The objectives of the Dissertation of the Final Year Project Report are to record all relevant findings from literature reviews relating to the project of Preparation and Characterization of Co-Ni/Al2O3 catalyst for steam reforming of acetic acid. The main objectives of this project are to study the methods of preparing the catalysts for steam reforming of acetic acid, to prepare the Co-Ni/Al2O3 catalysts for steam reforming of acetic acid using impregnation method and to study the properties of Co-Ni /Al2O3 to study catalysts. I would like to thank the Final Year Project Committee for arranging briefings and lecturers as support and knowledge to assist students in the project and report writing.
Furthermore, I want to thank all the lecturers, technicians and graduate assistants from Universiti Teknologi PETRONAS who have provided me guidance throughout the project period.
INTRODUCTION
BACKGROUND OF STUDY
Currently, most of the hydrogen is produced from fossil fuels such as natural gas, naphtha and coal. Due to environmental pollution and high dependence on fossil fuels, the world is now focusing on the production of hydrogen from alternative fuels such as biomass. The production of hydrogen from biomass is considered one of the most important renewable technologies because it can achieve zero emissions of carbon dioxide and make us less dependent on fossil fuels for power and transport (Czernik, 1999).
Therefore, some methods are explored to produce hydrogen from biomass, namely (i) gasification/water-gas shift technology or (ii) flash pyrolysis of biomass followed by steam reforming of biomass products.
PROBLEM STATEMENT
- Problem Identification
- Significant of the Project
Based on some research from literature reviews, the author has come up with some problematic statements; (i) how the preparation method affects the performance of the catalyst in the steam reforming of acetic acid. To find the answer, the author conducted several experiments by changing the methods of catalyst preparation to see the effect of the sequence of adding the support to the catalyst metal solution. In this project, the selectivity and activities of the catalyst will be studied for steam reforming using Ni-Co/Al2O3 to compare the findings with studies done using other catalysts previously.
The results of this project are expected to be better than those done before, as the catalyst used in this project are bimetallic catalysts - Ni-Co supported on alpha-alumina (Ni-Co/αAl2O3).
OBJECTIVES AND SCOPE OF STUDY
- Objectives
- Scope of Study
- The Relevancy of Project
- Feasibility of the Project
Later, the best condition for optimal hydrogen production of acetic acid reforming steam using Co-Ni/Al2O3 catalysts will be determined. The Co-Ni/Al2O3 Preparation and Characterization Project for Acetic Acid Steam Reforming is feasible to complete within two semesters. This project includes experiments to prepare and study the characteristics of Co-Ni/Al2O3 catalysts.
In addition, there will be several experiments to study the effects of the Co-Ni ratio on hydrogen production.
LITERATURE REVIEW
Acetic acid is produced by fermenting various substrates (starchy solutions, sugar solutions or alcoholic foods) with Acetobacter bacteria. Acetic acid (AcOH) is used to investigate the process of steam reforming as it is one of the main components of bio-oil. In the research conducted by Takanabe's team, the mechanical investigations of steam reforming of acetic acid over platinum on a zirconium oxide (Pt/ZrO2) catalyst were studied.
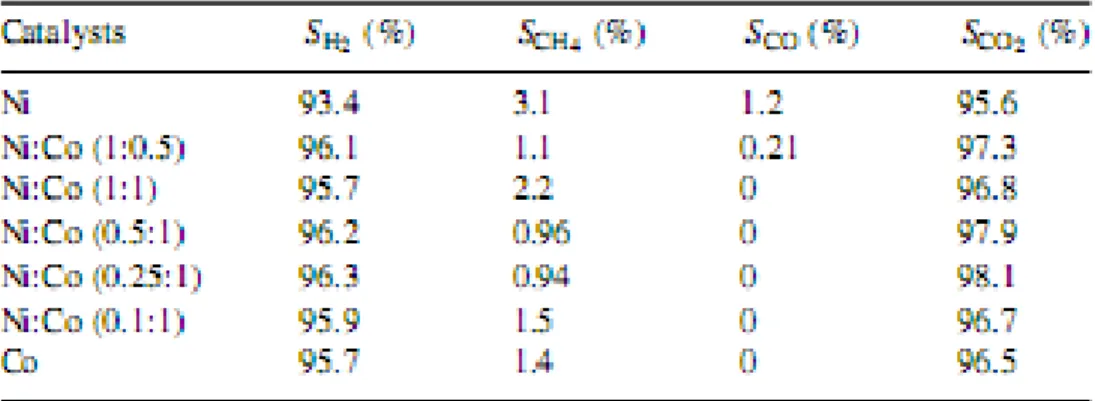
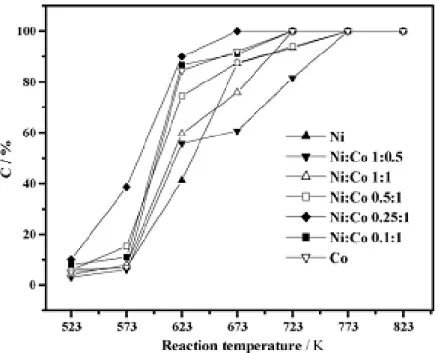
From the experiments conducted in the study of steam reforming of acetic acid to hydrogen over Ni-Co metal catalyst, it showed that the activities of the catalysts increased with the increase in Co content and reached the maximum at the ratio of 0.25:1 between Ni and Co For the steam reforming of acetic acid single metal Ni and Co catalysts were also active, but it was clear that they were inferior to the Ni-Co catalyst (0.25:1), both in terms of conversion of acetic acid and selectivities to products. It could be observed that the reaction temperature had a significant influence on acetic acid conversions and selectivities to the products in the investigated temperature range.
