Since CO is a dominant group of carbon in the ISM, this reaction sequesters large amounts available. A-amino acids and aldehydes extracted from his samples have δ13C measurements decrease with an increase in carbon number.
ORBITRAP ISOTOPE RATIO MASS SPECTROMETRY: A NEW METHOD TO PROBE ISOTOPIC STRUCTURES
Introduction
Even ultra-high-resolution IRMS have a mass resolution of 50,000 FWHM, so it can differentiate multiple isotopic substitutions from each other, including 13CH3D and 12CH2D2, but can only do so for low-molecular-mass gases (e.g. eg N2O, NO2, CO2, CH4) (Eiler et al. , 2013). An exception is the GC-combustion-GC-IRMS technique, which allows the specific analysis of carbon isotopes in a limited number of compounds for sample sizes down to nanomole (Dennis et al., 1998; Oba and Naraoka, 2006; Li et al. al. ., 2018).
Materials and Methods
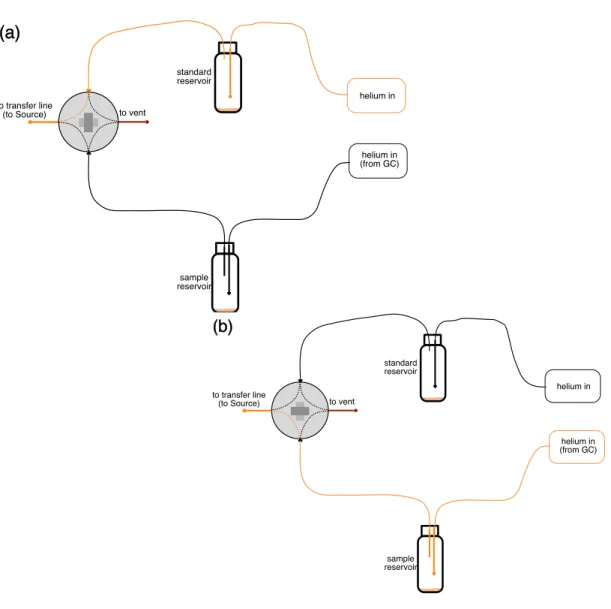
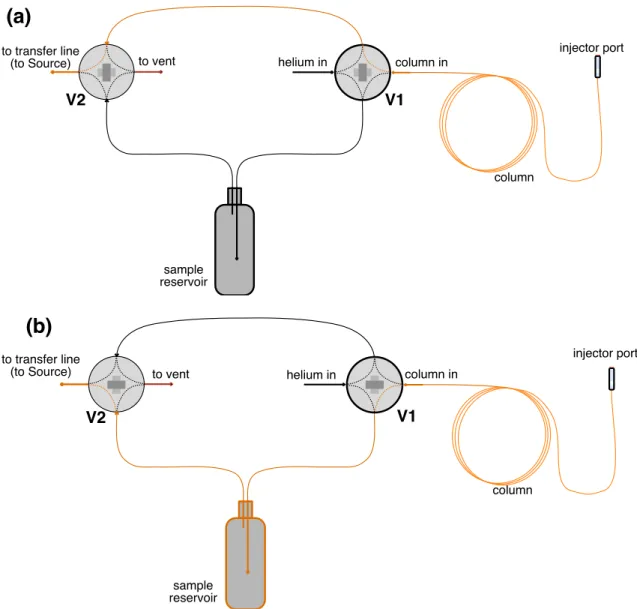
In (a), the orange trace traces the direct path from the pillar to the Orbitrap source and (b) the orange trace traces the path from the pillar to the source when the top is captured. In this mode, the valves are initially set to direct helium into the Orbitrap reservoir and source.
