Defense proteins from seed of
Cassia fistula
include a lipid
transfer protein homologue and a protease inhibitory plant
defensin
Ratna Wijaya
a,b, Gregory M. Neumann
a, Rosemary Condron
a, Andrew B. Hughes
b,
Gideon M. Polya
a,*
aDepartment of Biochemistry,La Trobe Uni6ersity,Bundoora,Vic.3083,Australia bDepartment of Chemistry,La Trobe Uni6ersity,Bundoora,Vic.3083,Australia
Received 15 March 2000; received in revised form 15 July 2000; accepted 17 July 2000
Abstract
A novel trypsin inhibitor was extracted from the seeds of Cassia fistulaby a process successively involving soaking seeds in water, extraction of the seeds in methanol, and extraction of the cell wall material at high ionic strength. The protease inhibitor (PI) was subsequently purified by chromatography on carboxymethylcellulose, gel filtration and reversed phase HPLC (RP-HPLC). Electrospray ionization mass spectrometry (ESMS) of the oxidized from of the PI yielded an average molecular mass of 5458.690.8 Da. Edman sequencing of the PI yielded a full-length 50 amino acid sequence inferred to contain eight cysteines and with a calculated average molecular mass (fully oxidized form) of 5459.3 Da, in agreement with the observed mass. TheC.fistula
seed PI is homologous to the family of plant defensins (g-thionins), which have four disulfide linkages at highly conserved locations. TheC.fistulaPI inhibits trypsin (IC502mM), and is the first known example of a plant defensin with protease inhibitory
activity, suggesting a possible additional function for some members of this class of plant defensive proteins.C.fistulaseeds also contain a 9378 Da lipid transfer protein (LTP) homologue, other LTPs, a 7117 Da protein copurifying with PI activity and a 5144 Da defensin which does not inhibit trypsin. The complete sequence of the 5144 Da defensin was determined by Edman sequencing, yielding a calculated average molecular mass (oxidized form) of 5144.1 Da, in agreement with the mass observed by ESMS. The likely trypsin inhibitory residue on the 5459 Da defensin is Lysine-25, the corresponding amino acid being Tyrosine-25 in the homologous 5144 Da defensin that is not a trypsin inhibitor. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Cassia fistula; Seeds; Protease inhibitor; Defensin
www.elsevier.com/locate/plantsci
1. Introduction
Plant defence against herbivores and fungal pathogens involves elaboration of a variety of bioactive secondary metabolites and defensive
proteins [1 – 5]. Plant antifungal proteins include enzymes such as glucanases and chitinases that can hydrolyse fungal cell walls [1,6], and a variety of proteins that can interact with membrane compo-nents, for example lipid transfer proteins (LTPs)
[7 – 9], thaumatin-related proteins [10,11], a- and
b-thionins [2,3,5], g-thionins (defensins) [4,12],
napins [13,14] and napin-like proteins [15,16]. In addition to providing a nitrogen-rich nutrient store in plant seeds, the napins can be protease inhibitors (PIs) [13,14] and a variety of other small plant defensive proteins are also PIs that can variously act as insect feedants and as anti-fungal proteins [1,17].
Abbre6iations: BAPNA, N%-benzoyl-arginine-p-nitroanilide; BYPNA, N%-benzoyl-tyrosine-p-nitroanilide; CaM, calmodulin; CDPK, Ca2+-dependent protein kinase; ESMS, electrospray
ioniza-tion mass spectrometry; LTP, lipid transfer protein; PI, protease inhibitor; PTH, phenylthiohydantoin; RP-HPLC, reversed phase HPLC; TFA, trifluoroacetic acid; TLCK, Na-p-tosyl-L-lysine chloromethyl ketone; TPCK, L -(1-tosylamido-2-phenyl)ethylchloro-methyl-ketone.
* Corresponding author. Tel.: +61-3-94792157; fax: + 61-3-94792467.
It is important to determine the precise bio-chemical interactions involved in the action of antifungal and anti-insect proteins, noting that such defensive proteins may have more than one mode of action. The antifungal napins provide a good example of defensive proteins that have evi-dent multiple functions and which have been shown to interact with more than one biochemical entity. Thus napins are glutamine-rich storage proteins and can also be PIs [13,14], cause fungal membrane changes and act synergistically with other membrane-altering antifungal proteins [18]. Napins and the napin-like proteins have a het-erodimeric subunit structure [15,16] and it has been found that particular napin [16] and napin-like protein [14] small and large subunits are
sub-strates for phosphorylation by plant
Ca2+-dependent protein kinase (CDPK), although
the biochemical significance of this has not been established. In addition, some of these oxidized complexes and their constituent small and large subunits can bind to calmodulin (CaM), as
de-tected through inhibition of CaM-dependent
myosin light chain kinase (MLCK) or through
their effect on Ca2+-dependent dansyl-CaM
fluorescence [16,19].
Some other plant antifungal proteins are also substrates for plant CDPK [19], including various LTPs [20,21], soybean Bowman – Birk PI BBI-I [22], potato tuber carboxypeptidase inhibitor
protein [23] and plant defensins (g-thionins) [12].
Some plant defensins can interact with Ca2+-CaM
[12] in addition to being CDPK substrates and antifungal proteins [4,5] and it is conceivable that further specific biochemical interactions may con-tribute to the defensive function of this group of plant bioactive proteins.
PIs have a major role in anti-insect plant de-fence [1,17]. Inhibition of serine proteases by plant protease inhibitory proteins or protease inhibitory secondary metabolites interferes with insect diges-tion and can inhibit larval growth and develop-ment [1,17]. As part of an investigation into the
anti-insect components of various IndonesianCas
-sia species, the nature and structure of Cassia
fistula PI proteins have been investigated. The present paper describes the purification and
se-quencing of a PI protein from the seeds of C.
fistulaand its identification as a plant defensin. To the authors’ knowledge this is the first demonstra-tion of a protease inhibitory funcdemonstra-tion associated
with a representative of this major class of plant defensive proteins.
2. Materials and methods
2.1. Materials
Seeds ofC. fistulawere collected from the Jambi
forest in Sumatra, Indonesia. N-benzoyl-D,L
-arginine-p-nitroanilide (BAPNA) and N
-benzoyl-L-tyrosine-p-nitroanilide (BYPNA) were obtained
from Sigma (St. Louis, MO). L
-(1-Tosylamido-2-phenyl)ethylchloromethyl-ketone (TPCK)-treated
bovine trypsin and Na-p-tosyl-L-lysine
chloro-methyl ketone (TLCK)-treated chymotrypsin were
obtained from Worthington (USA).
