This is to confirm that the thesis entitled "A study on multiple contaminant-soil interaction and its effect on contaminant fate prediction" submitted by Mrs. Poly Buragohain to the Indian Institute of Technology Guwahati, for the award of the degree of Doctor of Philosophy in Civil Engineering is a record of bonafide research work done by her under my supervision and guidance. Sreedeep for his guidance, encouragement, patience and support throughout my Ph.D. Most importantly, my deep gratitude goes to the person without whom the last two years of my life would not have been so happy, my husband Keyur.
During the later period of my PhD, the help and camaraderie from the fraternity of the Design Department was wonderful.
Abstract
Lists of Figures
List of Tables
Introduction
- Motivation for this study
Increased awareness of the harmful effects of pollution on both human life and the environment (Zhang et al., 2004; Du et al., 2006) has resulted in intensive research efforts to study the most significant geochemical process (retention and release) that affects the fate, transport and risk of disposed pollutants in the surface as well as in the subsurface (Kent et al., 2000; Davis et al., 2004a; Kohler et al., 2004). This knowledge is mandatory to design effective waste containment facilities for municipal and industrial wastes that may be hazardous or non-hazardous (Rowe and Booker 1985; Yong et al., 2001; Chang et al., 2005; Zhang et al., 2005). and also to decide appropriate remediation scheme for an already contaminated area and groundwater (Liu et al., 2004). Retention/release characterization of a soil pollutant is performed using laboratory tests or field experiments and quantified using mathematical models known as isotherms (Elkhatib et al., 1990; Hooda and Alloway 1998;.
It is noted that batch testing is one of the established methods for soil contaminant retention characterization (ASTM D 4606; Taha et al., 2002).
Literature Review
- Basic concept
- Soil Retention Studies
- Affinity trends according to properties of metal
- Properties related to electrostatic interactions (physical retention and/or ionic bonding)
- Properties related to covalent bonding, complexation and redox
- Other trends observed in experimental results Metal ion-
- Critical Appraisal
- Scope of the work
- General
- Soil retention theory
- Factors influencing retention of the soil
- Retention isotherm of soil
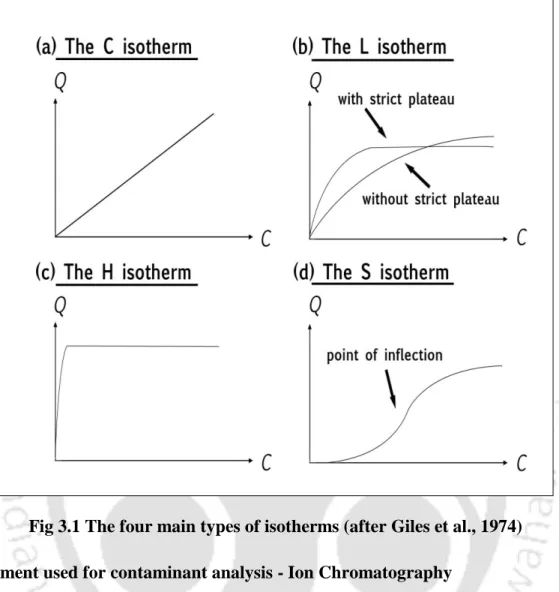
- Shapes of Isotherm
- Advection-dispersion equation (ADE) and role of isotherms
- Retardation factor for different isotherms .1 Linear isotherm
- Description on modelling and boundary conditions of the soil column
Both phenomena are well described by the Freundlich equation. The effects of organic matter, clay and pH content on the partition coefficient Kd between the soil and water were also investigated and it was observed that with the decreasing pH and increasing clay and organic matter content of the soil, the Kd value. Li et al., 2003 studied the retention and release of pesticides (4, 6-dinitro-o-cresol and dichlobenil) on clay minerals and humic acid complexes using batch equilibrations and X-ray diffraction method (XRD). Li et al., 2008 studied the retention and release behavior of bisphenol A (BPA) in sols, which affects ionic strength (Ca+2, NH4+), heavy metals (Cd+2, Pb+2) and surfactants (cetyltrimethylammonium bromide (CTAB)), cetylpyridine chloride (CPC).