Results and Discussion
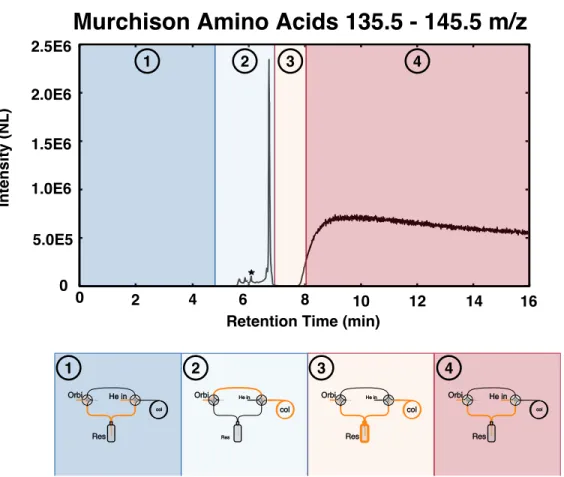
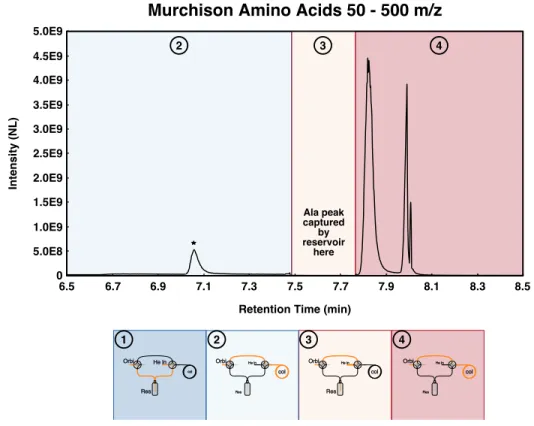
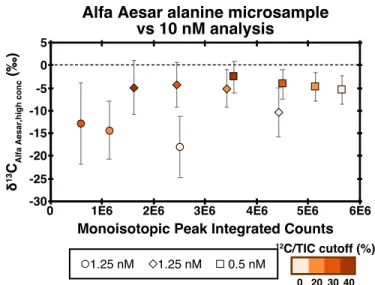
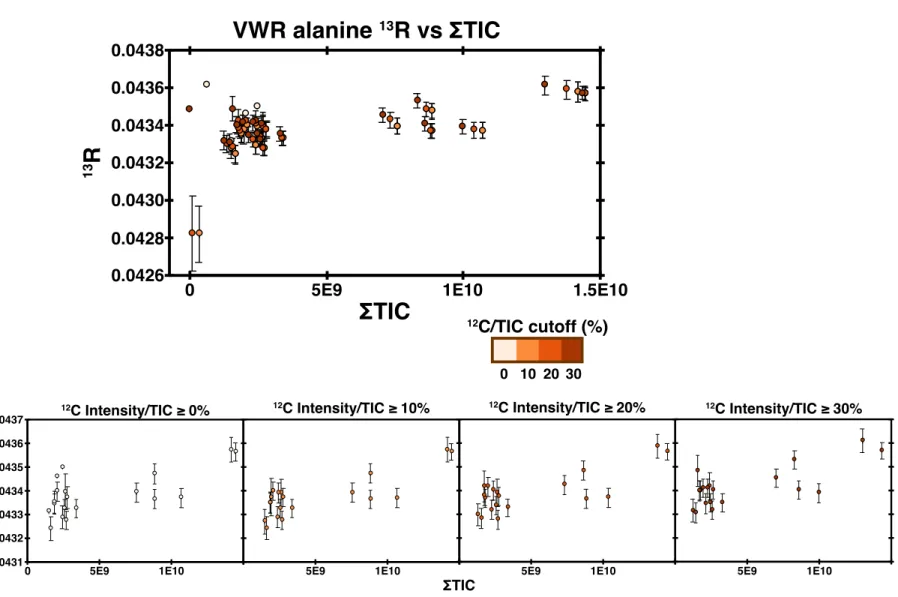
Single-tank studies enable three main observations: (1) the impact of peak IT on results, (2) the impact of microscanning connectivity on results, and (3) the impact of peak intensity relative to background. Finally, we exclude any scan for which the monoisotopic peak is less than 30% of the total ion intensity.