Carbo-xymethylcellulose (CM52) was obtained from Whatman (England). An Aquapore RP300 (C8)
RP-HPLC guard column (4.6 mm×30 mm; 7 mm
particle size, 300 A, pore size) was obtained from
Brownlee Laboratories (USA) and a Vydac C8
column (4.6 mm×25 cm; 5 mm particle size, 300
A, pore size) was obtained from Altech Associates
(USA).
2.2. Trypsin and chymotrypsin assays
Trypsin (or chymotrypsin) inhibitory activity of the isolated proteins was assayed in duplicate at
30°C in 96 well microtitre plates (100 ml well
volume) containing 50 mM Tris (Cl−, pH 8), 200
mM N-benzoyl-D,L-arginine-p-nitroanilide and 10
mg/ml trypsin (or 200 mM N-benzoyl-L-tyrosine-p
-nitroanilide and 10 mg/ml chymotrypsin) in the
presence and absence of added test proteins. Protease activity was determined from the ab-sorbance change at 405 nm, measured using a Spectra Max Pro 250 multiplate spectrophotome-ter (Molecular Devices). Alspectrophotome-ternatively, assays were conducted in duplicate in the same assay condi-tions but with a final assay volume of 1 ml and the absorbance changes were measured at 405 nm using an Hitachi 181 UV-VIS spectrophotometer.
IC50 values (concentrations for 50% inhibition) of
2.3. Purification of C. fistula PIs
Seeds ofC.fistula(200 g) were soaked overnight
in H2O at 20°C and were then homogenized in
methanol (600 ml) using an UltraTurrax blender (Janke Kunkel, Germany) at full power for 10 min. The homogenate was passed through Mira-cloth to recover the cell wall fraction. The cell wall
fraction was washed with 1.5 l H2O until no more
protein was detected in the eluate. The cell wall fraction was then extracted by suspension in 200
ml 2 M NaCl in 10 mM glycine (Na+, pH 9.5)
(the pH falling to 6 after addition of the cell wall material). The suspension was filtered through Miracloth and the filtrate (containing material ex-tracted at high ionic strength from cell wall
mate-rial) was centrifuged at 15 000×g for 15 min. The
supernatant was diluted 50-fold in 10 mM sodium phosphate (pH 6.0) buffer and applied to 60 g carboxymethylcellulose (Whatman CM52) on a Bu¨chner funnel. The CM52 was washed with 200
ml 10 mM phosphate (Na+, pH 6.0) and then the
bound protein was eluted with 100 ml 2 M NaCl –
50 mM glycine (Na+, pH 9.5). This
chromato-graphic step was repeated with the CM52 being eluted with a stepwise gradient of increasing NaCl
concentration in 50 mM glycine (Na+, pH 9.5), PI
activity being detected in fractions containing 100 – 700 mM NaCl. The PI fractions were pooled,
concentrated by pressure filtration (Amicon YM 3 membrane) and chromatographed on an Ultrogel
AcA 44 column (7 cm2×53 cm) which was eluted
with 0.5 M NaCl in 50 mM Tris (Cl−, pH 8.0).
Low molecular weight fractions containing PI ac-tivity (peak 2, Fig. 1) were pooled and
concen-trated by pressure filtration (Amicon YM3
membrane). The concentrated fraction was
brought to 0.1% trifluoroacetic acid (TFA),
cen-trifuged at 10 000×g for 5 min and filtered
through a 0.2 mm filter (Phenomenex). The
con-centrated acidified fraction was subjected to RP-HPLC employing an Aquapore RP300 (C8)
RP-HPLC guard column (4.6 mm×30 mm; 7 mm
particle size, 300 A, pore size) and a Vydac C8
column (4.6 mm×25 cm; 5 mm particle size, 300
A, pore size) which was eluted with a gradient of
increasing CH3CN concentration in 0.1% TFA
(0 – 100% CH3CN over 100 min; flow rate 1 ml/
min; UV detection at 240 nm). A major protein peak associated with trypsin inhibitory activity
was resolved (eluting at 33% CH3CN in 0.1%
TFA) (Fig. 2A) and was stored at −30°C before
further analysis.
A second procedure employed to purify the defensin was essentially the same as that described
above except that no overnight imbibing in H2O
was employed (seeds being frozen with liquid N2,
ground in a mortar and pestle and then extracted
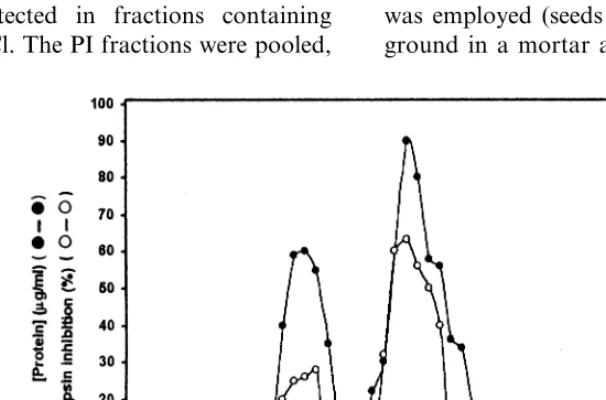
Fig. 1. Gel filtration of the basic, carboxymethylcellulose-binding fraction fromC.fistulaseeds on an Ultrogel AcA44 column. The gel filtration was conducted in 0.5 M NaCl – 50 mM Tris (Cl−pH 8.0) with a flow rate of about 0.5 ml/min. Fractions of
about 5 ml were collected. Protein was determined by the method of Sedmak and Grossberg [24]. Trypsin inhibition (%) by 10
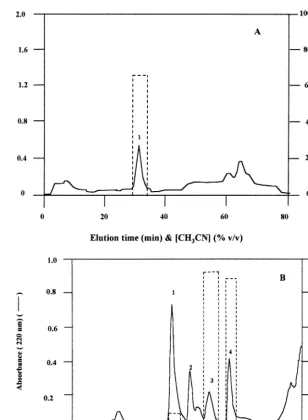
Fig. 2. Reversed phase HPLC (RP-HPLC) of the low molecular weight trypsin inhibitory basic protein fraction fromC.fistula
seeds. RP-HPLC and trypsin inhibition assays were conducted as described in Section 2. Continuous trace, absorbance at 240 nm (A) or 220 nm (B) (absorbance at 220 nm being used for greater measurement sensitivity); histogram (---), trypsin inhibition (%) by 10ml of the indicated fraction determined as described in the legend to Fig. 1. (A), trypsin inhibitory material from procedure 1; (B), trypsin inhibitory material from procedure 2.