Ni-Co catalyst could efficiently produce hydrogen via acetic acid steam reforming in a relatively low temperature range of 623–823K. LHSV had less effect on the reforming reactions, while reaction temperature and S/C had significant influence on the conversion of acetic acid and distributions of the products (Hu, 2007). In the experiment, the reaction scheme of steam reforming of acetic acid with respect to temperature was investigated by conducting series of transient experiments such as homogeneous reactions, reaction pattern over supports and reaction scheme over Ni/(La2O3/Al2O3) catalyst. The overall reaction network for acetic acid steam reforming is very complicated, with thermal decomposition taking place at intermediate temperatures producing CH4, CO, CO2 and H2, while reforming reactions dominate at elevated temperatures, resulting in the formation of H2 and carbon oxides.
Low reaction temperatures and high acetic acid to steam ratios promote carbon deposition on the catalyst surface. (Basaggianis, 2006). The typical activity order of different catalysts tested in steam reforming of acetic acid was: Ni-based catalysts >>Pt> Rh >Pd≈Ru.
METHODOLOGY
- CATALYST PREPARATION
- CATALYST CHARACTERIZATION
- TOOLS REQUIRED
- LIST OF CHEMICALS USED
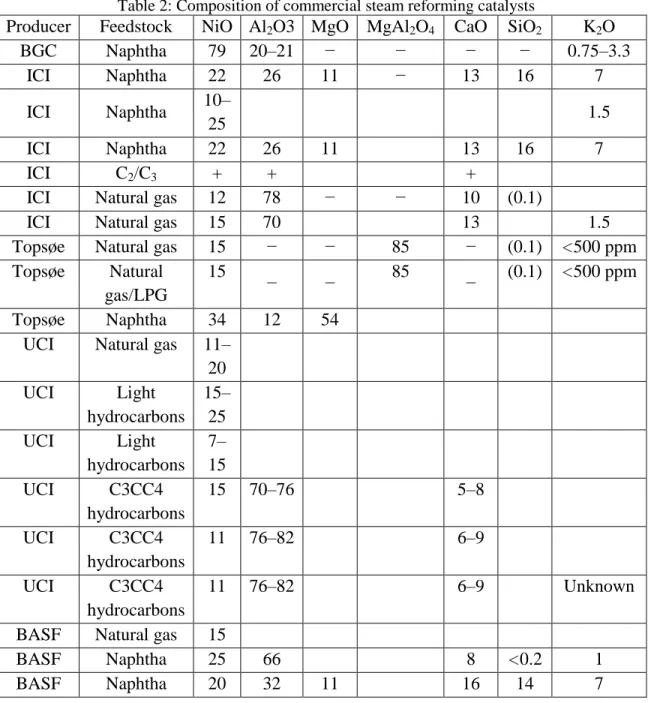
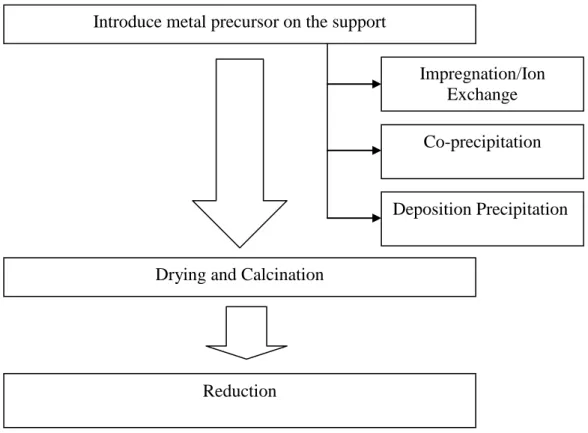
For the single metal catalyst, the metal precursor (nickel or cobalt) is introduced into the Al2O3 support by the impregnation method. The metal precursor is dissolved in sufficient amounts of deionized water before the Al2O3 support is added to the solution. The solution containing the metal precursor and the catalyst support was stirred for 6 hours on a magnetic stirrer.
For bi-metal sequential impregnation catalyst preparation, the first metal precursor is introduced to the support as in single-metal catalyst preparation. The single metal catalyst prepared earlier will become the support catalyst for the second metal precursor. The second metal precursor will be introduced to the support following the steps of preparing the single metal catalyst.