Conclusion
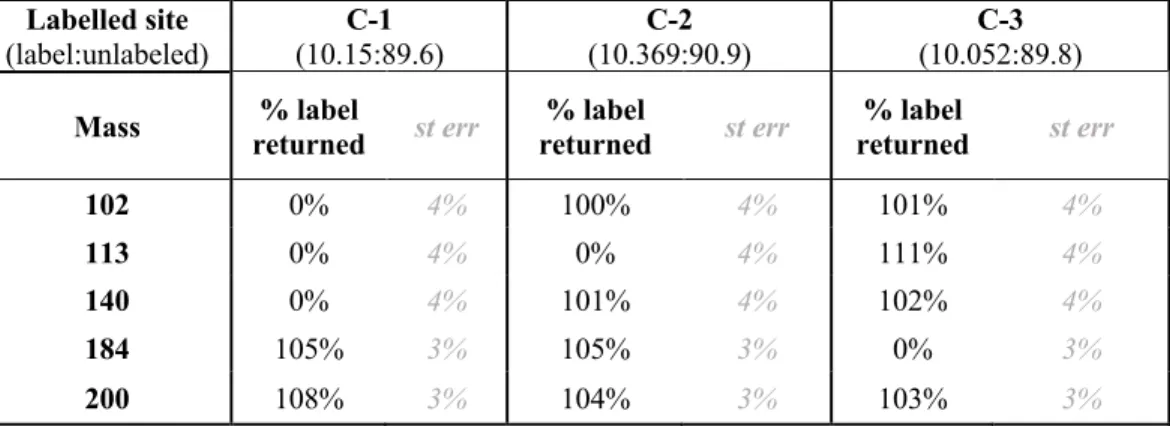
While smaller samples will have lower precision, we demonstrate the ability to measure alanine samples as small as 1 picomole for site-specific isotopic ratios for the C-1 site. Combined with conventional methods such as GC-C-IRMS, these analyzes allow natural samples to obtain site-specific isotopic structures.
ISOTOPE EFFECTS AT THE ORIGIN OF LIFE
FINGERPRINTS OF THE STRECKER SYNTHESIS
Introduction
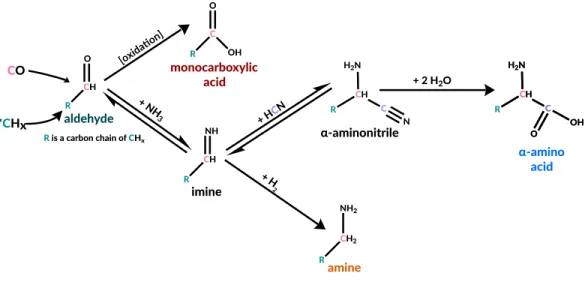
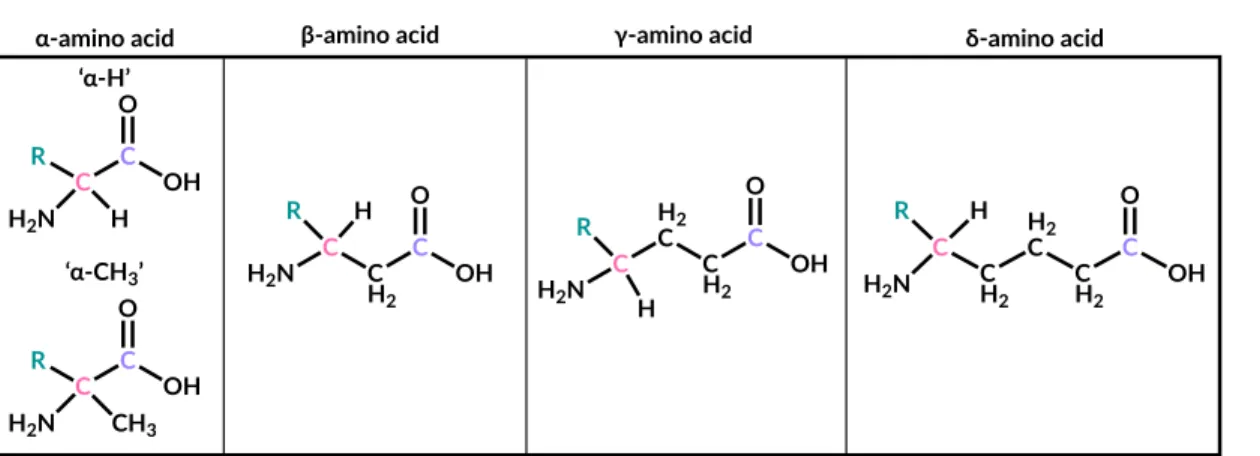
Specifically, an aldehyde or ketone reacts with ammonia and cyanide to form an α-aminonitrile, which is then hydrolyzed to an α-amino acid (Van Trump, 1975) (Figure 3.1). The Strecker synthesis is one of the simplest pathways by which abiotically available precursors produce α-amino acids, the subset of amino acids most abundant in known life.