as before in methanol), the cell wall fraction was
not washed with H2O and the cell wall fraction
was extracted with 2 M NaCl – 10 mM glycine (with the cell wall suspension in this solution having a final pH adjusted to 9.5 with NaOH). In addition, the CM52 chromatographic step was conducted only once, with the PI fraction being eluted batchwise in 2 M NaCl – 10 mM glycine (pH 9.5). This second procedure yielded a major peak of defensin on RP-HPLC that was associated with trypsin inhibitory activity (peak 4, Fig. 2B) together with three further peaks of protein eluting just prior to the defensin peak (peaks 1 – 3, Fig.
2B). All fractions were stored at −30°C before
further analysis. Protein was estimated from UV absorbance or by the method of Sedmak and Grossberg (1977) [24] using crystalline bovine serum albumin as a standard. SDS PAGE was conducted using 15% polyacrylamide BioRad Minigels following the method of Laemmli [25].
2.4. Amino acid sequencing
system calibrated with phenylthiohydantoin (PTH)-amino acid standards prior to each se-quencing run.
2.5. Electrospray ionization mass spectrometry
Prior to electrospray ionization mass spectrome-try (ESMS), protein fractions from RP-HPLC were concentrated under vacuum in a SpeedVac
concentrator and reconstituted with milli-Q H2O.
ESMS was carried out using 1 – 100 pmol protein
in 2 – 4 ml of 50% CH3CN containing 0.1% formic
acid. Samples were infused at a flow rate of 0.2
ml/min into a Perkin-Elmer Sciex API-300 triple
quadrupole mass spectrometer fitted with a micro-ionspray ion source and calibrated to an accuracy
equivalent to 90.01% using singly charged
poly(propylene glycol) ions. Mass spectra, typi-cally 30 – 100 scans, were recorded in the first quadropole (Q1) scan mode over the
mass-to-charge range m/z 200 – 3000 Da per unit charge,
using a constant peak width (at half peak height) of 0.6 Da per unit charge. Mass-to-charge ratio data were processed by signal-averaging, manual mass determination and transformation using PE-Sciex Biomultiview software.
3. Results
3.1. Purification of C. fistula PI
A PI was purified from C. fistula seeds by two
very similar procedures, both successively involv-ing methanol extraction of seeds to yield a crude cell wall fraction, extraction of the PI from the cell wall fraction at high ionic strength, elution from carboxymethylcellulose (CM52) at high ionic strength, gel filtration on an Ultrogel AcA 44 column and RP-HPLC on a C8 column.
Stepwise gradient elution from CM52 yielded one peak of trypsin inhibiting activity eluting be-tween 0.1 and 0.7 M NaCl in 10 mM glycine (pH 9.5). Subsequent gel filtration yielded two peaks of protein, one eluting at the void volume and the second corresponding to low molecular weight material. Trypsin inhibitory activity is associated with both peaks (Fig. 1).
RP-HPLC of the low molecular weight material from gel filtration in procedure 1 resolved one
major peak of material eluting at 33 – 34% CH3CN
in 0.1% TFA (Fig. 2A). SDS PAGE of this mate-rial (subsequently shown to be a defensin) revealed
only one major entity (Mr 6000 Da) (data not
shown). The high molecular weight material from gel filtration was not further analyzed.
As outlined in Section 2, a second abbreviated
procedure was used to isolate the C. fistula
de-fensin. This second procedure was the same as the
first but eliminated overnight imbibing in H2O,
extensive washing of the crude cell wall fraction in
H2O and a second gradient elution from CM52.
Further, in this second procedure the cell wall fraction was extracted in 2 M NaCl – 10 mM glycine at pH 9.5. This second procedure again yielded a protease inhibitory defensin peak on RP-HPLC (peak 4, Fig. 2B), but this final step also resolved three further less hydrophobic com-ponents (peaks 1 – 3, Fig. 2B) to be described further below.
Peaks 1 and 2 are not protease inhibitory at 5
and 3.8 mM, respectively, but peak 3 inhibits
trypsin with an IC50(concentration for 50%
inhibi-tion) value of 0.7 mM. The C. fistula 5459 Da
defensin (peak 4, Fig. 2B) inhibits bovine
TPCK-treated trypsin with an IC50of 2mM. This defensin
(at 4.4 mM) does not inhibit chymotrypsin.
3.2. Analysis of C. fistula basic proteins by mass spectrometry
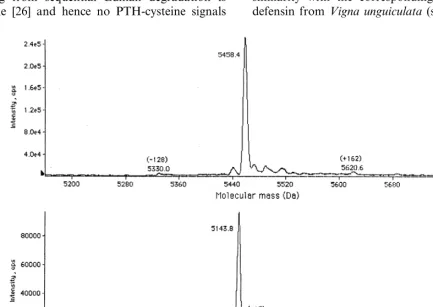
C. fistula seed defensin isolated by procedure 1 (Fig. 2A) was analyzed by ESMS in the conditions described in Section 2. The average molecular mass of the defensin in the fully oxidized form (no reductants having been added during the isolation
procedure) is 5458.690.8 Da (Fig. 3A). The
5458.6 Da component was effectively the only major entity present (Fig. 3A). Similar ESMS analysis of the protease inhibitory peak 4 from RP-HPLC of material from procedure 2 (Fig. 2B) revealed one major component with an average
molecular mass of 5458.890.8 Da, i.e. the same
mass within experimental error as the protein iso-lated by procedure 1.
Analysis of peaks 1 – 3 from the RP-HPLC step of procedure 2 (Fig. 2B) by ESMS revealed major
proteins with average molecular masses of
9377.991.2, 5143.690.8 (Fig. 3B) and 7117.19
2B) identified this entity as a defensin (Fig. 4) and N-terminal sequencing of peaks 1 and 2 from the RP-HPLC step of procedure 2 (Fig. 2B) identified these components as LTP and defensin homo-logues, respectively (Figs. 4 – 6). The minor 9177 and 9539 Da components in peak 1 can be related to the major 9378 Da component by differential processing as shown in Table 1. The minor 4928, 5015 and 5305 Da components in peak 2 can be similarly related to the major 5144 Da component (Table 1).
