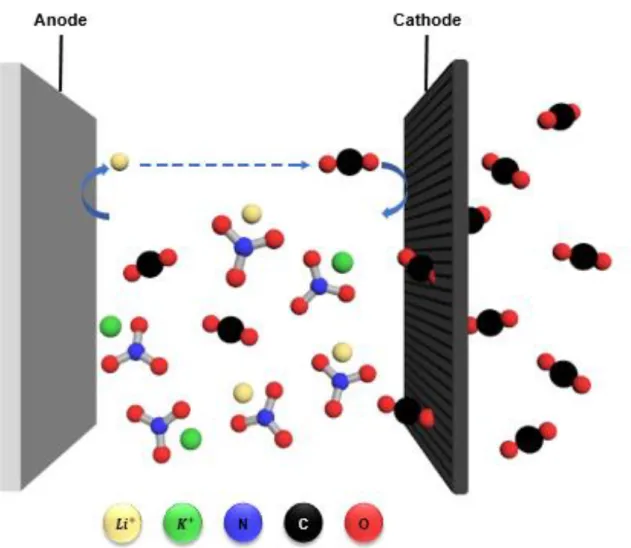
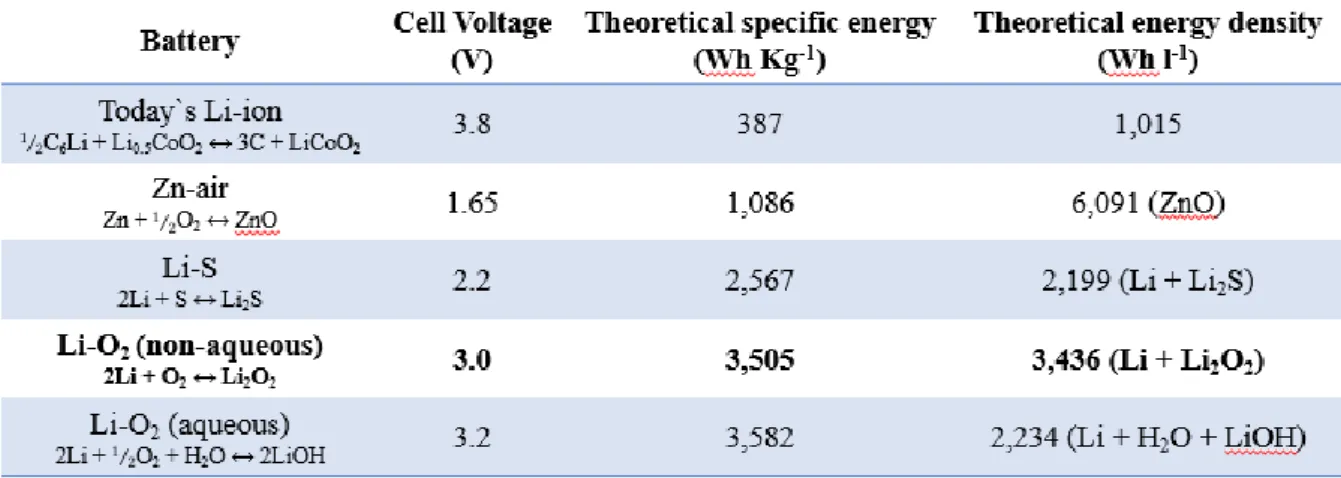
Aprotic electrolyte-based lithium-oxygen batteries are of great interest due to its ultrahigh theoretical specific energy density (1675 mAh per gram of oxygen) compared to the current lithium-ion battery. Despite the attractiveness of its high theoretical capacity, there are a number of disadvantages such as instability of electrochemical reaction of electrode and electrolytes. The molten electrolyte, which has a eutectic point of 65℃, has the advantages of high stability and high-temperature operation, thereby preventing harmful solvent by-products in lithium-oxygen batteries.
Our results showed that Li2O2, the discharge product, formed a stable hexagonal morphology in the lithium-oxygen battery after the discharge process by line electron microscopy and X-ray diffraction techniques. It also leads to improved oxygen mobility at high temperature, since molten salt was used as the electrolyte in lithium-oxygen batteries. Furthermore, we found that the kinetics improve with increasing operating temperature in molten salt electrolyte cells.