CHNH 2
CHNH 3
Materials and Methods
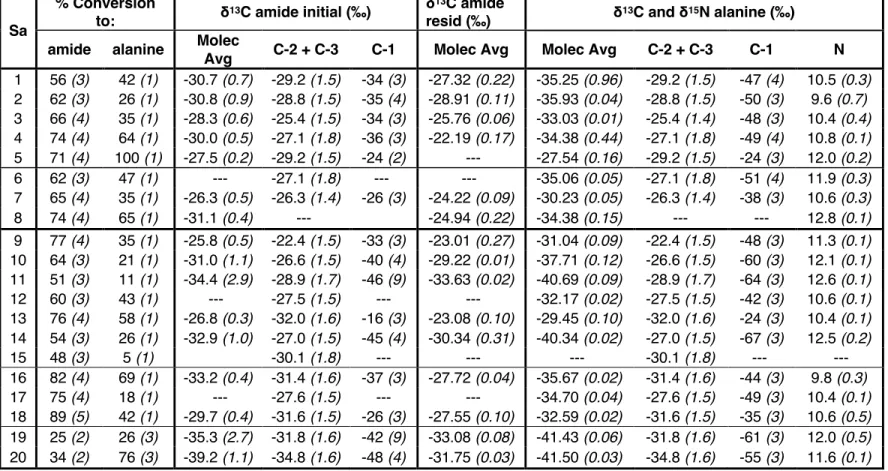
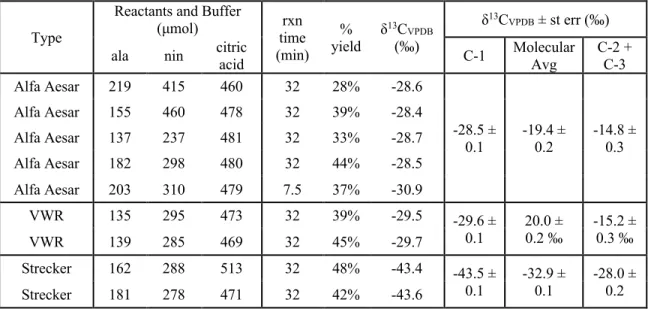
Final masses of alaninamide (ie, the residual alaninamide present at the end of the hydrolysis steps) and alanine were determined gravimetrically. Moles of initial alaninamide are calculated as the sum of moles of alanine and final alaninamide. For the initial compositions, the δ13C of the acetaldehyde and sodium cyanide and the δ15N of the ammonium chloride reactants added to the reaction vial are known from direct measurements.
Results
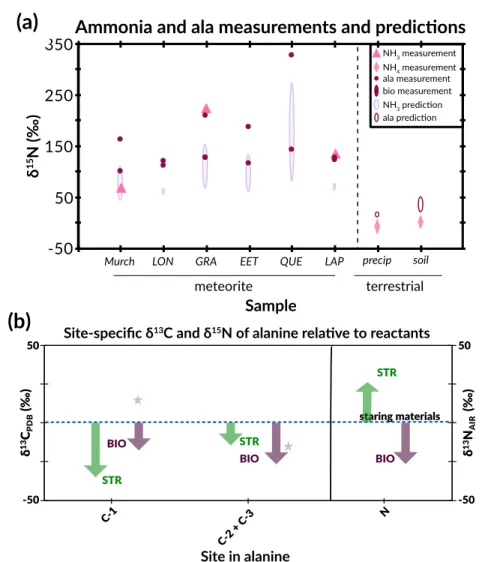
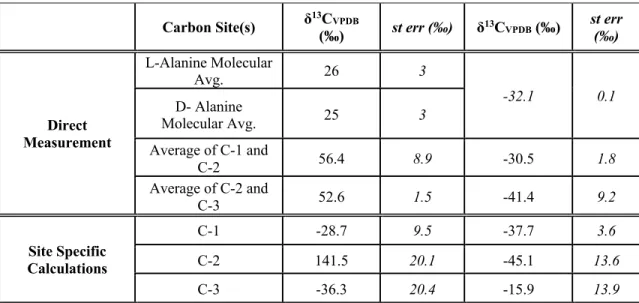
We calculate the average molecular δ13C values of α-APN as a weighted average of the C-1 site and the C-2 and C-3 sites in α-APN for each synthesis with the same weighting as above. We measured the δ13C of cyanide and calculated the δ13C of the C-1 site of alanine using the measured molecular average δ13C and the average δ13C of the C-2 and C-3 sites using equation 3.3 (we note again that all such calculations convert δ13C values to 13F values prior to such calculations mass balance). As with the molecular average δ13C values, for each synthesis the δ13C of the C-1 site decreases from α-APN to alaninamide to alanine (Figure 3.4a).