3.3. Edman sequencing of 5459 and 5144 Da C. fistula defensins
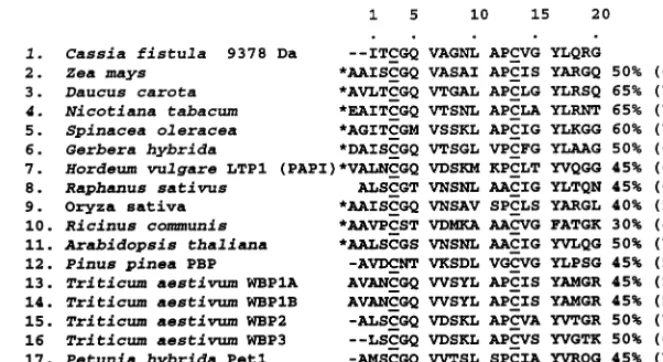
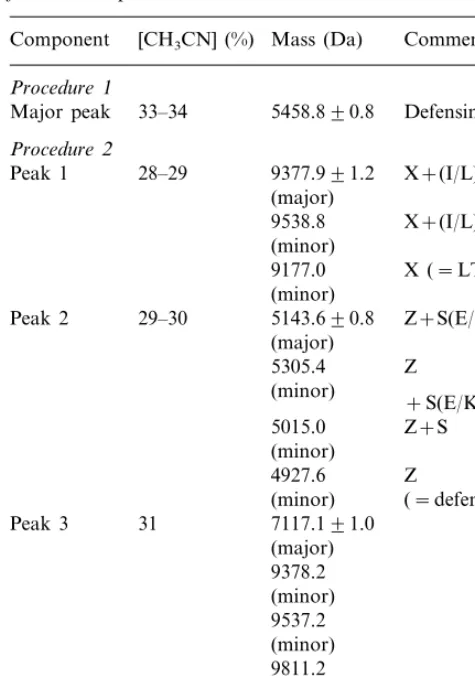
N-terminal sequencing of the 5459 Da defensin by Edman degradation yielded a sequence of 50 amino acids (Fig. 4). The amino acid residues in positions 3, 14, 23, 27, 38, 44, 46 and 50 are inferred to be cysteines in these positions for the following reasons. The PTH derivative of cysteine deriving from sequential Edman degradation is unstable [26] and hence no PTH-cysteine signals
are expected. However the PTH-cysteine-derived PTH-dehydroalanine was detected in positions 3, 14, 23 and 27, indicating cysteines in these
posi-tions. The cysteines in plant seed defensins (g
-thionins) are highly conserved in position (Fig. 5) and the cycle positions of major signal absences (positions 3, 14, 23) and possible signal carry-over from the preceding residue (positions 27, 38, 44, 46 and 50) (Fig. 4) exactly correspond with the eight cysteine locations found in other seed defensins (Fig. 5). The calculated mass of the fully reduced polypeptide from the deduced sequence (Fig. 4) is 5467.37 Da. Given eight cysteines involved in four disulphide linkages, the calculated mass of the
fully oxidized protein is 5467.37−8.06 Da=
5459.31 Da, which agrees within experimental er-ror with the observed average molecular mass of
5458.690.8 Da for the fully oxidized defensin
(Fig. 3A; Table 1).
The deduced sequence of the 5459 Da defensin as aligned in Fig. 5 has 52% identity and 62% similarity with the corresponding sequence of a
defensin from Vigna unguiculata (sequence 3, Fig.
Table 1
Electrospray ionization mass spectrometry of purifiedCassia fistulabasic proteinsa
Mass (Da) Comment Component [CH3CN] (%)
Procedure1
Major peak 33–34 5458.890.8 Defensin
Procedure2
aPossible interpretations of the mass differences between
the LTP homologues and defensins in peaks 1 and 2, respec-tively, are given based on inclusion of the following particular amino acids (average molecular mass (Da) contributions in parenthesis): I or L (113.16), E (129.12), K (128.17), Q (128.13), S (87.08) and Y (163.18).
residues than the legume defensins (an exception
being V. faba fabatin) (sequence 6, Fig. 5) in the
site corresponding to a position between C. fistula
residues 42 and 43 (Fig. 5).
Edman sequencing of the 5144 Da peak 2 mate-rial (eluting from the C8 column at 29 – 30%
CH3CN in 0.1% TFA) (Fig. 2B, Table 1) yielded a
sequence showing evident homology with the 5459 defensin (Fig. 4) and with other plant defensins (Fig. 5). The absence of a major PTH-amino acid signal in positions 3, 14, 23 and 27 is consistent with cysteines in positions 3, 14, 23 and 27, as is the presence of a PTH-dehydroalanine in these cycles. The cysteines in positions corresponding to positions 38 and 43 in the 5144 Da protein are highly conserved in the plant defensins (Fig. 5) and accordingly the PTH-threonine and PTH-ala-nine in cycles 38 and 43 may be due to carry-over from the threonine and alanine in positions 37 and 42, respectively (Fig. 4). No Edman sequencing was carried out for cycle 50 (Fig. 4) but a C-termi-nal cysteine is highly conserved in plant defensins (Fig. 5). All of the inferred cysteines align with the corresponding highly conserved cysteines found in other defensins (Figs. 4 and 5) and the mass of the deduced sequence is the same within experimental error as the observed mass. Thus the calculated average molecular mass for the fully reduced de-duced sequence (Fig. 4) is 5152.13 Da. Subtracting 8.06 Da for the formation of four disulphides yields a calculated oxidized mass of 5144.07 Da as
compared to the observed mass of 5143.690.8
Da.
The 5144 Da defensin exhibits 44% identity and 50% similarity with the 5459 Da defensin (se-quences 1 and 2, Fig. 5). However while the 5459
defensin is a trypsin inhibitor (IC50 2 mM), the
5144 Da defensin does not inhibit trypsin at 3.8
mM. The 5144 Da is similar to the 5459 Da C.
fistula defensin and other legume defensins in not having the additional residues present in Cruci-ferae defensins in a position between residues 41 and 43 (Fig. 5). Further, other legume and non-legume defensins variously lack residues present in both C. fistula defensins in positions 16 – 22 (Fig. 5).
