1
Journal of Chemical Technology and Metallurgy
Volume:55, No: 5, Oktober 2020, Pages: 1008-1018 ISSN 1314-3859 (print), 1314-7471 (on-line)
DOI:https://dl.uctm.edu/journal/node/j2020-5/12_19-213_p1008-1018.pdf H Index : 17
SJR Index : 0,190 (2019)
Turnitin similarity index : 11%
Penerbit: University of Chemical Technology and Metallurgy
Reputasi: Terindeks SCOPUS, Q3 JUDUL PAPER:
A Combine Ultrasound and Ozone (US/O3) Treatment Enhancing κ- Carrageenan Depolymerization
KORESPONDENSI PAPER
Item Halaman
Submission of Manuscripts_Aji Prasetyaningrum ( 27 September 2019)
2-26
Manuscript 19-213 review 1: 16 Desember 2019 27-29
Manuscript 19-213 review 2: 30 Juni 2020 30-58
Pemberitahuan accepted publikasi: (14 Juli 2020) 61
2 Submission of Manuscripts_Aji Prasetyaningrum: 27 September 2019
from: Aji
Prasetyaningrum <[email protected]>
date: Sep 27, 2019, 2:27 PM
subject: Submission of Manuscripts_Aji Prasetyaningrum mailed-
by:
che.undip.ac.id
Dear Editor,
I hereby submit the paper entitle “Combination Treatment of Ultrasound and Ozone (US/O3) to Enhance The Depolymerization of κ-Carrageenan” to Journal of Chemical Technology and Metallurgy. The paper is authored by Aji Prasetyaningrum, Bakti Jos, Ratnawati, Yudhy Dharmawan, and Andri C Kumoro with my self as corresponding author. We do state that this manuscript has been not submitted to other journal or elsewhere as well a had not been submitted earlier to Journal of Chemical Technology and Metallurgy.
We hope you find this paper interesting and publishable in your esteemed journal. We look forward to your decision.
Best regards
Dr. Aji Prasetyaningrum, S.T., M.Si.
Chemical Engineering Departement Diponegoro University
3 ORIGINAL PAPER
Combination Treatment of Ultrasound and Ozone (US/O3) to Enhance The Depolymerization of κ-Carrageenan
Aji Prasetyaningrum1*, Bakti Jos1, Ratnawati Ratnawati1, Yudhy Dharmawan2, and Andri C.
Kumoro1
1Department of Chemical Engineering, Faculty of Engineering, Diponegoro University, Jl. Prof.
Soedarto S.H., Tembalang, Semarang 50269, Indonesia
2Faculty of Public Health, Diponegoro University, Jl. Prof. Soedarto S.H., Tembalang, Semarang 50269, Indonesia
*Corresponding author, e-mail: [email protected]; phone number +628122856097; Semarang 50269, Indonesia.
Abstract
κ
-Carrageenan is a sulfated galactan derived from red algae. Low molecular weight fraction (LMWF) of κ-carrageenan exhibits many benefits in the pharmaceutical and biomedical fields.This study investigated the effects of individual and combined ultrasound and ozone (US/O3) treatments on the depolymerization of κ-carrageenan. The influence of a combination of US/O3
on the depolymerization of κ-carrageenan was indicated by the decreasing of molecular weight.
The sulfate content was characterized by the barium chloride-gelatin method. The characterized functional group analysis of native and treated κ-carrageenan was determined using FT-IR. The structural and morphological properties of the depolymerized κ-carrageenan were determined using SEM and XRD. The experimental results showed that the molecular weight of κ- carrageenan decreases appreciably with a combination of US/O3 treatment. Degree of depolymerization of κ-carrageenan at pH 7 leading to 18.36% for US only and 51.12% for O3
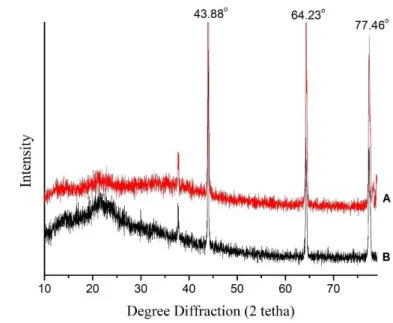
4 only after 20 min of treatment, but the combined of US and O3 led to achieving 88.36%. The depolymerization of κ-carrageenan becomes increase as the pH decrease, but the increasing of temperature no significant effect on reducing the molecular weight of κ-carrageenan. During US/O3 treatment, the sulfate content of κ-carrageenan tends to stable. FT-IR analysis shows there was no significant change in the functional properties of treated κ-carrageenan. The X-ray diffraction pattern of the native and treated κ-carrageenan by US/O3 showed a similar pattern with the main peaks at 43.88o, 64.23oand 77.46o. SEM analysis of the surface morphology of the κ-carrageenan after US/O3 treatment was amorphous and rough.
Keywords
ultrasound, ozone, depolymerization, κ-carrageenan
5 Introduction
Carrageenan is a natural polysaccharide sulfated galactan that is composed of alternating 3-linked β- D-galactopyranose and 4-linked α-D-galactopyranose or 4-linked 3,6-anhydro-α-D-galactopyranose [1]. Carrageenans were classified according to the number and position of sulfate groups. The commercial of κ-carrageenan or high molecular weight fractions (HMWF) of κ-carrageenan has an average molecular weight of 100-1000 kDa [1]. The depolymerization process may change HMWF of κ-carrageenan to low molecular weight fractions (LMWF) of carrageenan. LMWF of carrageenan has shown its biological activities, such as antiviral [2, 3, 4, 5, 6], anticoagulant [7, 8], antitumor [9,10]
and antioxidant [11,12]. The LMWF of κ-carrageenan has the ability to penetrate human cells more effectively compared to that of the high molecular weight fractions (HMWF) of κ-carrageenan [2].
LMWF of carrageenan is used with a size of a molecular weight of less than 20 kDa for biomedical applications [12]. The changes in the molecular structure of κ-carrageenan from HMWF of κ- carrageenan to LMWF of κ-carrageenan is presented in Figure 1.
HMWF of κ-carrageenan LMWF of κ-carrageenan Fig. 1 Depolymerization of κ-carrageenan
Thermal depolymerization [13], acid-catalyzed hydrolysis [9,14], enzymatic hydrolysis [10], irradiation [12,15], and sonication [16,17] are widely used in the depolymerization techniques. Acidic hydrolysis has been considered as a common and fast method to produce a series of increases in the level of environmental pollution [9,14]. The enzymatic method is not preferable because of the
6 relatively expensive and complex process [10]. Due to the high oxidation potential, ozone may be an alternative approach to achieving the degradation of organic and inorganic compounds [18,19].
Ozone is well known to act as a powerful oxidizing agent to remove the pollutants from water [20]
and to depolymerize polysaccharides because of its excellent oxidation ability [18]. Ozone was classified as generally recognized as safe (GRAS) for use in industrial food by the United States of Food and Drug Administration (FDA). However, the application of ozonation has been limited by the low ozone mass transfer rate. The ozone mass transfer rate may be increased by ultrasonication treatment [20]. The ultrasonication method can be considered as a type of green technology because it does not require the addition of chemicals. Acoustic cavitations produce a lot of hot spots with high temperatures and pressures causing the sonolysis of H2O. Therefore, radical species are formed which induced the degradation of the organic compound in aqueous solution. The polymer scission by the ultrasonic method has a unique character because that the depolymerization at the mid-point of the chain by non-random process. During ultrasonication, the radical generation initiates the depolymerization of polysaccharide especially for water-soluble polymers [17,21].
The combination of US/O3 has been reported to exhibit several advantages compared to the individual US or O3 process. The combination of US/O3 was selected to enhance the oxidation of organic compounds. Degradation of guar gum (GG) was significantly increased in about 99.1% using a combination of US/O3 [22]. Yue and co-workers [23] reported that the combination of US/O3 resulted in a significant decrease in the molecular weight of chitosan. Some previous studies, US and O3
methods were studied separately as the depolymerization of κ-carrageenan. Therefore, this study aims to investigate the effects of US and O3 treatments, both individually and combined, on depolymerization of κ-carrageenan. To verify the performance of the process, the physicochemical and morphological properties of native and treated κ-carrageenan were analyzed using the barium- chloride gelatin methods, FT-IR, SEM and XRD.
