. Evalua-tion of heated soluEvalua-tions of sulfur dioxide, ethanol, and hydrogen peroxide to control postharvest green mold of lemons. Plant Dis. 79, 742 – 747.
Sommer, N.F., Fortlage, R.J., Buckley, P.M., Maxie, E.C., 1967. Radiation-heat synergism for inactivation of market disease fungi of stone fruits. Phytopathol. 57, 428 – 433. Spotts, R.A., Chen, P.M., 1987. Prestorage heat treatments for
control of decay of pear fruit. Phytopathol. 77, 1578 – 1582. Stange, R.R., Midland, S.A., Sims, J.J., Eckert, J.W., 1993. Evidence that wound gum, not lignin, is deposited in infection-resistant injuries of citrus peel. Acta Hortic. 343, 347 – 352.
Trapero-Casas, A., Kaiser, W.J., 1992. Influence of tempera-ture, wetness period, plant age, and inoculum concentra-tion on infecconcentra-tion and development of Ascochta blight of chickpea. Phytopathol. 82, 589 – 596.
Wells, J.M., Harvey, J.M., 1970. Combination heat and 2,6-dichloro-4-nitroaniline treatments for control of rhizopus and brown rot of peaches, plums, and nectarines. Phyto-pathol. 60, 116 – 120.
Wild, B.L., Hood, C.W., 1989. Hot dip treatments reduce chilling injury in long-term storage of ‘Valencia’ oranges. HortScience 24, 109 – 110.
Williams, M.H., Brown, M.A., Vesk, M., Brady, C., 1994. Effect of postharvest heat treatments on fruit quality, surface structure, and fungal disease in Valencia oranges. Austr. J. Exper. Agricul. 34, 1183 – 1190.
Wilson, C.L., Ghaouth, A.El., Chalutz, E., Droby, S., Stevens, C., Lu, J.Y., Khan, V., Arul, J., 1994. Potential of induced resistance to control postharvest diseases of fruits and vegetables. Plant Dis. 78, 837 – 844.
Yao, B., Tuite, J., 1989. The effects of heat treatments and inoculum concentration on growth and sporulation of Penicillium spp. on corn genotypes in storage. Phytopathol. 79, 1101 – 1104.
Young, R.H., 1986. Fresh fruit cultivars. In: Wardowski, W.F., Nagi, S., Grierson, W. (Eds.), Fresh Citrus Fruits. AVI Publishing, pp. 101 – 126.