Li et al., 2008 studied the retention and release behavior of bisphenol A (BPA) in soil, affecting ionic strength (Ca+2, NH4+), heavy metals (Cd, Pb) and surfactants (cetyltrimethylammonium bromide (CTAB ), cetylpyridinium chloride (CPC)).
Experimental Investigations and Methodology
- X-ray diffraction (XRD) analysis
- Chemical characterization of soil
- Scanning electron microscope (SEM) and Energy dispersive X-ray spectroscopy (EDX)
- Soil pH
- Physical characterizations of soil .1 Specific gravity
- Gradational characterization
- Atterberg limits
- Soil classification
- Proctor compaction characteristics
- Batch method (ASTM D 4646-03)
- Preparation of single and multiple contaminant solution
This method involves saturating the cation exchange sites on the soil surface with ammonium and removing the excess ammonium with ethanol. Soil pH is a useful variable in determining the solubility of soil minerals and the mobility of ions in soil and in assessing the viability of the interaction between soil and pollutants. The temperature of the sample is measured and adjusted to the sample temperature with the temperature controller of the pH meter.
The results of the percentages of the size fractions of the soil, obtained from the grain size curve, are listed in Table 4.4. The consistency limits of the soil samples were determined as per the guidelines of ASTM D 4318 and IS: 2720 (Part V) for liquid limit (LL) and plastic limit (PL). Based on the physical characteristics, the classification of soil was carried out by following the Uniform Soil Classification System (USCS) (ASTM D 422).
Initial weight of the suspension along with the glass dish was measured using the precision balance and the dish was replaced in the desiccator for evacuation under vacuum. The glass dish was taken out of the drying machine, weighed and put back into it several times until it reached a constant weight. The compaction properties of the soil samples used in the study were determined using standard Proctor compaction procedure (IS: 2720: part 7; ASTM D 698).