7 Experimental
Materials
κ-carrageenan was produced from CV. Karagen Indonesia, Central of Java, Indonesia. Commercial κ- carrageenan was purified to obtain refined κ-carrageenan, by dilution of carrageenan powder in distilled water. The κ-carrageenan solution was completely dissolved by heating at 70 °C with stirring slowly. The polysaccharides in solution were precipitated by the dropwise addition of isopropyl alcohol (E. Merck, Catalog No. 818766) under vigorous stirring. The precipitate of refined κ- carrageenan was collected and dried at 60oC in a forced-air oven. Hydrochloric acid solution 37% (E.
Merck, Catalog No. 100314) and sodium hydroxide (E. Merck, Catalog No. 137020) have been used to arrange the initial of pHs. In this study, all the chemicals were used without any pretreatment.
Experimental Set-up
Depolymerization treatment of κ-carrageenan was performed in a transparent glass reactor with a capacity of 500 mL. Refined κ-carrageenan solution (1: 100, w/v) was prepared by dissolving it in distilled water at 70 0C. The experiments were carried out by different methods: the US only, O3 only and the combination of US/O3. The sonication process used an ultrasonic generator with the specification of 150W, 40 kHz. Ozonation process used different dissolved ozone concentrations (15, 25, 35 mg.min-1.L-1). Ozone gas was produced by an ozone generator (Dipo Technology Indonesia) and bubbled in the solution through a bubble diffuser. Inlet concentration of ozone determined by the indigo colorimetric method. This experiment was conducted at different times (0, 5, 10, 15 and 20 minutes). Initial pH was adjusted by the addition of HCl/NaOH and the temperature process was maintained using a water bath. The schematic experimental of US/O3 treatment of κ-carrageenan is depicted in Figure 2.
8 Fig. 2 Schematic experimental of US/O3 treatment
Analytical Procedure
Molecular Weight Determination
At regular intervals, samples were withdrawn, cooled to 25 0C ± 1 0C in chilled water to quench the reaction and the intrinsic viscosity analyzed using glass capillary Ubbelohde viscometer. The molecular weight of κ-carrageenan was determined according to the Mark-Houwink equation. Lai et al. [13] reported an equation that relates intrinsic viscosity to the molecular weight as follows:
[η] = kMH.Mα (1)
where [η] is intrinsic viscosity, kMH and α are constants, and M is the number-average of molecular weight. The values of kMH and α for the κ-carrageenan solution in water at 250C are 0.00778 and 0.90, respectively [24, 25]. The intrinsic viscosity is obtained using the Huggins equation:
= [η] + kH [η]2 C (2)
where ηsp, C, and kH are specific viscosity, the concentration of the solution, and Huggins constant of which value is 0.3 [24].
Sulfate Analysis
The sulfate contents of both non-treated and treated of κ-carrageenan were determined using the barium chloride-gelatin method [26]. A known number of the sample (W1 g) was hydrolyzed using 50 mL of 1 N HCl for 30 minutes by heating at boiling temperature. Ten milliliters of 0.25 M BaCl2
9 solution was added to the reaction mixture. The mixture was then cooled to room temperature and kept for 5 hours. The BaSO4 precipitate was filtered with ashless filter paper and incinerated in the muffle furnace at 700 0C for 1 hour. The ash was weighed as W2 g and the content of sulfate can be calculated according to the equation:
% Sulfate = (W2 / W1) × 41.16 (3)
FT-IR Spectroscopy
Fourier transform infrared (FT-IR) spectra of the native κ-carrageenan and depolymerized κ- carrageenan product were recorded with KBr powder (10 mg κ-carrageenan powder in 90 mg KBr powder) using a Perkin Elmer IR 10.6.1 Spectrophotometer, USA in the range from 400-2000 cm-1. X-ray Diffraction (XRD)
X-ray diffractograms of the native and treated κ-carrageenan by combination US/O3 were obtained with an X-ray diffractometer (XRD-7000, Shimadzu, Japan). The scanning region of the diffraction ranged from 10o to 80o with a target voltage of 30 kV, current of 30 mA and a scan speed of 5o/min.
The relative crystallinity (RC) of chitosan was calculated using an equation proposed by Klein et al.
[27].
(4)
Where is the crystalline area, and is the amorphous area.
Scanning Electron Microscopy (SEM)
SEM characterization was carried out using a Scanning electron microscope JSM-6510-LA JEOL series, Japan. Prior to analysis, the native and treated κ-carrageenan were sprinkled onto adhesive Al or C tapes and was supported on metallic discs and coated with Au. Images of the sample surfaces were recorded in different areas and magnifications.
10 Results and Discussions
Comparison of Ultrasonication and Ozonation Process for The Tepolymerization of κ- Carrageenan
The κ-carrageenan solution was treated with US only and O3 only at different concentrations of ozone.
The experiments were carried out at 29±1 0C and initial pH adjusted at pH 7. The US and O3
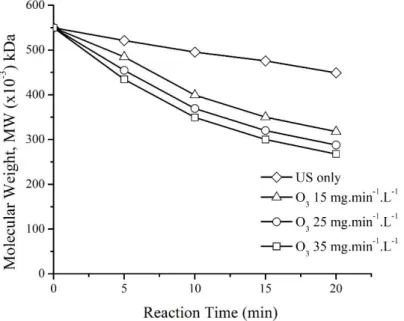
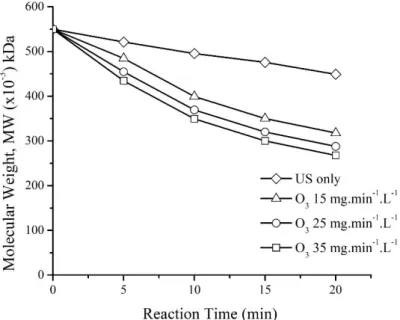
treatments were applied at 0, 5, 10, 15 and 20 minutes. The correlation between the molecular weight of κ-carrageenan with reaction time was shown in Fig 3.
Fig. 3 The correlation of reaction time on the molecular weight of κ-carrageenan.
In this research, the initial of κ-carrageenan molecular weight was 550 kDa in all treatment. The decreasing molecular weight of κ-carrageenan using US treatment only is marked with the symbol ( ).
The κ-carrageenan molecular weight was reduced from 550 kDa to 449 kDa for 20 minutes of ultrasonication. The reduction rate of the number-average molecular weight of κ-carrageenan using US methods was 18.36%. To compare the effect of individual treatment approaches for
11 depolymerization of κ-carrageenan, there were three different concentrations of ozone: 15 mg.min-1.L-
1 (marked with the symbol ∆), 25 mg.min-1.L-1 (marked with the symbol ○) and 35 mg.min-1.L-1, (marked with the symbol □). The results showed that κ-carrageenan molecular weight was decreased from 550 kDa to 317, 288 and 268 kDa in 20 minutes of ozonation, respectively. Therefore, the reduction of the molecular weight of κ-carrageenan was 42.36%, 47.64%, and 51.12%, respectively.
Thus it can be said that the percent reduction of the number-average molecular weight of κ- carrageenan for ozone treatments was higher than ultrasonic treatment. Similarly, Prajapat and Gogate [22] reported that the extent of reduction in intrinsic viscosity of guar gum for ozone treatment (96.20%) was only marginally higher as compared to that obtained for the ultrasonic radiation (95.59%). The ozone treatment was more effective than ultrasonic radiation in the degrading of chitosan [23]. It was also reported that during individual ultrasonic radiation, the viscosity-average molecular weight of chitosan was decreased by 12.74%. For ozone treatment, the extent of decrease in viscosity increased from 31.18 to 41.38% for the ozone dosage of 35±5 mg/min to 65±5 mg/min for 30 min of reaction. Overall, it can be said that the ozone treatment gives similar extents of viscosity reduction which is much higher than individual ultrasound treatment.