Discussion
The normal KIE of 15.4 ‰ for the hydrolysis of the C-1 site from an amide to a carboxyl group (Table 3.3) is approximately three times the KIE found for the full molecular average δ13C, further supporting the idea that the C-1 site is the only one that changes during H-2 (a consequence of our conclusion, above, that the average δ13C of the C-2 + C-3 sites does not change during the H-2 reaction). Because the C-3 site is a spectator atom during all equilibrium steps (i.e., it does not form or change bonds), we conclude that conversion of the carbonyl carbon of acetaldehyde to the C-2 site of α-APN carries all EIEs for the C-2 and C-3 Sites, and thus at equilibrium the carbonyl site of a-APN is 20 ‰ lower than that of acetaldehyde. The 15.4 ‰ KIE normal at the C-1 site is approximately three times the molecular average and both models for the C-1 site, and equal to the minimum value for δ13C of the C-1 site of the amide subtracted from that of α -APN .
Implications
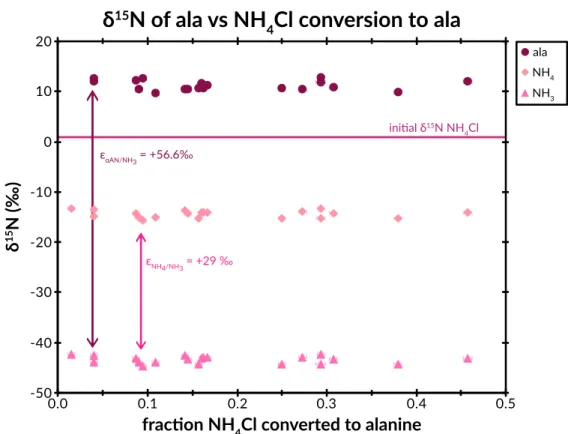
The left panel shows δ13C ranges relative to CN and acetaldehyde for C-1 and C-2 + C-3 respectively for Strecker synthesis (green) and CO2 for biosynthesis (burgundy) (Abelson and Hoering, 1961). The right panel shows δ15N ranges relative to input ammonium for Strecker synthesis and biosynthesis. This suggestion was recently supported by a study of the site-specific carbon isotope structure of alanine (Chimiak et al., 2020).
Conclusions
If this interpretation is correct, we can combine the experimental constraints on N isotopic fractions from the Strecker. To better understand the isotope chemistry of the H-1 reaction step, future experiments should focus on α-APN hydrolysis alone. However, this uncertainty does not affect our evaluation of the equilibrium isotope effects or the isotope effects.
CARBON ISOTOPE EVIDENCE FOR THE SUBSTRATES AND MECHANISMS OF PREBIOTIC SYNTHESIS IN THE EARLY SOLAR SYSTEM
Introduction
Proposed mechanisms for meteoritic amino acid synthesis include i) ion/radical-molecule reactions in the interstellar medium (ISM) (e.g. with irradiation of methanol ice (Bernstein et al., 2002)), ii) Fischer-Tropsch type (FTT) ) synthesis in the protosolar nebula (Botta and Bada, 2002) and/or iii) aqueous chemistry (e.g. Strecker synthesis or reductive amination) of ISM-derived precursor molecules that were accumulated in ice of the meteorite parent body during aqueous reactions. change (Kerridge, 1999;. The molecular average δ13C values1 of individual α-amino acids from Murchison CM2 CC decrease systematically with increasing carbon number (Pizzarello et al., 1991; Sephton, 2002; Elsila et al., 2012; Glavin et al. , 2018), suggesting that they may have been assembled from smaller precursors where each newly added carbon atom is lower in 13C than its source due to kinetic isotope effects (KIEs) (Yuen et al., 1984; Engel et al., 1990; Sephton, 2002. Alternatively, these trends could reflect the dilution of a high-δ13C carbon inherited from ISM-derived CO with carbon from other, lower-δ13C precursors (Elsila et al., 2012 ).