3.4. Edman sequencing-based identification of a C. fistula LTP
Edman sequencing of the 9378 Da peak 1 mate-rial yielded a 20 residue N-terminal amino acid 5) and 52% identity and 64% similarity to the
corresponding sequence of the Pisum sati6um
pI230 defensin (sequence 4, Fig. 5). However the C. fistula 5459 Da defensin has much less homol-ogy to some other legume defensins, namely the Pisum sati6um pI39 (sequence 5, Fig. 5) andVicia
faba fabatin (sequence 6, Fig. 5), and to a variety
of other plant defensins (Fig. 5). It should be noted that the pI230 and pI39 sequences (Fig. 5)
derive from translation of cDNAs [27,28]. C.
fistuladefensin has extra residues in positions 20 – 23 compared to most of the other plant defensins. Conversely, in general the non-legume plant
de-fensins (an exception being Sorghum bicolor
sequence (sequence 1, Fig. 6) that is clearly related to the N-terminal sequences of plant LTPs (Fig.
6). Thus theC. fistula9378 Da protein N-terminal
amino acid sequence has 60 – 65% identity and 70 – 75% similarity with the corresponding
se-quences of LTPs from Daucus carota, Nicotiana
tabacum, Spinacea oleracea and Petunia hybrida (LTP Pet 2) (Fig. 6, sequences 3, 4, 5 and 18, respectively). In contrast, there is a much less
homology with LTPs from Triticum aesti6um and
LTPs Pet 1, Pet 3 and Pet 4 from Petunia hybrida
(see sequences 13 – 20, Fig. 6). The 9378 Da protein does not inhibit trypsin, in keeping with the observations that, while plant LTPs exhibit some sequence homology to PIs [46], there are no published reports of LTPs actually shown to in-hibit proteases.
3.5. Edman sequencing of the peak 3 protease inhibitory fraction
Peak 3 from RP-HPLC of C. fistula seed basic
cell wall proteins (Fig. 2B) contains trypsin
in-hibitory activity (IC50 0.7 mM). ESMS established
that the major component is a 7117 Da entity but there are also minor components present of about 9378, 9537 and 9811 Da (Table 1) that have masses similar to the masses of LTP homologues resolved in peak 1 (Table 1). Edman sequencing of the peak 3 material yielded a major 20 amino acid
N-terminal sequence, namely VN( –
)SPVALS-PLLGAIT(Y/S)(S/L)S(P/K) (noting that ( – ) in
cy-cle 3 indicates that no significant PTH-amino acid signal was observed). Minor sequence components
were also detected. No homology of the major amino acid sequence with sequences of other proteins was detected from database analysis.
4. Discussion
The procedures outlined in this paper permit purification of several kinds of plant defensive
proteins from seeds ofC. fistula, a plant employed
in Indonesia for its anti-insect properties. The
defensive proteins isolated from C. fistula seeds
include a LTP homologue (oxidized mass 9378 Da) and two distinct types of defensins (with oxidized masses of 5144 and 5459 Da, respec-tively) of which the 5459 Da defensin is a trypsin inhibitor. In addition a further protease inhibitory fraction was resolved (peak 3, Fig. 2B) that con-tains circa 9 kDa proteins as well as a major 7117 Da protein. The purification procedure involved successive extraction of cell wall material at high ionic strength at pH 9.5, cation exchange chro-matography on CM52, gel filtration and RP-HPLC. A procedure involving extraction of basic proteins from the crude cell wall fraction at high ionic strength but at a pH of 6 evidently results in a more selective extraction of the 5459 Da protease inhibitory defensin without the 5144 Da defensin, the 9378 Da LTP homologue, and fur-ther circa 9 – 10 kDa and circa 7 kDa entities (Fig. 2A; Table 1).
The basic cell wall proteins of C. fistula seeds
were characterized by a combination of ESMS and
Wijaya
et
al
.
/
Plant
Science
159
(2000)
243
–
255
251
Fig. 6. Sequence homologies of the 9378 DaC.fistulaprotein with plant lipid transfer protein N-terminal sequences. Sequence 1 is from Edman sequencing of peak 1 (Fig. 2B) in which the major component is a 9378 Da entity. The absence of a major signal in cycle 3 has been interpreted as due to the presence of a cysteine [26]. Cysteines are underlined; *, preceding sequences of cDNA-derived sequences are not shown. The % identity with the first 20 amino acids of the 9378 Da protein are shown (% similarity is shown in parenthesis and was calculated on the basis of I=L=V=M, A=G, S=T, D=E, R=K, N=Q, Y=W=F). The LTP sequences derive from the sources listed under ‘Ref.’[7,20,21,41 – 50].
Edman sequencing, this analysis identifying (in order of elution from RP-HPLC and hence in order of increasing hydrophobicity) a 9378 Da LTP (together with minor co-purifying 9539 and 9177 Da components that can be related to the 9378 Da components by N- or C-terminal process-ing), a 5144 Da defensin (together with minor, co-purifying 4928, 5015 and 5305 Da components that can be related to the 5144 Da component by N- or C-terminal processing), a major 7117 Da protein (together with minor 9378, 9537 and 9811 Da components) and a 5459 Da defensin with protease inhibitory activity. The defensin and LTP components were established as such by Edman sequencing and database analysis together with ESMS analysis that, coupled with Edman N-ter-minal sequence information, provided evidence for possible processing-based inter-relatedness of the 9177, 9378 and 9539 Da LTP homologues and of the 4928, 5015, 5144 and 5305 Da defensins, re-spectively (Table 1). No homologues of the major N-terminal sequence found for the peak 3 protease inhibitory fraction (Fig. 2B) were found on data-base analysis and either the major 7117 Da protein or the minor circa 9 kDa entities (Table 1) could be responsible for the protease inhibitory activity of this fraction.
A number of plant defensins have been resolved at both the gene and the processed protein level and amino acid sequences variously determined
from cDNA sequence translation [9], Edman se-quencing or Edman sese-quencing coupled with ESMS [20]. The latter procedure is particularly effective, allowing for a final confirmation of the sequencing result from comparison of the calcu-lated and experimentally observed masses. The sequence of the more hydrophobic 5459 Da de-fensin described here (Fig. 4) yields a calculated fully oxidized mass of 5459.31 Da, in excellent agreement with the observed average molecular
mass of the fully oxidized defensin of 5458.690.8
Da (Table 1). Similarly the fully oxidized average molecular mass calculated from the deduced se-quence of the more hydrophilic 5144 Da defensin (Fig. 4) is in excellent agreement within experi-mental error with the observed mass (5144.07 Da
calculated, 5143.690.8 Da observed, Table 1).