The compaction characteristics of the soils are expressed in the form of the relationship between the dry unit weight, γd, and the moisture content, w, as shown in Figure 4.27. The salient features such as maximum dry unit weight, γdmax, optimum moisture content (OMC) of the soil are shown in Table 4.5.
Experimental evaluation of soil-single contaminant interaction
- General
- Batch results for Na + ion from NaCl solution
- Batch results for K + ion from KCl solution
- Influence of range of concentration on retention characteristics
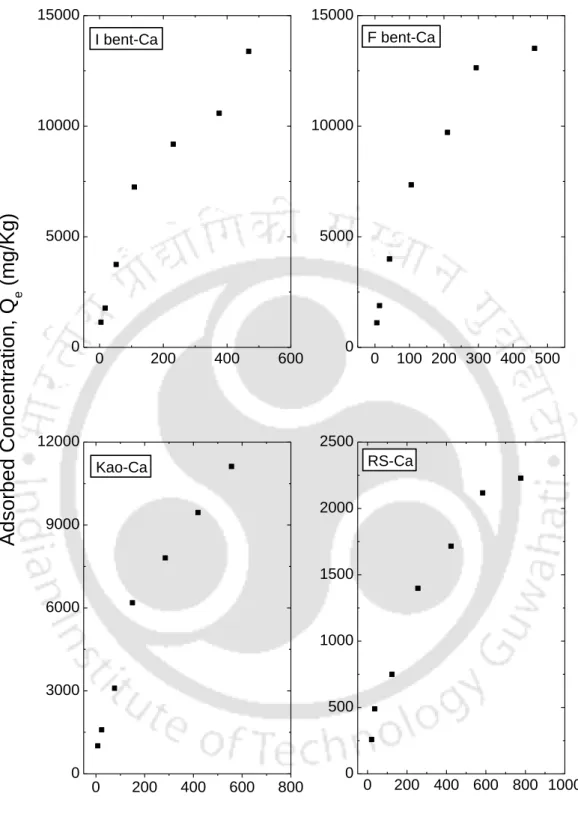
- Batch results for Ca +2 ion from CaCl 2 solution
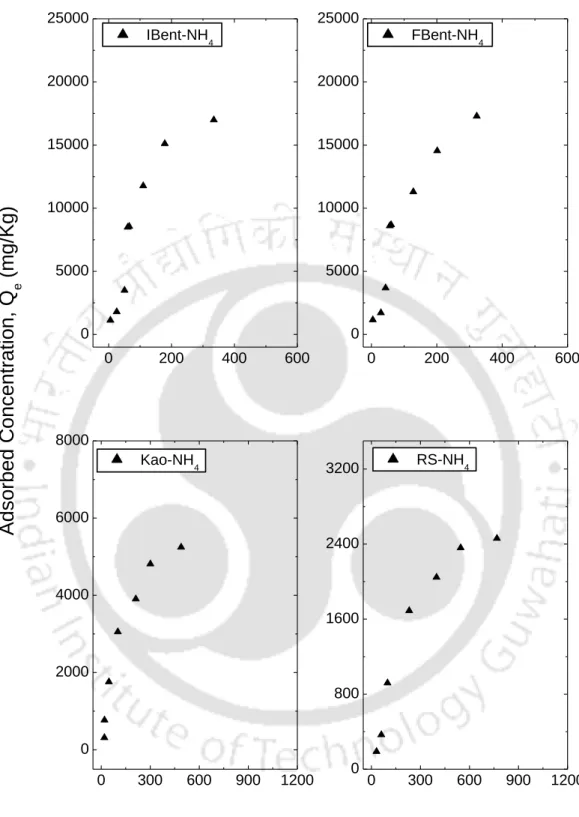
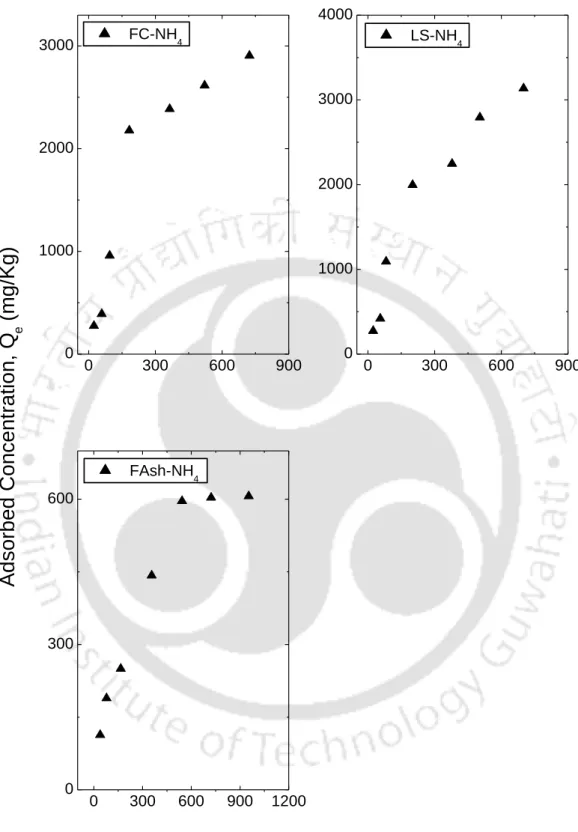
- Batch results for ammonium (NH 4 + ) ion from NH 4 Cl solution
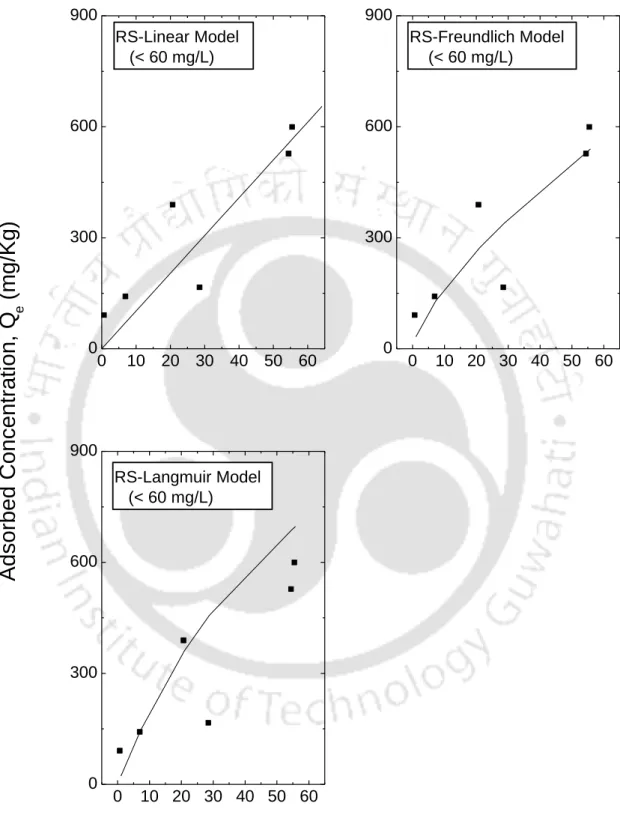
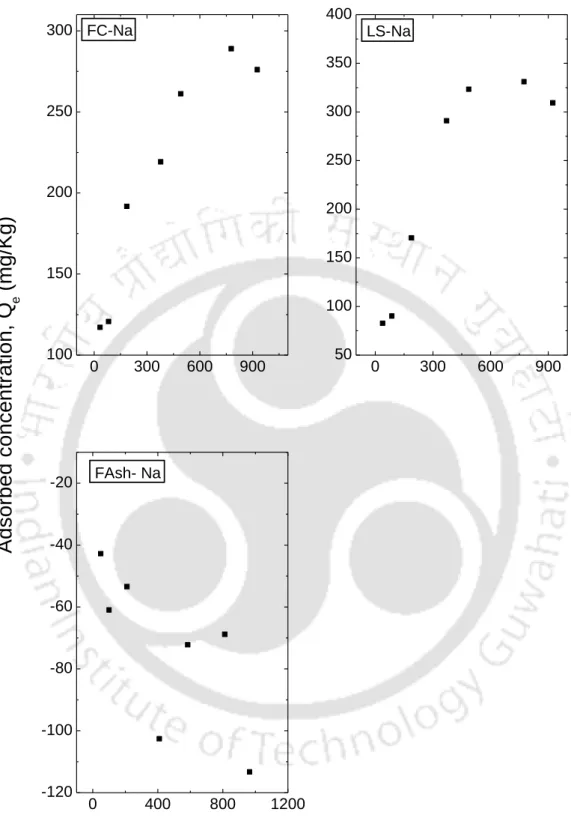
In this study, smectite-rich soil such as IBent and FBent showed high K+ retention. In this case, K+ has a smaller hydrate radius than Na+ (the hydrate radius of K+ is 0.330 nanometers and 0.360 nanometers for Na+). In Figure 5.6, it can be seen that the dispersion of the data is greater for the area Ce<.