In the case of ultrasonication treatment, reactions included pyrolysis and chemical reactions with radicals produced from H2O. The hydroxyl radicals are generated from the sonication of H2O [23].
The equation is shown below:
(5)
During ozonation treatment alone, O3 transferred from the gas phase into the liquid phase. The hydroxyl radicals are produced. Ozone was immediately reacted with the κ-carrageenan solution through direct reaction and indirect reactions with radicals generated by ozone auto decomposition [18], as shown in the equation below:
(6)
12 (7)
Synergetic Effect of US and O3
The US/O3 applications were carried out simultaneously resulted in a synergetic effect, which leads to an increase in the depolymerization of κ-carrageenan. Three different rates of ozone concentration: 15, 25 and 35 mg.min-1.L-1 were also used to study the effect of the combination of US/O3. The molecular weight of κ-carrageenan was decreased from 550 to 114, 84 and 64 kDa for 20 minutes of treatments, respectively (Figure 4). The percentage of reduction of κ-carrageenan molecular weight using US/O3
treatment with 15, 25 and 35 mg.min-1.L-1of ozone concentration was 79.27%, 84.47%, and 88.36%, respectively. It is intriguing that in the O3/US reaction system combination, the average molecular weight of κ-carrageenan decreases much higher when compared to the amount that occurs in the US process only and ozone processing only. In addition, the rate of decrease in viscosity-average molecular weight of carrageenan in the combination of US/O3 is much higher than the summation of degradation value obtained by individual ultrasonic radiation and individual ozone treatment. The extent of degradation carrageenan in the combination system of US/O3 can be achieved to 88.36%
while the summation of both processes only to 69.48% (51.12% of ozone treatment only and 18.36%
of ultrasonication only).
Fig. 4 Combination of ultrasonication and ozonation on depolymerization of molecular weight of κ- carrageenan. The reaction was kept at 29±1 0C and initial at pH 7.
When an organic substance such as carrageenan solution is treated with ozone in the presence of ultrasonic radiation, the hydroxyl radicals can be produced from four mechanism reaction. The first
13 reaction system is sonication by H2O. The equation of the reaction can be seen in the Eq. (5). The second reaction system is ozone self-decomposition, for the equation as follows in the Eq. (6) and (7).
The third reaction system is the production of hydroxyl radicals attacking the -D-(1,3)-galactose linkages of κ-carrageenan that is radiolysis of ozone by ultrasonic radiation, as follows the equation below:
(8) (9)
The last reaction system is the dissociation of oxygen molecules in the vapor phase of the cavitation bubble. Under a treated with ozone in the presence of ultrasonic radiation, the addition ●OH radicals are generated by molecular oxygen dissociation in the bubble:
(10) (11)
The reaction mechanism of the depolymerization of κ-carrageenan might be affected by each other during the combination of US/O3 system. Similar to the result, Yue et al. [23] reported that the combination of US/O3 was an effective system for the degradation of chitosan. The combination process of US/O3 can significantly produce hydroxyl radicals, which are powerful oxidizing species and can attack the -D-(1,4)-glucosidic linkages of chitosan and make the linkages break.
Ultrasonic reactions will increase the mass transfer of ozone into the κ-carrageenan solution thereby increasing the value of the volumetric mass transfer coefficient. During ozonation, the addition of the ultrasonication treatment can produce turbulent flow resulting in smaller ozone bubbles. The microbubbles of ozone have the function of the sonolysis cavitation nucleus to generate more acoustic cavitations, which improves US/O3 treatment. This will encourage the process of diffusion of ozone molecules into a κ-carrageenan solution, increasing contact area and oxidation rate. The ultrasound treatment enhanced the ozone to produce more ●OH to improve the oxidation rate. Ozone was
14 decomposed rapidly in the vapor phase of a cavitation bubble during the combination of US/O3
reaction. Furthermore, the ultrasonic reaction will also increase the decomposition reaction of ozone to produce more ●OH which will react with κ-carrageenan. There was more opportunity for the hydroxyl radical attacking the -D-(1,3)-galactose linkages of κ-carrageenan.
Effect of The US/O3 Operating Conditions Effect of pH
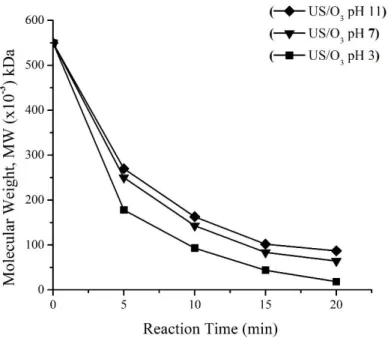
Fig. 5 shows the influence of pH on carrageenan depolymerization by a combination of the US and O3
processes on the depolymerization of κ-carrageenan. The initial pH was adjusted at pH 3, 7 and 11.
Ozone gas was bubbled in the κ-carrageenan solution with a concentration of ozone 35 mg.min-1.L-1. At the 20 minutes reaction, the molecular weight of κ-carrageenan decreased from 550 kDa to 18, 64 and 87 kDa, respectively. Whereas the percentage of depolymerization κ-carrageenan at initial pH of 3, 7 and 11 were 96.73; 88.36 and 84.18%, respectively.
The results showed that during US/O3 treatment, pH has significant effects on the depolymerization of κ-carrageenan. The overall observed results were that the reaction becomes faster as the pH decreases.
It was previously mentioned that in acidic conditions, the concentration of proton (H+) becomes higher and thus more β-1,4-glycosidic bond being attacked [28]. Yoon et al. [29] reported that the depolymerization of seaweed extracts process like that of cellulose. The glycosidic oxygen in the β- 1,4-glycosidic bond of cellulose rapidly interacts with the ion H+ of the acid to produce a conjugated acid. Therefore, the C-O bond slowly cleavage to form two fragments. One fragment has OH linkage as the end group while the other fragment ends with a cyclic carbocation.
Fig. 5 Effect of pH on depolymerization of molecular weight of κ-carrageenan. Reaction was kept at 29±1 0C and O3 (35 mg.min-1.L-1).
15 This research showed that the percentage of depolymerization of κ-carrageenan at low pH was higher than alkaline conditions. Under acidic conditions, the ozonation reaction was dominated by the direct ozonation process. On the other hand, indirect ozonation was predominant under neutral and alkali conditions [6]. Lemeune et al. [19] reported that the decomposition of ozone initiated by ●OH ions produces hydroxyl radicals that can very rapidly attack glucose or cellobiose with a low reaction selectivity. Chirat and Lachenal et al. [30] concluded that molecular ozone is mainly responsible for cellulose degradation and the cellulose is less degraded at high pH.
The effect of pH on the number-average molecular weight can also be viewed in terms of the rate of reaction as well as the amount of hydrolysis product. Lenihan et al. [31] hydrolyzed lignocellulosic biomass from potato peels using phosphoric acid as the catalyst with various concentrations, i.e. 2.5, 5.0, 7.5, and 10% w/w which are equivalent to pH of 1.40, 1.23, 1.14, and 1.10 respectively. They reported that the reaction rate increased as the acid concentration rose or the pH decreased. Kumar et al. [32] who hydrolyzed sugar cane bagasse using sulfuric acid as the catalyst reported that the hydrolysates (xylose, glucose, and furfural) increase as the percentage of sulfuric acid increases.
Myslabodski et al. [33] who hydrolyzed κ-carrageenan at different temperatures with various pHs, reported similar results to this work. It was reported that to reduce 25% of molecular weight, the hydrolysis with pH 6, 5, 4, and 3 required 12 days, 2 days, 8 h, and 1.4 h respectively.
Effect of Temperature
The effect of US/O3 treatment of κ-carrageenan depolymerization with a temperature range of 30-50
◦C is shown in Figure 6.