Methods and Materials
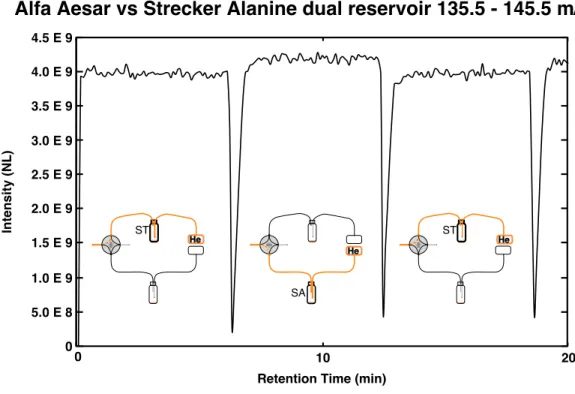
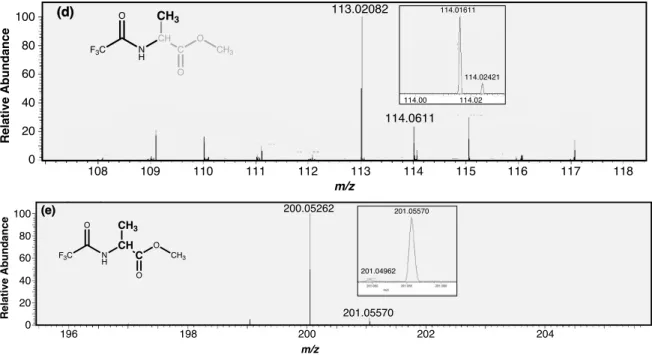
Here we present site-specific δ13C measurements of three carbon sites in alanine extracted from a Murchison sample and measured using new techniques performed with an Orbitrap mass spectrometer. A split of the analytical sample extract and Caltech alanine standards were also derivatized as N-trifluoroacetyl-O-isopropyl esters in GSFC following protocols from (Elsila et al., 2012) in GSFC. Alanine measurements were collected for 10 to 60 minutes depending on the size of the reservoir and the abundance of the fragments of interest (Figure 4.2d).
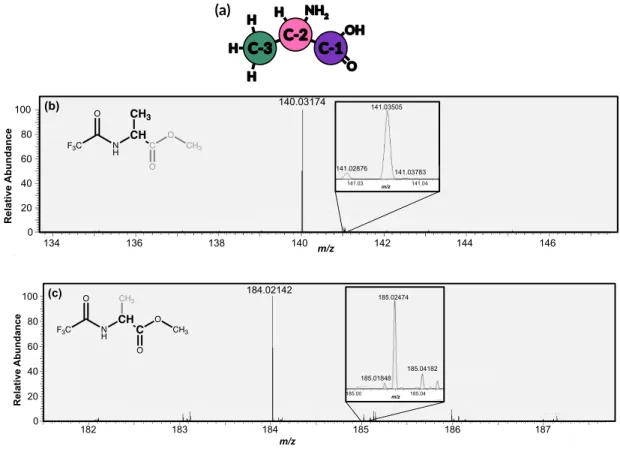
- Results
- Discussion
- Conclusions
Error bars for carboxylic acids are smaller than the symbols and are not included in the data for Engel et al. Our predictions represent the largest change in δ13CVPDB in amino acids (purple arrowheads in Figure 4.4). The success of a simple reaction model (Figure 4.3) in explaining most of the previously measured δ13C values for these various compounds supports the idea that the various required chemical reactions occurred simultaneously, in a single environment, and stemmed from a common set of precursors. , some of which likely originate in the ISM (de . Marcellus et al., 2015).