Gas evolution profile during the charging process of the Li-CO2 cell with limited capacity of 0.25 mAh obtained using in situ DEMS analysis using organic electrolyte (c) and inorganic electrolyte (d).
Organic-free electrolyte for stable lithium-oxygen batteries
- Introduction of rechargeable lithium-oxygen batteries
- Challenges of lithium-oxygen batteries
- Organic-free electrolyte for high oxygen efficiency
- A molten salt electrolyte
- Experimental
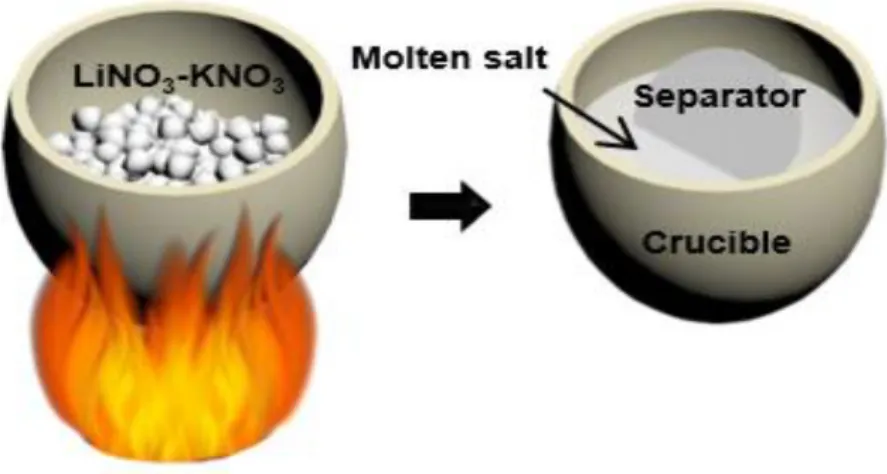
- Preparation of a molten salt electrolyte and electrode
- Assembly of the lithium-oxygen cell
- Designing cell kit for high-temperature operation
- Oxygen efficiency measurement using differential electrochemical mass spectrometry
- Result and Discussion
- Conclusion
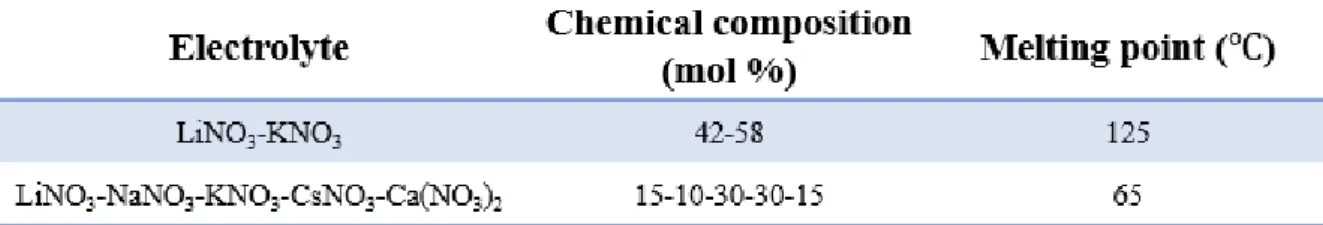
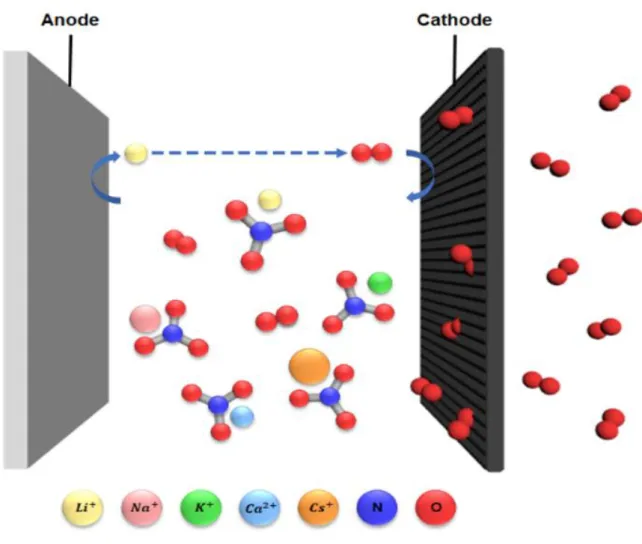
In particular, Li2O2 is produced during the consumption of lithium ions and oxygen during discharge, and this reaction takes place in the pores of the oxygen cathode. An important obstacle is their low round-trip efficiency (65%) due to parasitic side reactions and instability of the electrolyte.9-10 These problems are closely related to the technical barriers, which include instability between electrolyte and carbon electrode, kinetics of charging and discharge with slow discharge at the oxygen electrode. In addition, we will demonstrate the temperature effect of the molten salt electrolyte with lower eutectic points such as Li, Na, K, Ca, Cs-NO3 (eutectic point: 65 ℃) mixtures.