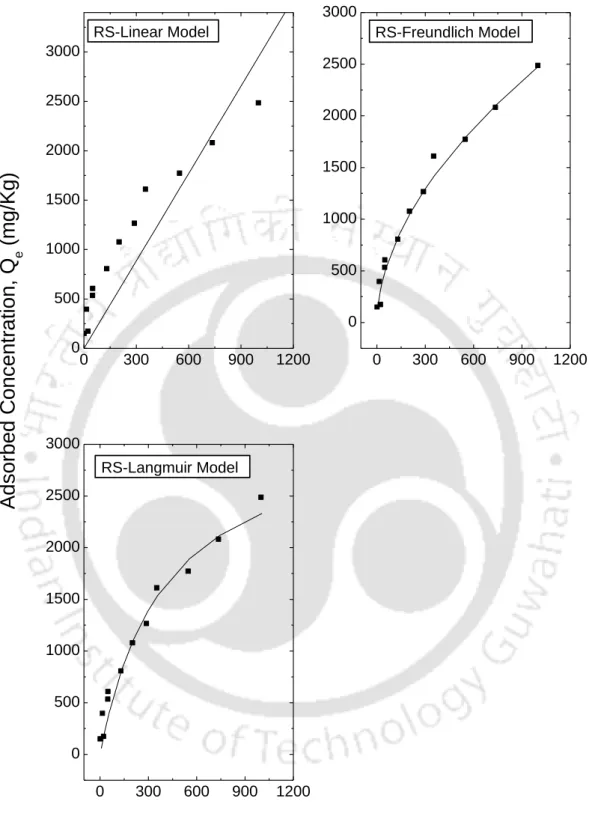
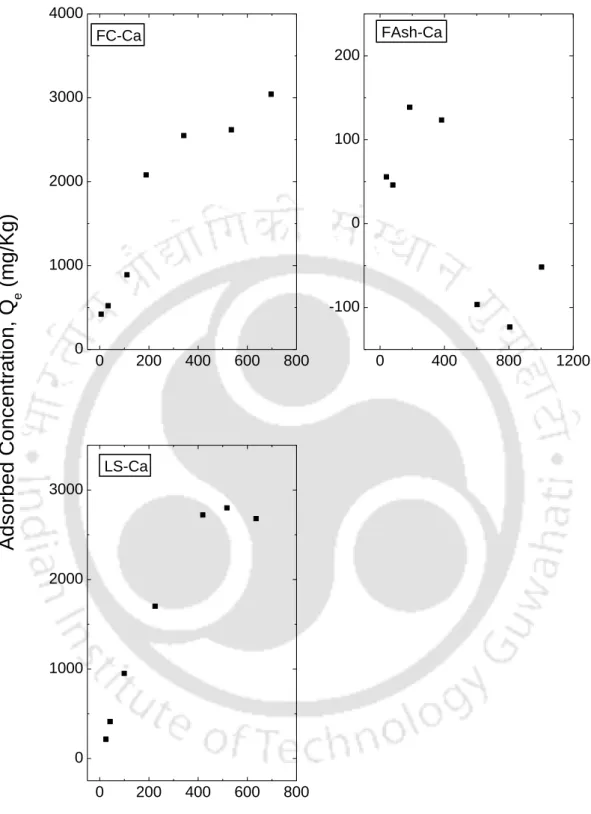
This observation is consistent with observations reported in the literature that linear isotherms are suitable for low ranges of The observation clearly shows that the range of Ce affects the fit of the isotherm and therefore the batch test should be performed for the predicted concentration range in the field. The resulting fitted curves are classified according to the Giles model (Sposito, 1989) as an L-type for I Bent, F Bent and Kao that showed an initial curvature, implying that the soil sites are rapidly filled by the added Ca+2. , and subsequent growth of the solute on the soil surface becomes difficult.
The S-type isotherms are followed by RS and FC and LS, where the initial S-type curvature shows a gradual retention in the initial phase with the addition of Ca+2 ions in the solution. Studies conducted by Wang et al. (2011), state that retention of the NH4+ ion below pH 6 is relatively less due to the competition of hydrogen ions present in solution for exchange sites. From the experimental data, it can be seen that an increase in the initial concentration resulted in an increase in the amount of NH4+ retained by almost all soils.
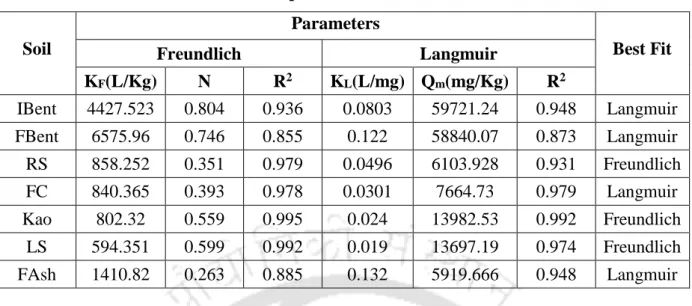
The results also show that an increase in the initial NH4+ concentration led to a reduction in the retention at higher concentration, showing that the process is highly dependent on the initial concentration, this could lead to a possible saturation of the monolayer coverage on the surface of almost all the soils by the NH4+ ions. It can be observed from table 5.5 that although both the isotherms (Langmuir and Freundlich) fitted the experimental data well, there were some discrepancies in the order of NH4+ retention of soils based on Freundlich KF.