Fig. 6 Effect of temperature on depolymerization of molecular weight of κ-carrageenan. The reaction was adjusted at an initial pH 7 and O3 (35 mg.min-1.L-1).
16 The reaction was adjusted at an initial pH 3and concentration of ozone 35 mg.min-1.L-1. Temperatures were adjusted at 30, 40 and 50 ◦C and the viscosity-average molecular weight of κ-carrageenan reduced from 550 kDa to 18 kDa; 16 kDa and 12 kDa. The depolymerization of κ-carrageenan slightly increased from 96.73% to 97.82% if the reaction temperature increased from 30 oC to 50 o C at 20 min of US/O3 treatment.
A combination of US/O3 treatment has a positive and negative influence on the increase in κ- carrageenan depolymerization. The ozone concentration in the κ-carrageenan solution will decrease with increasing reaction temperature. On the other hand, increasing temperature lead to improving the rate of mass transfer during the ozonation process. Simoes and Castro [34] reported the depolymerization rate of hollocellulose increase markedly with temperature.
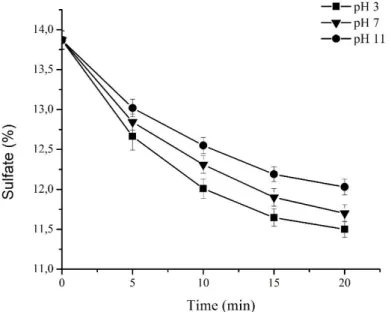
Effect of US/O3 Treatment on Sulfate Content of κ-carrageenan
The low molecular weight of κ-carrageenan and their sulfated derivatives exhibited beneficial biological activities. The remaining sulfate in the treated κ-carrageenan calculated using barium- chloride gelatin methods. As shown in Fig.7, the sulfate content after the US, O3 and US/O3 reaction does not significantly decrease. The sulfate content of native carrageenan is 13.87±0.25 %. After 20 minutes of US/O3 treatment at pH 11, pH 7 and pH 3 the sulfate contents are reduced to 12.03±0.25
%, 11.78±0.19 %, and 11.50±0.27 %, respectively. The results of this study suggest that the sulfate groups are only slightly reduced during US/O3 process at different pH. Some other groups of researchers reported that κ-carrageenan relative stable during the depolymerization process [8, 14, 15, 35]. The sulfate residues after irradiation, approximately 90 %, 83 % and 71 % for κ-, ι-, and λ- carrageenan, respectively [15].
Fig. 7 Sulfate content in κ-carrageenan US/O3 treatment at various pH.
17 Sun et al. [36] reported a comparative investigation of the remaining sulfate content in κ-carrageenan after H2O2 depolymerization, enzymatic digestion, partial reductive hydrolysis, and HCl hydrolysis.
With initial content of sulfate of 15.66 ± 0.30 %, the sulfate content after H2O2 depolymerization, enzymatic digestion, partial reductive hydrolysis, and HCl hydrolysis, were 15.47 ± 0.43 %, 15.27 ± 0.35 %, 15.55 ± 0.16 %, and 11.87 ± 0.51 %, respectively. Karlsson et al. [14] reported that no significant change of sulfate content under the depolymerized of κ-carrageenan using mild acidic hydrolysis. Prasetyaningrum et al. [37] also reported that the sulfate content of κ-carrageenan does not significantly decrease after the ozonation reaction process.
The biological activity of low molecular of κ-carrageenan depends on chemical structure, molecular weight, and chain conformations. Wang et al. [6] reported that oligosaccharides of κ-carrageenan and their sulfated derivatives effectively inhibit anti-influenza A virus. The degree of sulfation and molecular weight were the main factors that influenced the anti-influenza A virus activity of κ- carrageenan oligosaccharide. The most active κ-carrageenan oligosaccharide had a sulfate content of 0.8-1.0 mole/mole of disaccharide and a molecular weight of 1-3 kDa [6]. Yuan and Song [9] studied the depolymerization of κ-carrageenan by acid hydrolysis and tested its activity in the biomedical field. Bioassay tests showed that κ-carrageenan oligosaccharides 1.2 kDa and 8.98% sulfate content had relatively high antitumor activity compared to carrageenan polysaccharide. The extract of low molecular weight fragments of κ-carrageenan with an average molecular weight (Mw) ranging from 2.3 kDa to 5.0 kDa obtained by gamma irradiation in solution exhibits antioxidant properties [38-39].
Relleve et al. [35] reported that the oligomer obtained from κ-carrageenan with molecular weight 10 kDa showed a strong growth promotion effect on potato in tissue culture. Oligoalginate prepared by irradiation with a molecular weight of approximately 14.3 kDa was found to be the highest positive effect on the propagation of flower plants in tissue culture [40]. Based on existing research, oligo κ-
18 carrageenan obtained from this experiment with a molecular weight of approximately 12 - 18 kDa and sulfate content from 11.50 % to 12.03 % have prospected in biological effect as vitro propagation in tissue culture.
FT-IR Analysis
The method of the infrared spectroscopy was utilized to control the structure modification of κ- carrageenan which could appear after chemical depolymerization. The FT-IR spectra of native and degraded κ-carrageenan at different methods are shown in Fig. 8. The characteristic absorption peaks of κ-carrageenan appearing from 1220 to 1440 cm-1 determines the presence of ester sulfate (S=O).
Wavelengths from 1010 to 1080, 928 to 930, 840 to 850 cm-1 attributed the presence of glycosidic linkage, 3,6-AG galactose-4-sulfate, galactose-4 sulfate, respectively. The results of FT-IR spectra shows that the molecules present in native and treated κ-carrageenan are quite similar. The peak absorption at 928 cm-1 was a characteristic absorption of C-O-C in 3,6 anhydro-D-galactose-4-sulfate.
The absorption peak of the treated κ-carrageenan at 840-1 showed the presence of the sulfate groups.
The sulfate content of κ-carrageenan more stable than i-carrageenan and λ-carrageenan during the acid hydrolysis process [14]. Relleve et al. [41] observed that depolymerization of κ-carrageenan using gamma X-ray radiation. It was reported that the sulfate groups of κ-carrageenan are not preferentially or selectively removed. About 90%, 83% and 71% of the sulfate remained in the irradiated κ-, i- and λ-carrageenan, respectively.
Fig. 8 FTIR spectra of κ-carrageenan: (A) native, (B) US treatment, (C) O3 treatment, (D) US/O3 treatment
19 Wavelength (cm-1) ranging from 1010 to 1080 is assigned to the glycosidic bond of κ-carrageenan.
The results of this study show that there is a decrease in intensity at the wavelength of 1027 cm-1 for treated κ-carrageenan, which indicate the cleavage of β-1,4-glycosidic linkages of κ-carrageenan after US/O3 treatment. Relleve et al. [42] reported that there important functional groups were retained even after irradiation. There was a considerable decrease in the peak heights corresponding to the glycosidic, 3,6 anhydrogalactose and methylene groups of κ-carrageenan after radiation treatment. In addition, Li et al. [43] also reported that there was a decrease in the intensity of the FT-IR spectrum in degraded polysaccharide rhamnogalacturonan using a combination of hydrogen peroxide oxidation and ultrasound treatment. For this research, FT-IR results have clearly established that there was no significant change in the functional groups and the chemical structure of κ-carrageenan during the US/O3 treatment. The free radical-induced chain scission only breaks the polymer structure giving molecular weight reduction without any changes in the activity or the functional properties κ- carrageenan.
X-ray Diffraction Analysis
The X-ray diffraction pattern of the native κ-carrageenan and treated κ-carrageenan by US/O3
combination are presented in Fig. 9. The diffraction pattern of native κ-carrageenan and degraded κ- carrageenan by the combination of US/O3 showed a similar pattern with the main peaks at 43.88o, 64.23oand 77.46o. Elsupikhe et al. [44] reported that the result X-ray diffraction of κ-carrageenan showed similar degree diffraction at the main peak that appeared. This research shows that there was a decrease in the main peak for treated κ-carrageenan when compared to native κ-carrageenan.