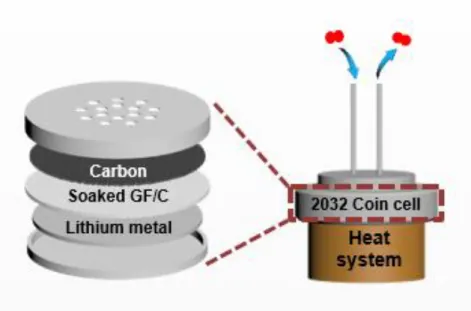
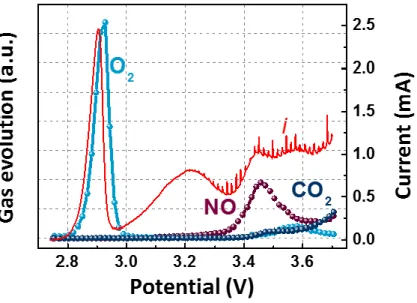
The following Figure 1.1 shows a molten salt electrolyte of five different nitrate salts applied to a lithium-oxygen battery. Therefore, the oxygen efficiency of the OER/ORR can be obtained through the DEMS instrument shown in Figure 1.5 below. A glass fiber (Whatman) with a diameter of 19 mm containing 150 μl of electrolyte was used in the battery.
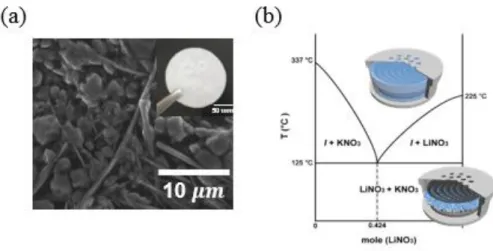
Generally, in the case of the cathode, the cell was disassembled after cooling and the cathode was withdrawn and washed with NMA solvent to remove the nitrate salt from the residue. The coin-type cell inserted in figure 1.b shows before (two solid phases) and after (one liquid phase) the eutectic point. It is confirmed that the binary molten salt shows very low voltage gap (~0.1 V) in accordance with the results of the previous paper.18 Figure 1.7.b shows the results of the in situ gas analysis, in which the oxygen gas increases at the beginning of the charge and remains constant throughout the charge cycle.
These would arise from oxidation reactions of the carbon electrode material and the electrolyte materials, NO3-. As the temperature increases, the overpotential of the cell decreases (in particular, the charging voltage is greatly reduced) and the oxygen evolution reaction increases. This is because not only the mobility of oxygen in the electrolyte is increased, but also the viscosity of the electrolyte is decreased, and the resistance required for the decomposition of Li2O2, the discharge product is reduced by increasing the solubility of Li2O2.
For the characterization of discharge products, the oxygen side of the cathode was analyzed by XRD and SEM after it was fully discharged in an oxygen atmosphere (Figure 1. 11). The XRD results clearly show that crystalline Li2O2 is clearly formed on the surface of the cathode after discharge depending on the temperature. SEM analysis was also performed to investigate the morphology of the discharge products at the oxygen electrode.
At 150℃, the kinetics of the electrode would increase and the ionic conductivity of the molten salt electrolyte would increase and the internal resistance of the cell would decrease. In all cases, the decomposition of the molten salt electrolyte results in the generation of nitric oxide gas from 3.4 V. As the temperature increases, the viscosity of the electrolyte decreases, which leads to the effect of facilitating the movement of oxygen ions and lithium.
The oxygen evolution reaction proceeded with gas analysis and observed not only ideal oxygen evolution but also electrolyte decomposition at 3.4 V by LSV-DEMS analysis.