- Batch results for divalent lead (Pb +2 ) ion from Pb(NO 3 ) 2 solution
- Batch results for divalent Ni +2 ion from (Ni(NO 3 ) 2 .6H 2 O) solution
- Batch results for divalent Zn +2 ion from Zn(NO 3 ) 2 solution
- Summary
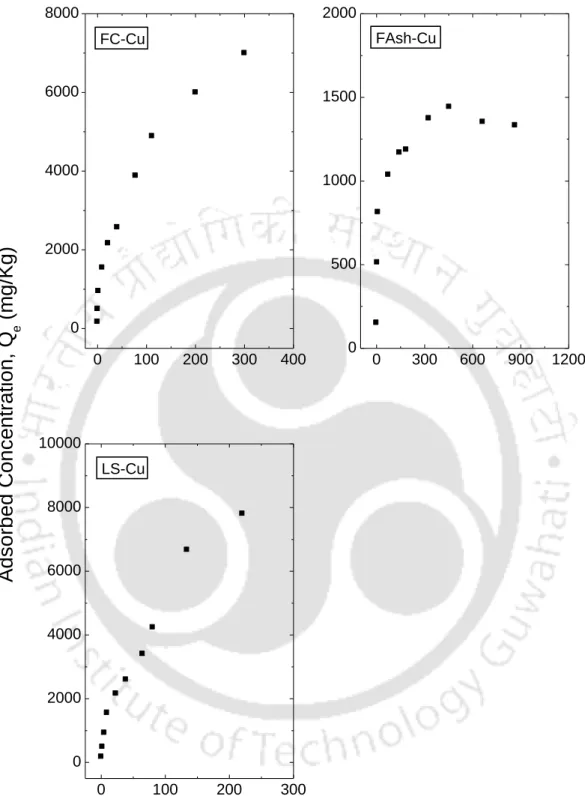
This is consistent with the findings of McLaren et al., (1981) who stated that at low concentrations metal retention is essentially linear. Based on the classification of the isotherms described by Giles et al., (1960) it can be noted that for the retention data Pb+2 followed the H and L type curve, which implies a strong affinity between the metal cations and the surface of of the sorbent for the entire concentration range and the lower range respectively. As the metal concentration increased, more sites were filled and Cu+2 retention was reduced (Petruzzelli et al., 1985).
Similar results were obtained by Jordão et al., (2000), who demonstrated the occurrence of two phases in the retention curve. Thus, each linear part of the retention curve suggests different types of retention sites responsible for the retention of Cu+2 in the soil. Harter (1983) and Beukes et al., (2000) reported that metal retention was pH dependent and that the intensity of the phenomenon increased dramatically above pH 7.0.
Based on the classification of isotherms described by Giles et al. (1960), it was observed that the Ni+2 retention data followed an S-type for LS, FC and FAsh, characterized by a small initial slope that remained constant and was not related to the equilibrium concentration (Sposito, 1989). According to Gomes et al. (2001), soils with higher pH values had the greatest relative capacity to absorb metal ions. The findings obtained in this study confirm that soil retention capacity cannot be controlled by its pH value alone.
Indeed, it was observed by Fotovat et al., (1996) that the solubility of Zn+2 in soil is directly related to pH, independent of total Zn+2 concentration. According to Giles et al., 1960 is thought to reflect the type of retention mechanism that is occurring.