Prasetsung et al. [45] reported that degraded chitosan by plasma has a lower intensity on the main peak than native chitosan. The relative crystallinity (RC) of treated κ-carrageenan (57,03%) was lower than native κ-carrageenan (72,54%). These results indicated that the crystal structure of treated κ-
20 carrageenan was destroyed during US/O3 treatment. Moreover, the morphological structure of treated κ-carrageenan was became amorphous. Rokhati et al. [46], reported that there was a decrease of RC after chitosan experienced degradation process.
.
Fig. 9 X-ray diffraction pattern: (A) native κ-carrageenan, (B) treated κ-carrageenan SEM Analysis
The surface topography of the solid samples of native κ-carrageenan and κ-carrageenan after US/O3
treatment was obtained using SEM (Fig.10). The results indicate that the surface morphology of treated κ-carrageenan was relatively more rough and amorphous than the native κ-carrageenan. Fojas et al. [47] reported that the surface morphology of the κ-carrageenan after irradiation treatment was amorphous and rough. Shahbazi et al. [48] also reported that the thermal degradation of κ-carrageenan made a rougher and more porous surface. The morphological structure of κ-carrageenan becomes an amorphous and irregular shape. This is due to the cleavage of the glycosidic linkage of κ-carrageenan during the depolymerization process.
(A)
(B)
Fig 11. SEM Analysis: (A) native κ-carrageenan, (B) treated κ-carrageenan using US/O3
Conclusions
κ-carrageenan was efficiently depolymerized by ozone in the presence of ultrasonic radiation. This research demonstrated that a combination of US/O3 treatment simultaneously resulted in a synergetic effect, the synergistic effects of ozone and ultrasound treatment will improve the performance of the κ-carrageenan depolymerization process. During US/O3 treatment, pH has significant effects on the depolymerization of κ-carrageenan but increasing of temperature no significant with reducing the
21 molecular weight of κ-carrageenan. There are no significant changes in sulfate content under depolymerized using US, O3, and US/O3 treatment. FT-IR analysis of native and degraded κ- carrageenan showed there was no significant change in the functional properties of κ-carrageenan.
XRD analysis showed that there was a decrease in the main peak for treated κ-carrageenan when compared to native κ-carrageenan. The morphological properties of the treated κ-carrageenan become amorphous and rough.
Acknowledgment
This work was supported by the PDUPT Research Grant, The Ministry of Reseach Technology and Higher Education of Indonesia with the research contract number: 257-67/UN7.P4.3/PP/2019. The authors also would like to acknowledge the technical assistance of Ms. Dwi Purwati for the laboratory analyses of samples.
References
1. V.L. Campo,D. F Kawano, D.B. da Silva Jr, I. Carvalho, Carrageenans: Biological properties, chemical modifications, and structural analysis–a review, Carbohydr. Polym., 77,2009, 167-180.
2. I. Wijesekara, R. Pangestuti, S.K. Kim, Biological activities and potential health benefits of sulfated polysaccharides derived from marine algae, Carbohydr. Polym., 84, 1, 2011, 14-21.
3. T. Yamada, A. Ogamo, T. Saito, H. Uchiyama, Y. Nakagawa, Preparation of O-acylated low- molecular-weight carrageenans with potent anti-hiv activity and low anticoagulant effect, Carbohydr. Polym., 41, 2000, 115-120.
4. Y.H. Chiu,Y.L. Chan, L.W. Tsai, T.L. Li, C.J. Wu, Prevention of human enterovirus 71 infection by kappa carrageenan, Antivar Res, 95, 2012, 128-134.
22 5. A.A. Kalitnik, A.O.B. Barabanova, V.P. Nagorskaya, A.V. Reunov, V.P. Glazunov, T.F.
Solov’eva, I.M. Yermak, Low molecular weight derivatives of different carrageenan types and their antiviral activity, J. Appl.Phycol., 25, 2013, 65-72.
6. W. Wang, P. Zhang,G.L. Yu, C.X. Li, C. Hao, X. Qi, L.J. Zhang, H.S. Guan, Preparation and anti-influenza A virus activity of κ-carrageenan oligosaccharide and its sulphatedderivatives, J.
Food Chem., 133, 2012, 880-888.
7. F.R.F. Silva, C.M.P.G. Dore, C.T. Marques, M.S. Nascimento, N.M.B. Benevides, H.A.O.
Rocha,S.F. Chavante, E.L. Leite, Anticoagulant activity, pawedema and pleurisy induced carrageenan: action of major types of commercial carrageenans,Carbohydr. Polym.,79, 2010, 26-33.
8. C.A. deAraújo, M.D. Noseda, T.R. Cipriani, A.G. Goncalves, M.E.R. Duarte, D.R.B. Ducatti, Selectivesulfation of carrageenans and the influence of sulfate regionchemistry on anticoagulant properties,Carbohydr. Polym.,91, 2013,483-491.
9. H. Yuan,J. Song, Preparation structural characterization and in vitro antitumor activity of kappa carrageenan oligosaccharide fraction from kappaphycusstriatum, J. Appl. Phyc., 17,2005, 7-13.
10. M. Haijin, J. Xiaolu, G. Huashi, A carrageenan derived oligosaccharide prepared by enzymatic degradation containing anti-tumor activity, J. Appl. Phyc., 15, 297-303.
11. E. Gomez-Ordonez, A. Jimenez-Escrig, P. Rupérez,Bioactivity of sulfated polysaccharides from the edible red seaweed, Mastocarpusstellatus Bio Carbohydr.and Diet. Fibre, 3, 2014, 29-40.
12. L.A.R. de Souza, C.M.P.G. Dore, A.J.G. Castro, T.C.G. de Azevedo, M.T.B. de Oliveira, M.F.V. Moura, N.M.B. Benevides, E.L. Leite, Galactans from the red seaweed Amansiamultifida and their effects on inflammation, angiogenesis, coagulation and cell viability, Biom. and Preventive Nutri., 2, 2012, 154-162.
23 13. V.M.F. Lai, C.Y. Lii,W.L. Hung, T.J. Lu, Kinetic compensation effect in depolymerisation of
food polysaccharides, F.Chem., 68, 2000, 319-325.
14. A. Karlsson and S.K. Singh, Acid hydrolysis of sulphated polysaccharides: desulphation and the effect on molecular mass,Carbohydr. Polym., 38, 1999, 7-15.
15. L.V. Abad, H. Kudo, S. Saiki, N. Nagasawa, M. Tamada, H. Fu, Y. Muroya, M. Lin, Y.
Katsumura, L.S. Relleve, C.T. Aranilla, A.M. DeLaRosa, Radiolysis studies of aqueous κ- carrageenan, Nuclear Instrum. and Methods. Phys. Res. B, 268, 2010, 1607-1612.
16. M.T. Taghizadeh, R. Abdollahi, Influence of different degradation techniques on the molecular weight distribution of κ-Carrageenan, Int. J. Biochem and Biophys.,3, 2015, 25-33.
17. R. Ratnawati, A. Prasetyaningrum, and D.H. Wardhani, Kinetics and thermodynamics of ultrasound-assisted depolymerization of κ-carrageenan, Bull. Chem. React. Eng. & Catalyst.,11, 2016,48-58.
18. Z.B. Guzel-Seydim, A.K. Greene,A.C. Seydim, Use of ozone in the food industry, LWT, Food Sci. and Tech., 37,4, 2010, 453-460.
19. S. Lemeune, J.M. Barbe, A. Trichet, R. Guilard, Degradation of cellulose models during an ozone treatment: ozonation of glucose and cellobiose with oxygen or nitrogen as carrier gas at different pH,Ozone : Science & Engineering, J. Int. Ozone Association, 22, 5, 2000, 447-460.
20. E. Cséfalvay, T. Nöthe, P. Mizsey, Modelling of wastewater ozonation-determination of reaction kinetic constants and effect of temperature, Period. Polym. Chem. Eng., 51, 2, 2007,13- 17.