The 5144 and 5459 Da defensins resemble all the other defensins analyzed in Fig. 5 in having eight cysteines that are located in the same highly conserved positions. The three dimensional struc-tures of several plant defensins have been
deter-mined, this structure involving a triple stranded b
sheet disulphide linked to an a-helix [31]. These
elements are sequentially linked thus: b1 strand –
loop –a-helix –b2 strand –b3 strand. The cysteine
connectivities are conveniently described using the amino acid numbering shown in Fig. 5, with the N-terminal region Cys-3 linked to the C-terminal
strand Cys-38 and the a-helix Cys-25 and Cys-27 residues linked to the second and third last
cys-teine residues of theb3 strand (Cys-44 and Cys-46,
respectively, sequence 1, Fig. 5). It is useful to adopt this conserved 3-dimensional structure as a model for the structures of the other plant
de-fensins and in particular in the following
discussion.
The 5144 and 5459 Da defensins are more
simi-lar to thePisumand Vignadefensins (sequences 1,
3, 4 and 5, Fig. 5) than to the V. faba defensin
fabatin (sequence 6, Fig. 5) and the other de-fensins compared (sequences 7 – 24, Fig. 5).
Never-theless both the C.fistula 5459 and 5144 defensins
differ from the Vigna and Pisum defensins in
having a serine in position 7, serine being present in this position in all the defensins compared (sequences 1,2 and 6 – 22, Fig. 5) except for the Vigna, and Pisum defensins (sequences 3 – 5, Fig.
5). This serine of Sinapis alba defensins is
phos-phorylated in vitro by plant CDPK [12].
Unlike the protease inhibitory 5459 Da
de-fensin, the C. fistula 5144 Da defensin does not
exhibit trypsin inhibitory activity. A likely site for a trypsin inhibitory sequence would be an exposed region containing a positively charged residue (such as arginine or lysine) as found with the trypsin inhibitory domains of PIs such as
Bow-man – Birk PIs [22,51]. The C. fistula 5144 Da
defensin amino acid sequence has only 44% iden-tity (50% similarity) with the 5459 Da defensin sequence (Fig. 5). However it is notable that the non-protease inhbitory 5144 Da defensin differs from the protease inhibitory 5459 Da defensin in
having a sequence C23DY25LC27 as compared to
C23DK25TC27 for the 5459 Da defensin (Fig. 5).
Inspection of the 3-dimensional structures of re-lated plant defensins [31] suggests that the posi-tively charged Lysine-25 of the 5459 Da defensin is
located within the a-helical domain (Fig. 5). If
Lysine-25 is indeed the key trypsin inhibitory ele-ment in the 5459 Da defensin, substitution of Tyrosine-25 for Lysine-25 (as in the 5144 Da defensin) would abolish trypsin inhibitory activity. Accordingly it is suggested that Lysine-25 is likely to be the critical trypsin inhibitory residue in the 5459 Da defensin.
Plant defensins have antifungal activity but the precise mechanism(s) involved have not yet been determined [4,5,19]. While particular plant de-fensins can increase the permeability of fungal
membranes to K+ ions, they do not have this
effect with artificial membranes, suggesting that these defensins might act via some kind of
mem-brane receptor [52]. The Hordeum 6ulgare g
-hor-dothionin and v-hordothionin defensins inhibit
protein synthesis [32,34] as does a plant defensin
(HvAMP1) isolated from the legume Hardenbergia
6iolacea [53]. Like the protease inhibitory 5459 Da
C. fistula defensin, HvAMP1 has a Lysine-25 [53]. Sorghum defensins are a-amylase inhibitors [37], suggesting that they may act in part to inhibit
pathogen or herbivore nutrient acquisition
through inhibiting starch digestion. Many small plant defensive proteins are PIs, examples includ-ing the napins [13,14], Bowman – Birk PIs [22,51], potato carboxypeptidase inhibitors [23] and some other small, disulphide-rich plant proteins [19,54]. Sequence similarities between potato tuber de-fensins and particular PIs have been noted [39] and the pattern of cysteine locations within some
small, circa 6 kDa cysteine-rich PIs from Brassica
napus is quite similar to that found in the plant defensins [55]. Nevertheless, to the authors’
knowl-edge, the C. fistula 5459 Da is the first plant
defensin shown to be a PI. Since plant PIs can have antifungal activity as well as having anti-di-gestive effects on herbivores [1,17,19], the present demonstration of protease inhibitory activity asso-ciated with a plant defensin suggests a potential anti-insect as well as antifungal function for plant defensins.
Acknowledgements
We gratefully acknowledge an Asian Develop-ment Bank Scholarship to Ratna Wijaya and an Australian Research Council Small Grant to Dr G.M. Polya.
References
[1] D.J. Bowles, Defence-related proteins in higher plants, Annu. Rev. Biochem. 59 (1990) 873 – 907.
[2] H. Bohlmann, K. Apel, Thionins, Ann. Rev. Plant Phys-iol. Mol. BPhys-iol. 42 (1991) 227 – 240.
[3] H. Bohlmann, The role of thionins in plant protection, Crit. Rev. Plant Sci. 13 (1994) 1 – 16.
[5] W.F. Broekaert, B.P.A. Cammue, M.F.C. De Bolle, K. Thevissen, G.W. De Samblanx, R.W. Osborn, Antimi-crobial peptides from plants, Crit. Rev. Plant Sci. 16 (1997) 297 – 323.
[6] N.V. Raikhel, H.-I. Lee, W.F. Broekaert, Structure and function of chitin-binding proteins, Annu. Rev. Plant Physiol. Plant Mol. Biol. 44 (1993) 591 – 615.
[7] F.R.G. Terras, I.J. Goderis, F. Van Leuven, J. Vander-leyden, B.P.A. Cammue, W.F. Broekaert, In vitro anti-fungal activity of a radish (Raphanus sati6us L.) seed
protein homologous to nonspecific lipid transfer proteins, Plant Physiol. 100 (1992) 1055 – 1058.