Experimental evaluation of soil -multiple contaminant interaction
- General
- Batch tests results for common ions
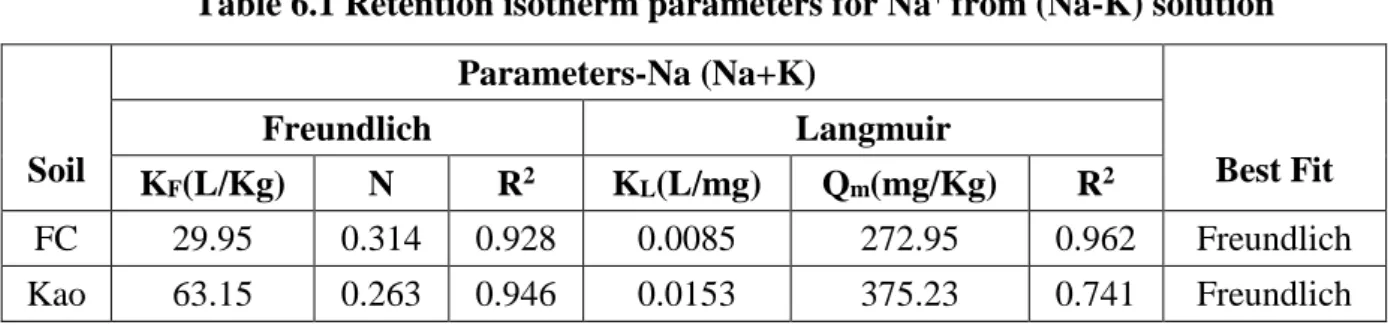
- Batch test results for Na + and K + in binary (Na +K) solution
- Investigation on the range of concentration
- Comparison of Na + and K + corresponding to single and binary (Na+K) solution An effort was made to compare the retention results of Na + and K + corresponding to
- Batch test results for NH 4 + and K + in binary (NH 4 +K) solution
- Batch test results for Na + , K + and Ca +2 in tertiary (Na+K+Ca) chloride solution Solutions of different concentrations were prepared by mixing equivalent amount of
- Comparison of K + and Ca +2 corresponding to single, binary, and tertiary (Na+K+Ca) solution
- Batch test results for heavy metals
- Batch test results for Ni +2 and Cu +2 in binary (Ni+Cu) solution
- Batch test results for Cu +2 and Pb +2 in binary (Pb+Cu) solution
- Batch tests results for Cu +2 and Zn +2 in binary (Cu+Zn) solution
- Batch tests results for Cu +2 , Pb +2 and Ni +2 in tertiary (Pb+Cu+Ni) solution
- Batch tests results for Cu +2 in quaternary (Cu+Ni+Pb+Zn) solution
- Comparison of Cu +2 retention in single, binary, tertiary and quaternary (Cu+Ni+Pb+Zn) solution
Fig.6.4a Comparison of retention characteristics of Na+ on 4 soils corresponding to single and binary (Na+K) solution. Fig.6.4b Comparison of retention characteristics of Na+ on 3 soils corresponding to single and binary (Na+K) solution. Fig.6.5a Comparison of retention characteristics for K+ on 4 soils corresponding to single and binary (Na+K) solution.
Figure 6.5b Comparison of K+ retention properties of 3 soils corresponding to single and binary (Na+K) solutions. Figure 6.7a Comparison of K+ retention properties of 4 soils corresponding to single and binary (NH4+K) solutions. Figure 6.7b Comparison of K+ retention properties of 3 soils corresponding to single and binary (NH4+K) solutions.
Fig.6.11b Comparison of retention characteristics for Ca+2 on 3 soils corresponding to simple and tertiary (Na+K+Ca) solution. Fig.6.12b Comparison of retention characteristics of Cu+2 and Ni+2 on 3 soils from binary (Cu+Ni) solution. Fig.6.13a Comparison of retention characteristics for Ni+2 on 4 soils corresponding to single and binary (Cu+Ni) solution.
Fig.6.13b Comparison of Ni+2 retention characteristics in 3 soils corresponding to single and binary solutions (Cu+Ni). Fig.6.14a Comparison of Cu+2 retention characteristics in 4 soils corresponding to single and binary solutions (Cu+Ni). Fig.6.19a Comparison of Cu+2 retention characteristics in 4 soils corresponding to single and binary solutions (Cu+Zn).
Figure 6.19b Comparison of Cu+2 retention properties on 3 soils corresponding to single and binary (Cu+Zn) solutions.