21. A.L. Prajapat,P.B. Subhedar, P.R. Gogate, Ultrasound assisted enzymatic depolymerization of aqueous guar gum solution, Ultrason.Sonochem., 29, 2016, 84-92.
24 22. A.L. Prajapat, P.R. Gogate, Intensification of degradation of guar gum: Comparison of approaches based on ozone, ultraviolet and ultrasonic irradiations,Chem. Eng. And Process., 98, 2015,165–173.
23. W. Yue,P. Yao, Y. Wei, H. Mo, Synergetic effect of ozone and ultrasonic radiation on degradation of chitosan, Polym. Degrad. and Stab., 93, 2008, 1814–1821.
24. H.J. Vreeman, T.H.M. Soneren, and T.A.J. Payens, Physicochemical investigation of κ- carrageenan in the random state, Biopolym., 19, 1980, 1357-1354.
25. C. Rochas, M. Rinaudo, and S. Landry, Role of the molecular weight on the mechanical properties of kappa carrageenan gels, Carbohydr. Polym.,12, 1990, 255-266.
26. B. Klein, N.L. Vanier, K. Moomand, V.Z. Pinto, R. Colussi, E. da Rosa Zavareze, A.R.G. Dias, Ozone oxidation of cassava starch in aqueous solution at different pH, Food. Chem, 155, 2014, 167-173.
27. K.S. Dodgson,R.G. Price, A note on the determination of the ester sulphate content of sulphated polysaccharides, Biochem. J. , 84,1962,106-110.
28. Q. Xiang, Y.Y. Lee, P.O. Petterson, and R.W. Torget, Heterogeneous aspects of acidhydrolysis of α-cellulose, Appl. Biochem. and Biotech., 107, 2003, 505-514.
29. Y.S. Yoon,Method for producing biofuels via hydrolysis of seaweed extract using heterogeneous catalyst, US Patent WO 2010098585 A2,2010.
30. C. Chirat and D. Lachenal, Effect of hidroxyl radicals on cellulose and pulp and their occurrence during ozone bleaching, Holzforschung ,51, 1997, 147-154.
31. P. Lenihan, A. Orozco, E. O’Neill, M.N.M. Ahmad, D.W. Rooney, and G.M. Walker, Dilute acid hydrolysis of lignocellulosic biomass, Chem. Eng. J., 156, 2010,395-403.
32. S. Kumar, P. Dheeran, S.P. Singh, I.M. Mishra, and D.K. Adhikari, Kinetic studies of two stage sulphuric acid hydrolysis of sugarcane bagasse, Renew. Energy, 83, 2015, 850-858.
25 33. D.E. Myslabodski, D. Stancioffh, and R.A.Heckertt, Effect of acid hydrolysis on the
molecularweight of kappa carrageenan by GPC-IS, Carbohydr. Polym., 31, 1-2 1996, 83-92.
34. R. Simoesand J. Castro, Ozone depolymerization of polysaccharides in different materials, J.
Pulp and Paper Sci., 27(3), 2001.
35. L. Relleve, N. Nagasawa, L.Q. Luan, T. Yagi, C. Aranilla, L. Abad, T. Kume, F. Yoshii, A.
Rosa dela,Degradation of carrageenan by radiation, Polym. Degrad. and Stab., 87, 2005.
36. Y. Sun, B. Yang, Y. Wu, Y. Liu, X. Gu, H. Zhang, C. Wang, H. Cao, L. Huang, Z. Wang, Structural characterization and antioxidant activities of κ-carrageenan oligosaccharides degraded by different methods, Food. Chem., 178, 2015, 311-318.
37. A. Prasetyaningrum, B. Jos, R. Ratnawati,Effect of ozonation process on physicochemical and rheological properties of κ-carrageenan, Sci. Study & Res. Chem. & Chem. Eng., Biotech., Food Indusrty., 18, 1, 2017, 009-018.
38. L.V. Abad, L.S. Relleve, T. Racadio,C. Darwin, Aranilla, T. Charito, De la Rosa, M.
Alumanda,Antioxidant activity potential of gamma irradiated carrageenan, Appl. Rad. and Isotop.,79, 2013, 73-79.
39. L. Relleve, L. Abad,Characterization and antioxidant properties ofalcoholic extracts from gamma irradiated κ-carrageenan, Rad. Physic. and Chem., 112, 2015, 40-48.
40. L.Q. Luan, N.Q. Hien, N. Nagasawa, T. Kume, F. Yoshii, T.M.Nakanishi, Biological effect of radiation-degraded alginate on flower plants in tissue culture, Biotech. Appl. Biochem, 38, 2003, 283-288.
41. L. Relleve, N. Nagasawa, L.Q. Luan, T. Yagi, C. Aranilla, L. Abad, T. Kume, F. Yoshii, A.
dela Rosa, Degradation of Carrageenan by Radiation, Polym. Degrad. and Stab., 87, 2005, 403- 410.
26 42. L. Relleve, A. Delarosa, L. Abad, C. Aranilla, and Aliganga, A. Kathrina, Biological Activaties
of Radiation Degraded Carrageenan, Jaeri Conf., 005, 2001, 44-62.
43. J. Li, S. Li, Y. Zheng, H.Zhang, J. Chen, L. Yan, T. Ding, R.J. Linhardt, C. Orfila, D. Liu, X.
Ye, S. Chen, Fast Preparation of Rhamnogalacturonan I Enriched Low Molecular Weight Pectic Polysaccharide by Ultrasonically Accelerated Metal-Free Fenton Reaction, F. Hydroc., 2018, 001-047.
44. R.F. Elsupikhe, K. Shameli, M.B. Ahmad, N.A. Ibrahim, N. Zainudin, Green Sonochemical Synthesis of Silver Nanoparticles at Varying Concentration of κ-carrageenan, Nanoscale Res.
Lett., 10, 302, 2015.
45. I. Prasetsung, S. Damrongsakkul, N. Saito, Degradation of -chitosan by Solution Plasma Process (SPP), Polym. Degra. and Stab., 98, 2013, 2089-2093.
46. N. Rokhati, H. Susanto, K. Haryani, B. Pramudono, Enhanced Enzymatic Hydrolysis of Chitosan by Surfactant Addition, Period. Polym. Chem. Eng., 10, 2016, 001-007.
47. J.J.R. Fojas, R.L. De Leon, L.V. Abad, Effect of Irradiation to Morphological. Physicochemical and Biocompatibility Properties of Carrageenan, Inter. J. Biotech. and Bioeng., 7, 5, 2013, 320- 323.
48. M. Shahbazi, G. Rajabzadeh,R. Ettelaie, and A. Rafe, Kinetic Study Of Rmkappa-Carrageenan Degradation And Its Impact On Mechanical And Structural Properties Of Chitosan/Rmkappa- Carrageenan Film. Carbohydr. Polym., 2016, 001-052.
27
Manuscript 19-213 review 1: 16 Desember 2019
from: Bogdana
Koumanova <[email protected]>
to: Aji Prasetyaningrum
date: Dec 16, 2019, 8:32 PM subject: manuscript 19-213 review
mailed- by:
uctm.edu
signed- by:
uctm-edu.20150623.gappssmtp.com
security: Standard encryption (TLS) Learn more : Important according to Google magic.
Dear author,
please find attached the review for your manuscript 19-213.
It is accepted for publication.
Kind regards JCTM
28 JOURNAL OF CHEMICAL TECHNOLOGY AND METALLURGY
REVIEW JCTM 19-213 Type of manuscript:
o Review X Full paper
o Short communication
No. Queries Yes No See
comments 1. Does the title of the manuscript comply
with the fields of research covered by the Journal?
X
2. Are the data interpreted correctly? X
3. Are the studies presented in the manuscript new and original?
X 4. Does the title correspond to the text? X
5. Are the requirements for length, chronology and presentation of the results fulfilled?
X
6. Would you recommend additions or changes (words, phrases), or statements that could improve the quality of the paper, so that it might be interesting for readers from other countries as well?