AVERAGES TO INSIGHTS
USING MOLECULAR-AVERAGE ISOTOPE VALUES TO MODEL ISOTOPIC STRUCTURES AND METEORITE ORGANIC SYNTHESIS
Introduction
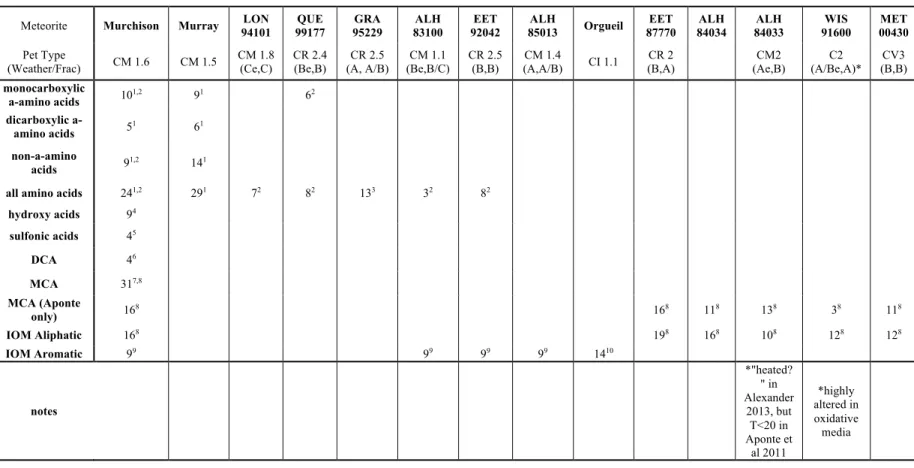
Carbonaceous chondrites (CC) – a class of undifferentiated meteorites that have 2–5 wt. % C (Glavin et al., 2018) – are among the most primitive samples of solar system material. CC includes soluble and insoluble organic matter (SOM and IOM, respectively), includes more than 80 amino acids and 100 polyaromatic hydrocarbons (PAHs), and is dominated by the IOM component, comprising ~70% of organic matter (OM) (Glavin et al., 2018 ). However, measurements of ketones in 13C are significantly lower than aldehydes of the same size (Simkus et al., 2019; Aponte et al., 2019).
Methods
These isotope effects are combined with assigned δ13C values of precursors for each reaction step to predict the δ13C value of the product. The molecular average δ13C of the product was then calculated as the weighted average of the site-specific carbon isotope contents of its carbon atoms. These errors are lower than many of the analytical errors associated with aldehyde δ13C measurements used in the model.
Results
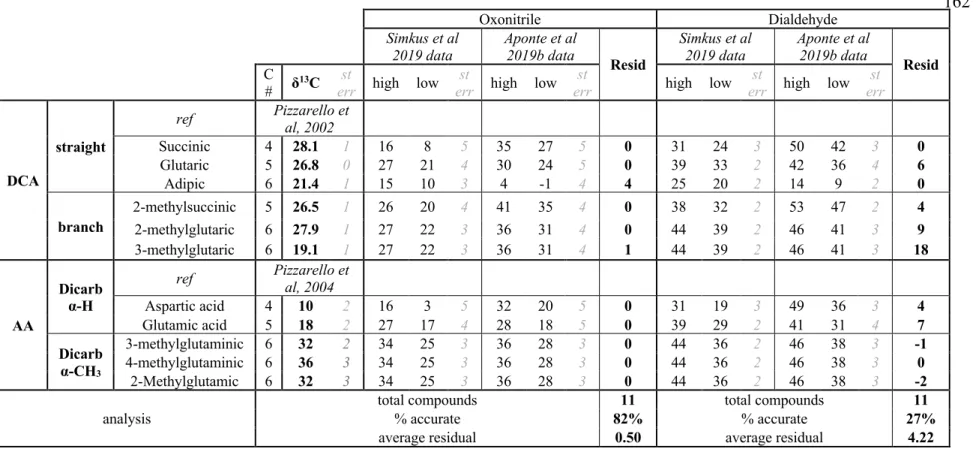
The key features of our proposed reaction model most essential to observed descriptive patterns of SOM δ13C values are: (1) the integrated aldehyde chemistry (see 5.2.2.2 for description), which creates straight-chain and α-H bonds; (2) addition of aldehyde to these straight-chain compounds (typically drawing on formaldehyde as a precursor, but using acetaldehyde in the case of 3-pentylamine) to form branched and α-CH3 species; and (3) formation of dicarboxylic species through chemistry similar to that forming monocarboxylic species, but starting with oxonitrile precursors rather than aldehyde precursors (and accompanied by hydrolysis of the nitrile moiety to another carboxyl group on these oxonitriles). Focusing first on the data for the Murchison meteorite, if we 'saw' the proposed reaction network with the known δ13C values of straight-chain aldehydes, we can predict the δ13C values for the straight-chain amines, α-H-amino acids and monocarboxylic acids resulting from amination, respectively , Strecker synthesis and oxidation (Table S5.4). The fit of the models is improved with the integrated aldehyde network used for straight chain and α-H compounds and uses the measured straight chain and α-H compounds and formaldehyde to predict values for the branched and α-CH3 compounds.