[8] A. Molina, A. Segura, F. Garcia-Olmeda, Lipid transfer proteins (nsLTPs) from barley and maize leaves are potent inhibitors of bacterial and fungal plant patho-gens, FEBS Lett. 316 (1993) 119 – 122.
[9] J.-C. Kader, Lipid-transfer proteins, Annu. Rev. Plant Physiol. Plant Mol. Biol. 47 (1996) 627 – 654.
[10] J. Hejgaard, S. Jacobsen, I. Svendsen, Two antifungal thaumatin-like proteins from barley grain, FEBS Lett. 291 (1991) 127 – 131.
[11] C. Ogata, P.F. Gordon, A.M. de Vos, S.H. Kim, Crystal structure of a sweet tasting protein thaumatin I, at 1.65 A, resolution, J. Mol. Biol. 228 (1992) 893 – 908. [12] G.M. Neumann, R. Condron, G.M. Polya, Purification
and mass spectrometry-based sequencing of yellow mus-tard (Sinapis alba L.) 6 kDa proteins. Identification as antifungal proteins, Int. J. Peptide Protein Res. 47 (1996) 437 – 446.
[13] I. Svendsen, D. Nicolova, I. Goshev, N. Genov, Isola-tion and characterizaIsola-tion of a trypsin inhibitor from the seeds of kohlrabi (Brassica napusvar rapifera) belonging to the napin family of storage proteins, Carlsberg Res. Commun. 54 (1989) 231 – 239.
[14] G.M. Neumann, R. Condron, I. Thomas, G.M. Polya, Purification and sequencing of multiple forms of Bras
-sica napus seed napin large chains that are calmodulin antagonists and substrates for plant calcium-dependent protein kinase, Biochim. Biophys. Acta 1295 (1996) 34 – 43.
[15] S.D. Irwin, J.N. Keen, J.B.C. Findlay, M.J. Lord, The
Ricinus communis 2S albumin precursor: a single pre-protein may be processed into two different hetero-trimeric storage proteins, Mol. Gen. Genet. 222 (1990) 400 – 408.
[16] G.M. Neumann, R. Condron, G.M. Polya, Purification and sequencing of napin-like protein small and large chains fromMomordica charantiaandRicinus communis
seeds and determination of sites phosphorylated by plant Ca2+-dependent protein kinase, Biochim. Biophys. Acta
1298 (1996) 223 – 240.
[17] P.R. Shewry, J.A. Lucas, Plant proteins that confer resistance to pests and pathogens, Adv. Bot. Res. 26 (1997) 135 – 192.
[18] F.R.G. Terras, H.M.E. Schoofs, K. Thevissen, R.W. Osborn, J. Vanderleyden, B.P.A. Cammue, W.F. Broekaert, Synergistic enhancement of the antifungal activity of wheat and barley thionins by radish and oil-seed rape 2S albumins and by barley trypsin in-hibitors, Plant Physiol. 103 (1993) 1311 – 1319.
[19] G.M. Polya, The structure and function of plant defen-sive proteins, Recent Res. Dev. Phytochem. 1 (1997) 95 – 110.
[20] G.M. Neumann, R. Condron, I. Thomas, G.M. Polya, Purification and sequencing of a family of wheat lipid transfer protein homologues phosphorylated by plant calcium-dependent protein kinase, Biochim. Biophys. Acta 1209 (1994) 183 – 190.
[21] G.M. Neumann, R. Condron, I. Thomas, G.M. Polya, Purification, characterization and sequencing of Petunia
petal lipid transfer proteins phosphorylated by calcium-dependent protein kinase, Plant Sci. 107 (1995) 129 – 145. [22] G.M. Neumann, R. Condron, G.M. Polya, Phosphory-lation of a plant protease inhibitor protein by wheat calcium-dependent protein kinase, Plant. Sci. 96 (1994) 69 – 79.
[23] G.M. Neumann, I. Thomas, G.M. Polya, Identification of the site on potato carboxypeptidase inhibitor that is phosphorylated by plant calcium-dependent protein ki-nase, Plant. Sci. 114 (1996) 45 – 51.
[24] J.J. Sedmak, S.E. Grossberg, A rapid, sensitive and versatile assay for protein using Coomassie brilliant blue G250, Anal. Biochem. 79 (1977) 544 – 552.
[25] U.K. Laemmli, Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature 227 (1970) 680 – 685.
[26] M.W. Hunkerpiller, PTH amino acid analysis, Applied Biosystems Protein Sequencer User Bulletin No. 14 (1985) Nov. 18.
[27] N. Ishibashi, D. Yamaguchi, T. Minamikawa, Stored mRNA in cotyledons of Vigna unguiculataseeds: nucle-otide sequence of cloned cDNA for a stored mRNA and induction of its synthesis by precocious germination, Plant Mol. Biol. 15 (1990) 59 – 64.
[28] C.C. Chiang, L.A. Hadwiger, The Fusarium solani -in-duced expression of a pea gene family encoding high cysteine content proteins, Mol. Plant-Microbe Interact. 4 (1991) 324 – 331.
[29] Y. Zhang, K. Lewis, Fabatins: new antimicrobial plant peptides, FEMS Microbiol. Lett. 149 (1997) 59 – 64. [30] A.L. Vilas Alves, G.W. Samblanx, F.R.G. Terras,
B.P.A. Cammue, W. Broekaert, Expression of functional
Raphanus sati6usantifungal protein in yeast, FEBS Lett.
348 (1994) 228 – 232.
[31] M. Bruix, M.A. Jimenez, J. Santoro, C. Gonzalez, F.J. Colilla, E. Mendez, M. Rico, Solution structure ofg1-H and g1-P thionins from barley and wheat endosperm determined by1H-NMR: a structural motif common to
toxic arthropod proteins, Biochemistry 32 (1993) 715 – 724.
[34] E. Mendez, A. Rocher, M. Calero, T. Girbes, L. Citores, F. Soriano, Primary structure of v-hordothionin, a member of a novel family of thionins from barley en-dosperm, and its inhibition of protein synthesis in eu-karyotic and procaryotic cell-free systems, Eur. J. Biochem. 239 (1996) 67 – 73.
[35] M. Colilla, A. Rocher, E. Mendez, g-Purothionins: amino acid sequence of two polypeptides of a new family of thionins from wheat endosperm, FEBS Lett. 270 (1990) 191 – 194.