X
7. Do you recommend general shortening of the paper or deleting of particular paragraphs?
X
29 8. Is the quality of the English language
up to the Journal standards?
X
9. Are all figures and tables needed and does their quality meet the
requirements of the Journal?
X
10. Are the cited references sufficient and are they presented according to the requirements of The Journal?
X
11. Do the abstract and keywords present adequately the paper?
X
12. Would you recommend publication of the paper?
а. as presented
b. with minor corrections c. with major corrections after which the
manuscript should be reviewed again
d. not suitable for publication X
This manuscript describes the depolymerization of κ-carrageenan by ozone in the presence of ultrasonic radiation. This research demonstrate that a combination of ultrasonic radiation and ozone treatment lead to a synergetic effect. The results of this research are proved by using the appropriate analytical methods and techniques: X–ray diffraction (XRD), Fourrier transform infrared (FTIR), and scanning electron microscope (SEM).
The manuscript provides a good amount of results and it is relevant to the JCTM.
This article is well written, and arranged according to the requirements of the journal. I recommend this manuscript for publication in Journal of Chemical Technology and Metallurgy.
30
Manuscript 19-213 review 2: 30 Juni 2020
from: Bogdana
Koumanova <[email protected]>
to: Aji Prasetyaningrum
date: Jun 30, 2020, 9:46 PM subject: manuscript 19-213
mailed- by:
uctm.edu
signed- by:
uctm-edu.20150623.gappssmtp.com
security: Standard encryption (TLS) Learn more : Important according to Google magic.
Dear author,
Your manuscript has passed technical and English corrections (made in red colour). Please answer as soon as possible if you agree with them.
Kind regards JCTM
31
from: Aji
Prasetyaningrum <[email protected]>
to: aji prasetyaningrum <[email protected]>
date: Jul 4, 2020, 1:02 PM subject: Fwd: manuscript 19-213
mailed- by:
che.undip.ac.id
Dear Editor in Chief JCTM
Thank you for the information that my manuscript has passed technical and English corrections.
I agree with the corrections.
Best regards
Dr. Aji Prasetyaningrum, S.T., M.Si.
Chemical Engineering Departement Diponegoro University
32
REVISED VERSION_2
A COMBINED ULTRASOUND AND OZONE (US/O3) TREATMENT ENHANCING k-CARRAGEENAN DEPOLYMERIZATION
Aji Prasetyaningrum1, Bakti Jos1, Ratnawati Ratnawati1, Yudhy Dharmawan2, Andri C. Kumoro1
1Department of Chemical Engineering, Faculty of Engineering, Diponegoro University Jl. Prof. Soedarto S.H., Tembalang, Semarang 50269, Indonesia
E-mail: [email protected]
2Faculty of Public Health, Diponegoro University, Jl. Prof. Soedarto S.H., Tembalang, Semarang 50269, Indonesia
27 September 2019
15 December 2019
ABSTRACT
κ
-Carrageenan is a sulfated galactan derived from red algae. The low molecular weight fraction (LMWF) of κ-carrageenan exhibits many benefits in the pharmaceutical and biomedical fields.This study investigates the effects of individual and combined ultrasound and ozone (US/O3) treatments on the depolymerization of κ-carrageenan. The influence of a combination of US/O3
on the depolymerization of κ-carrageenan is indicated by the molecular weight decrease. The sulfate content is characterized by the barium chloride-gelatin method. The functional group analysis of the native and the treated κ-carrageenan is determined by FT-IR. The structural and
33 morphological properties of the depolymerized κ-carrageenan are determined by SEM and XRD. The experimental results show that the molecular weight of κ-carrageenan decreases appreciably as a result of the combined US/O3 treatment. The degree of depolymerization of κ- carrageenan within 20 min of treatment at pH 7 is equal to 18.36 % in case of US application, it amounts to 51.12 % in case of O3 treatment, but it reaches a value of 88.36 % under the joint effect of US and O3. The depolymerization of κ-carrageenan increases with a pH decrease, while the temperature increase has no significant effect. The sulfate content of κ-carrageenan tends to stay stable during the US/O3 treatment. The FT-IR analysis shows no significant change of the functional properties of the treated substance. The X-ray diffraction patterns of the native and that treated by US/O3 have a similar pattern of main peaks at 43.88o, 64.23oand 77.46o. The SEM analysis of κ-carrageenan morphology upon US/O3 treatment indicates an amorphous and rough surface
Keywords: ultrasound, ozone, depolymerization, κ-carrageenan.
34 INTRODUCTION
Carrageenan is a natural polysaccharide sulfated galactan composed of alternating 3-linked β- D-galactopyranose and 4-linked α-D-galactopyranose or 4-linked 3,6-anhydro-α-D- galactopyranose [1]. Carrageenans are classified according to the number and position of the sulfate groups. The commercial κ-carrageenan or the high molecular weight fractions (HMWF) of κ-carrageenan have an average molecular weight of 100 kDa - 1000 kDa [1].
The depolymerization process may change HMWF of κ-carrageenan to low molecular weight fractions (LMWF). The latter have biological activities, such as antiviral [2 - 6], anticoagulant [7, 8], antitumor [9, 10] and antioxidant [11, 12] one. The LMWF of κ- carrageenan can penetrate the human cells more effectively when compared with HMWF [2].
The LMWF of carrageenan of a molecular weight size of less than 20 kDa are used for biomedical applications [12]. The changes of the molecular structure of κ-carrageenan from HMWF to LMWF is presented in Fig. 1.
HMWF of κ-carrageenan LMWF of κ-carrageenan Fig. 1. Depolymerization of κ-carrageenan.
Thermal depolymerization [13], acid-catalyzed hydrolysis [9, 14], enzymatic hydrolysis [10], irradiation [12, 15], and sonication [16, 17] are widely used as depolymerization techniques.
The acidic hydrolysis is a common fast method which leads to an increase of the environmental pollution level [9, 14]. The enzymatic method is not preferable because of the relatively expensive and complex process used [10].
35 Due to its high oxidation potential, ozone can be alternatively used to degrade organic and inorganic compounds [18, 19]. It is well known as a powerful oxidizing agent [18] removing pollutants from the water bodies [20] and depolymerizing polysaccharides. Ozone is generally recognized as safe (GRAS) for use in industrial food preparation by the United States of Food and Drug Administration. However, the application of ozonation has been limited by the low ozone mass transfer rate. The latter may be increased by an ultrasonication treatment [20], which in correspondence with the green technology requirements because no chemicals addition is required. The acoustic cavitations produce [21] a lot of hot spots of high temperatures and pressures causing H2O sonolysis. Thus, radical species are formed, which induce the degradation of the organic compound present in the aqueous solution. The polymer scission by the ultrasonic method is unique because the depolymerization proceeds non-randomly at the mid-point of the chain. The radical generation during ultrasonication (US) initiates the depolymerization of polysaccharides especially in case of water-soluble polymers [17, 21].
The combination of US/O3 has been reported to exhibit several advantages compared to the individual US or O3 processes. It is selected to enhance the organic compounds oxidation.
The degradation of guar gum (GG) is significantly increased by about 99.1% in case of US/O3 application [22]. Yue et al. [23] report that the combined US/O3 treatmentresults in a significant decrease of chitosan molecular weight. US and O3 treatment procedures have not been so far applied to the depolymerization of κ-carrageenan. Therefore, this study aims to investigate their individual and combined effects in this case. The physicochemical and morphological properties of the native and the treated κ-carrageenan are analyzed using the barium chloride - gelatin method, FT-IR, SEM and XRD to follow the performance of the processes investigated.
36 EXPERIMENTAL
Materials
The commercial κ-carrageenan used in this study was provided by CV. Karagen, Indonesia.
The carrageenan powder was dissolved in distilled water. A complete dissolution was achieved by heating at 70°C combined with slow stirring. The polysaccharides in the solution were precipitated by a dropwise addition of isopropyl alcohol (E. Merck, Catalog No.