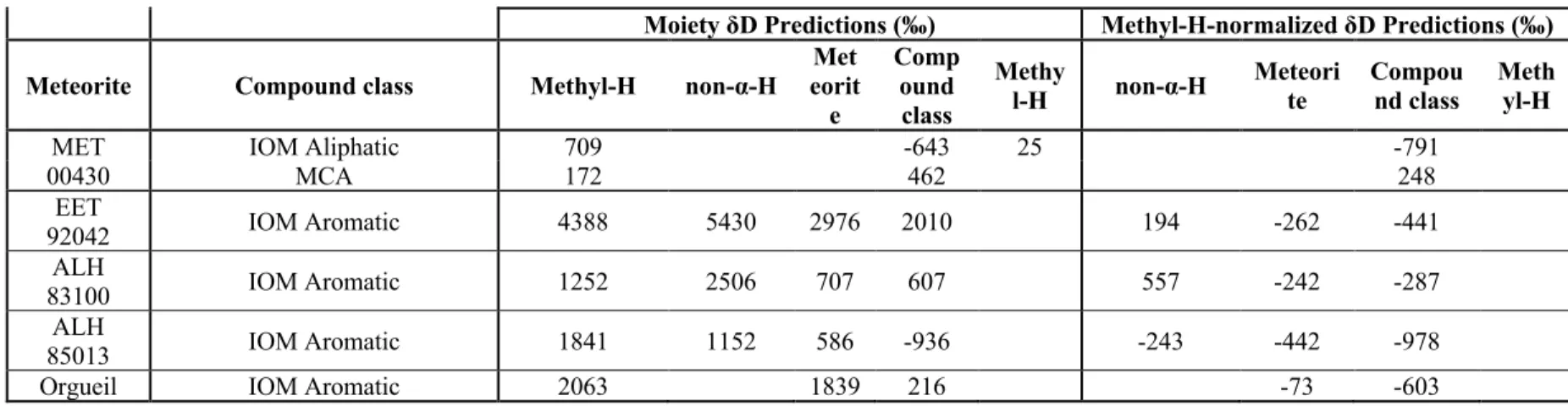
Discussion
Existing ISM chemical models also predict 13C isotopic enrichments at specific sites in organic compounds (Charnley et al., 2004). A common premise is that aldehyde chemical networks create amines, MCAs, hydroxy acids, and α-amino acids (Aponte et al., 2017). It is conceivable that changes in pH would change the δ15N of ammonia by up to 30‰ (Walters et al., 2019), the approximate value of the EIE between NH4+ (aq) and NH3 (aq) at relevant temperatures.
Summary and Conclusions
Organic material in meteorites: molecular and isotopic analyzes of the Murchison meteorite. 1995) Properties and Formation of Amino Acids and Hydroxy Acids from the Murchison Meteorite. 2018) Effect of polychromatic X-ray microtomography imaging on the amino acid content of the Murchison CM chondrite. Van Slyke D D, DA MacFadyen and Hamilton P. 1941) Determination of free amino acids by titration of the carbon dioxide formed in the reaction with ninhydrin.