[36] K.M. Kragh, J.E. Nielsen, K.K. Nielsen, S. Dreboldt, J.D. Mikkelsen, Characterization and localization of new antifungal cysteine-rich proteins fromBeta6ulgaris, Mol. Plant-Microbe Interact. 8 (1995) 424 – 434. [37] C. Bloch, M. Richardson, A new family of small (5 kDa)
protein inhibitors of insect a-amylases from seeds of sorghum (Sorghum bicolor (L) Moench) have sequence homologies with wheatg-purothionins, FEBS Lett. 279 (1991) 101 – 104.
[38] Q. Gu, E.E. Kawata, M.-J. Morse, H.-M. Wu, A.Y. Cheung, A flower-specific cDNA encoding a novel thionin in tobacco, Mol. Gen. Genet. 234 (1992) 89 – 96. [39] W.J. Stieckma, F. Heidekamp, W.G. Dirkse, J. Van Beckum, P. De Haan, C. Ten Bosch, J.D. Louwerse, Molecular cloning and analysis of four potato tuber mRNAs, Plant Mol. Biol. 11 (1988) 255 – 269.
[40] B. Karunanandaa, A. Singh, T.-H. Kao, Characteriza-tion of a predominantly pistil-expressed gene encoding a
g-thionin-like protein ofPetunia inflata, Plant Mol. Biol. 26 (1994) 459 – 464.
[41] F. Tchang, P. This, P. Stiefel, V. Arondel, M.D. Morch, M. Pages, P. Puigdomenech, F. Grellet, M. Delseny, P. Bouillon, J.-C. Huet, F. Guerbette, F. Beauvais-Cante, H. Duranton, J.-C. Pernollet, J.-C. Kader, Phospholipid transfer protein: full-length cDNA and amino acid se-quence in maize. Amino acid sese-quence homologies be-tween plant phospholipid transfer proteins, J. Biol. Chem. 263 (1988) 16849 – 16855.
[42] P. Sterk, H. Booij, G.A. Scheleekens, A. Van Kammen, S.C. de Vries, Cell-specific expression of the carrot EP2 lipid transfer protein gene, Plant Cell 3 (1991) 907 – 921. [43] A.J. Fleming, T. Mandel, S. Hofmann, P. Sterk, S.C. de Vries, C. Kuhlemeier, Expression pattern of a tobacco lipid transfer protein gene within the shoot apex, Plant J. 2 (1992) 855 – 862.
[44] W.R. Bernhard, S. Thoma, J. Botella, C.R. Somerville, Isolation of a cDNA clone for spinach lipid transfer protein and evidence that the protein is synthesized by the secretory pathway, Plant Physiol. 95 (1991) 164 – 170. [45] M. Kotilainen, Y. Helariutta, P. Elomaa, L. Paulin, T.H. Teeri, A corolla- and carpel-abundant, non-specific
lipid transfer protein gene is expressed in the epidermis and parenchyma of Gerbera hybrida var Regina (Com-positae), Plant Mol. Biol. 26 (1994) 971 – 978.
[46] B. Svennson, K. Asano, I.B. Jonassen, J. Poulsen, J. Mundy, I.B. Svendsen, A 10 kD barley seed protein homologous with an a-amylase inhibitor from Indian finger millet, Carlsberg Res. Commun. 51 (1986) 493 – 500.
[47] F. Vignols, G. Lund, S. Pammi, D. Tre´mousaygue, F. Grellet, J.-C. Kader, P. Puigdome`nech, M. Delseny, Characterization of a rice gene coding for a lipid transfer protein, Gene 142 (1994) 265 – 270.
[48] S. Tsuboi, T. Suga, K. Takishima, G. Mamiya, K. Matsui, Y. Ozeki, M. Yamada, Organ-specific occur-rence and expresssion of the isoforms of nonspecific lipid transfer protein in castor bean seedlings, and molecular cloning of a full-length cDNA for a cotyledon-specific isoform, J. Biochem. 110 (1991) 823 – 831.
[49] S. Thoma, U. Hecht, A. Kippers, J. Botella, S. De Vries, C.R. Somerville, Tissue-specific expression of a gene encoding a cell wall-localized lipid transfer protein from
Arabidopsis, Plant Physiol. 105 (1994) 35 – 45.
[50] G.M. Polya, S. Chandra, R. Chung, G.M. Neumann, P.B. Hoj, Purification and characterization of wheat and pine small basic protein substrates for plant calcium-de-pendent protein kinase, Biochim. Biophys. Acta 1120 (1992) 273 – 280.
[51] M.H. Werner, D.E. Wemmer, Three-dimensional struc-ture of soybean trypsin/chymotrypsin Bowman – Birk in-hibitor in solution, Biochemistry 31 (1992) 999 – 1010. [52] K. Thevissen, A. Ghazi, G.W. De Samblanx, C.
Brown-lee, R.W. Osborn, W.F. Broekaert, Fungal membrane responses induced by plant defensins and thionins, J. Biol. Chem. 271 (1996) 15018 – 15025.
[53] S.J. Harrison, J.P. Marcus, K.C. Goulter, J.L. Green, D.J. Maclean, J.M. Manners, An antimicrobial peptide from the Australian native Hardenbergia 6iolacea
pro-vides the first functionally characterised member of a subfamily of plant defensins, Aust. J. Plant Physiol. 24 (1997) 571 – 578.
[54] F. Ogata, T. Miyata, N. Fujii, N. Yoshida, K. Noda, S. Makisumi, A. Ito, Purification and amino acid sequence of a bitter gourd inhibitor against an acid amino acid-specific endopeptidase of Streptomyces griseus, J. Biol. Chem. 266 (1991) 16715 – 16721.
[55] F. Ceciliani, F. Bortolotti, E. Menegati, S. Ronchi, P. Ascenzi, S. Palmieri, Purification, inhibitory properties, amino acid sequence and identification of the reactive site of a new serine protease inhibitor from oil-rape (Brassica napus) seed, FEBS Lett. 342 (1994) 221 – 224.

![Fig. 5. Sequences of C[12,27–40]. Also shown is the pattern of disulphide linkages found forof theintroduced for alignment of related sequences; * indicates additional cDNA-derived sequences that are not shown; g1-H, gamma-1-hordothionin; o-H, omega-hordot](https://thumb-ap.123doks.com/thumbv2/123dok/1032278.928346/9.792.141.677.84.411/sequences-disulphide-theintroduced-alignment-sequences-additional-sequences-hordothionin.webp)