818766) under vigorous stirring. The precipitate of refined κ-carrageenan was collected and dried at 60oC in a forced-air oven. 37 % hydrochloric acid solution (E. Merck, Catalog No.
100314) and sodium hydroxide (E. Merck, Catalog No. 137020) were used to adjust the initial pH value. All the chemicals were used without any pretreatment.
An experimental Set-up
The depolymerization treatment of κ-carrageenan was performed in a transparent glass reactor of a capacity of 500 mL. The refined κ-carrageenan solution (1:100, w/v) was prepared by dissolving it in distilled water at 70 0C. The experiments were carried out by different methods: by US only, by O3 only and by a combination of US/O3. The sonication process used an ultrasonic generator of 150W and 40 kHz. Different dissolved ozone concentrations (15 mg min-1L-1, 25 mg min-1L-1, 35 mg.min-1L-1) were used during the ozonation process. The ozone was produced by an ozone generator (Dipo Technology Indonesia) and bubbled in the solution through a bubble diffuser. The inlet ozone concentration was determined by the indigo colorimetric method. This was done at different times (0 min, 5 min, 10 min, 15 min and 20 min). The initial pH value was adjusted by the addition of HCl/NaOH, while the temperature of the process was maintained by a water bath.
The schematic presentation of the equipment used is shown in Fig. 2.
37 Fig. 2. A schematic presentation of the experimental equipment used for US/O3 treatment.
Analytical Procedures
A Molecular Weight Determination
At regular intervals samples were withdrawn, cooled to 25oC ± 1oC in chilled water to quench the reaction and the intrinsic viscosity was analyzed using a glass capillary Ubbelohde viscometer. The molecular weight of κ-carrageenan was determined according to the Mark- Houwink equation. Lai et al. [13] advanced an equation relating the intrinsic viscosity to the molecular weight as follows:
[η] = kMH.Mα (1)
where [η] was the intrinsic viscosity, kMH and α were constants, while M was the number- averaged molecular weight. The values of kMH and α for the κ-carrageenan aqueous solution at 25oC referred to 0.00778 and 0.90, respectively [24, 25]. The intrinsic viscosity was obtained using the Huggins equation:
= [η] + kH [η]2 C (2)
where ηsp, C, and kH were the specific viscosity, the concentration of the solution, and the Huggins constant of 0.3 [24], correspondingly.
38 A Sulfate Analysis
The sulfate content of both the non-treated and the treated κ-carrageenan was determined using the barium chloride-gelatin method [26]. A known amount of the sample (W1, g) was hydrolyzed using 50 mL of 1 N HCl for 30 min by heating at the boiling temperature. Ten milliliters of 0.25 M BaCl2 solution were added to the reaction mixture, which was then cooled to a room temperature and kept for 5 h. The BaSO4 precipitate was filtered with ashless filter paper and incinerated in a muffle furnace at 700oC for 1 h. The ash was weighed (W2,g) and the sulfate content was calculated in accordance with the equation:
% Sulfate = (W2 / W1) × 41.16 (3)
FT-IR Spectroscopy
The Fourier transform infrared (FT-IR) spectra of the original and the depolymerized κ- carrageenan were recorded with KBr powder (10 mg κ-carrageenan powder in 90 mg KBr powder) using a Perkin Elmer IR 10.6.1 Spectrophotometer (USA) in the range from 400 cm-1 to 2000 cm-1.
X-ray Diffraction (XRD)
The X-ray diffractograms of the original and the treated κ-carrageenan were obtained with an X-ray diffractometer (XRD-7000, Shimadzu, Japan). The scanning region of the diffraction ranged from 10o to 80o with a target voltage of 30 kV, a current of 30 mA and a scan speed of 5o/min. The relative crystallinity (RC) of chitosan was calculated using the equation proposed by Klein et al. [27]:
(4)
where was the crystalline area, while was the amorphous area.
39 Scanning Electron Microscopy (SEM)
The SEM characterization was carried out using a Scanning electron microscope JSM-6510- LA JEOL series (Japan). Prior to the analysis, the original and the treated κ-carrageenan samples were sprinkled onto adhesive Al or C tapes and were supported on metallic discs coated with Au. The images of the sample surfaces were recorded at different magnifications.
RESULTS AND DISCUSSION
A Comparison of Ultrasonication and Ozonation in Respect to κ-Carrageenan
Depolymerization
The κ-carrageenan solution is treated by US only and O3 only at different ozone concentrations. The experiments are carried out at 29±1oC and an initial pH value adjusted at 7. The US and O3 treatments are applied at 0-th min, 5-th min, 10-th min, 15-th min and 20-th min. The correlation between the molecular weight of κ-carrageenan and the reaction time is illustrated in Fig 3.
Fig. 3. A dependence of κ-carrageenan
40 molecular weight on the reaction time.
The initial molecular weight of κ-carrageenan refers to 550 kDa in all treatment procedures.
The decrease of κ-carrageenan molecular weight by US treatment only is marked by the symbol (. ). The molecular weight decreases from 550 kDa to 449 kDa within 20 min of ultrasonication. The decrease in this case is found equal to 18.36 %. Aiming to compare the effect of the individual treatment approaches to κ-carrageenan depolymerization, three different concentrations of ozone are used: 15 mg min-1L-1 (marked by ∆), 25 mg min-1L-1 (marked by ○) and 35 mg min-1L-1 (marked by □). The results show that κ-carrageenan molecular weight decreases from 550 kDa to 317 kDa, 288 kDa and 268 kDa within 20 min of ozonation, respectively. Therefore, the decrease observed in this case refers to 42.36 %, 47.64 %, and 51.12 %, correspondingly. Thus, it can be concluded that the percentage decrease of the number-averaged molecular weight of κ-carrageenan in case of an ozone treatment is higher than that obtained by an ultrasonic treatment. Prajapat and Gogate [22]
find that the decrease of the intrinsic viscosity of guar gum by an ozone treatment (96.20 %) is only marginally higher when compared to that obtained by an ultrasonic radiation (95.59
%). The ozone treatment is more effective than the ultrasonic radiation in respect to chitosan degradation [23]. It is also reported that the chitosan viscosity is decreased by 12.74 % during an individual ultrasonic radiation. In case of an ozone treatment, it increases within 30 min from 31.18% to 41.38% for an ozone dosage ranging from 35±5 mg/min to 65±5 mg/min. In general, it can be said that the ozone treatment provides a much greater viscosity decrease than that in case of an individual ultrasound treatment.
The reactions proceeding in presence of ultrasonication refer to pyrolysis and chemical interactions with radicals produced from H2O. The hydroxyl radicals are generated by the sonication of H2O [23]. The corresponding equation is shown below:
41 (5) During the ozonation treatment alone, O3 is transferred from the gas phase into the liquid one.
It reacts immediately with the κ-carrageenan solution through direct and indirect interactions with the participation of radicals generated by ozone auto decomposition [18] as shown below:
(6) (7)
A Synergetic Effect of US and O3
The US/O3 joint application results in a synergetic effect, which leads to an increase of κ- carrageenan depolymerization. Three different ozone concentration (15 mg min-1L-1, 25 mg min-1L-1, and 35 mg min-1L-1) are used. The molecular weight of κ-carrageenan decreases within 20 min of a treatment from 550 kDa to 114 kDa, 84 kDa and 64 kDa, respectively (Fig. 4). The percentage decrease of κ-carrageenan molecular weight in this case refers to 79.27 %, 84.47 %, and 88.36 %, correspondingly. It is intriguing that the average molecular weight decrease observed in case of the joint O3/US treatment is much higher when compared to that found when US and ozone applications are considered. In addition, the viscosity-averaged molecular weight decrease is much greater under the effect of US/O3 than that obtained by summation of the degradation values reached by individual ultrasonic radiation and ozone treatments. The extent of carrageenan degradation obtained by the joint effect of US/O3 equals 88.36 %, while the value found by summation of those referring to the both processes amounts only to 69.48 % (51.12 % in case of an ozone treatment and 18.36 % obtained by an ultrasonication).