Organic-free electrolyte for rechargeable lithium-carbon dioxide batteries
- Introduction of rechargeable lithium-carbon dioxide batteries
- Experimental
- Results and Discussion
- Conclusions
Here, we made a Li-CO2 battery consisting of a lithium foil anode and an organic-free binary molten salt electrolyte (LiNO3-KNO3) and a Super P cathode. The same as the above-mentioned experimental method in Chapter 1, but the only difference is in by changing the reactant by injecting carbon dioxide instead of oxygen. The composite Li-CO2 battery operates in a coin-shaped cell with a fixed capacity of 1 mAh.
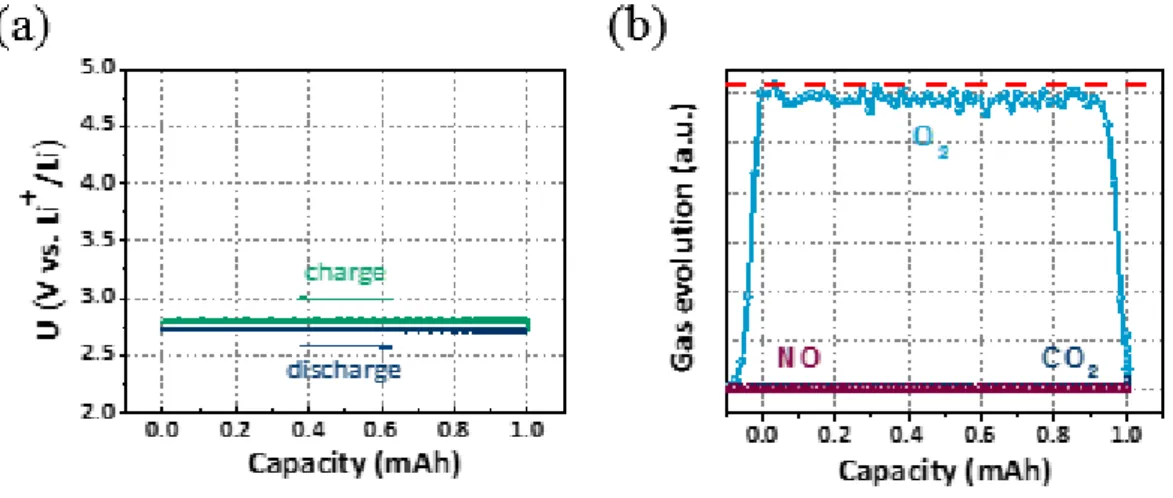
In addition, to understand the electrochemical oxidation of Li2CO3, an electrode filled with Li2CO3 and a conductive carbon material that showed a discharged state was used.30 A DEMS instrument was used to analyze the gas generated during charging with this prefilled electrode , and XRD was used to analyze the components of the electrode. The charging voltage of the inorganic (molten salt, LiNO3-KNO3) electrolyte was 3.2 V, which was lower than the charging voltage of organic electrolyte of 4.5 V, but the inorganic electrolyte did not generate carbon dioxide. XRD analysis of the Li-CO2 cell shown in Figure 2.3 shows that the discharge product, Li2CO3, is more evident in the inorganic electrolyte (LiNO3-KNO3 molten salt) after discharge.
As shown in Figure 2.2, only Li2CO3 was decomposed in the organic electrolyte, but no gas was formed in the inorganic electrolyte. When organic electrolyte and inorganic electrolyte are used, conductive carbon material and iridium with excellent catalytic activity for Li2CO3 are added to the electrode, and the charging profile of the electrode is shown in Figure 2.4. It was found that the charging voltage decreased slightly when the Ir catalyst was used, and the efficiency of Li2CO3 decomposition increased in the case of the organic electrolyte.
However, it was confirmed that Li2CO3 was decomposed and not released into CO2 even in the case of molten salt electrolyte when using an Ir catalyst. In summary, we investigated the electrochemical reactions of Li2CO3 formation and oxidation using organic electrolytes and inorganic electrolytes. Furthermore, DEMS analysis confirmed that CO2 was detected as the main gas product during the charging process using the Li2CO3 electrode.
In the case of organic electrolytes, the excellent catalytic activity of Ir@KB lowers the charging voltage and increases the amount of CO2 produced.