To prepare the bimetal co-impregnation catalyst, both metal precursors are dissolved in sufficient amounts of deionized water before the support is added to the solution.
RESULT AND DISCUSSION
XRD Result
- Sample 1
- Sample 2
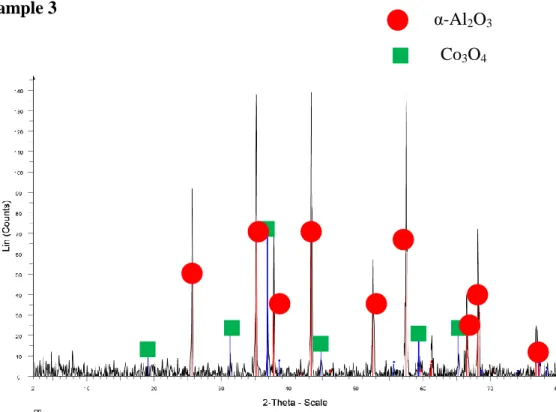
- Sample 3
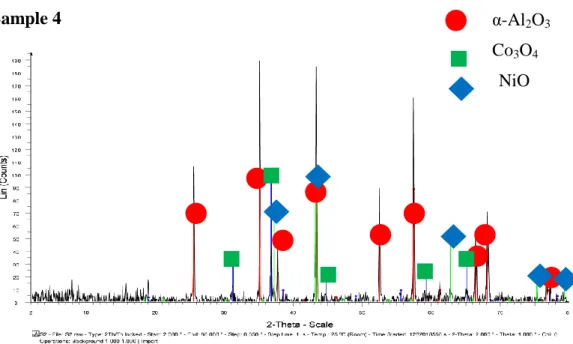
- Sample 4
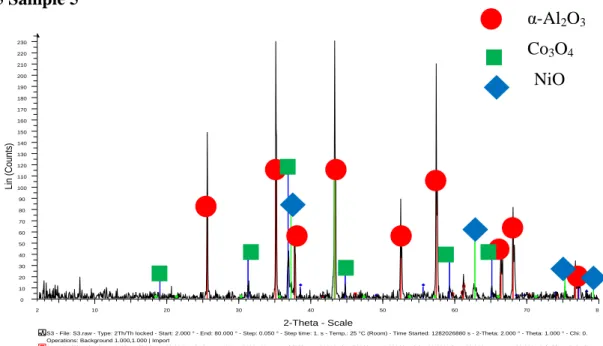
- Sample 5
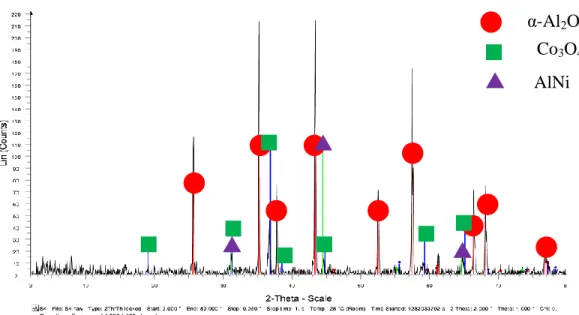
- Sample 6
- Sample 7
- Sample 8
- Comparison of All XRD Results
- Comparison with Literature Finding
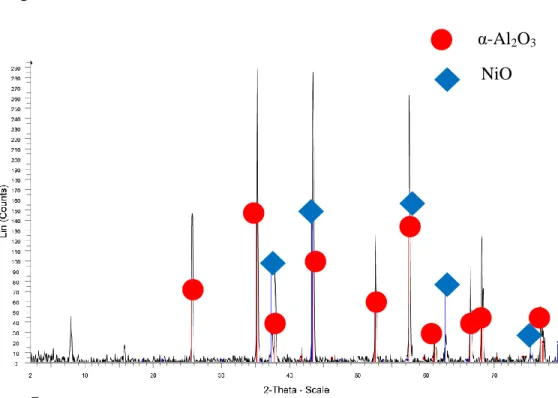
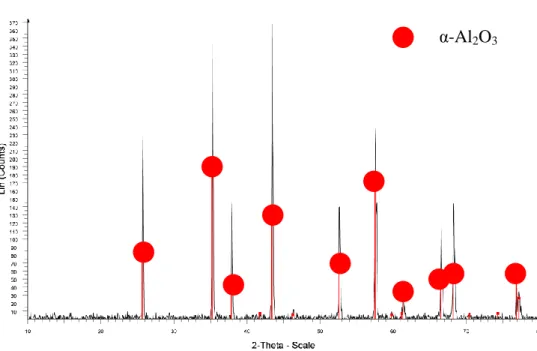
The diffraction peaks indicate the presence of corundum oxide in the sample, which is consistent with the claim that the sample contains Al2O3. The diffraction peaks indicate the presence of corundum oxide and NiO in the sample, which is consistent with the claim that the sample contains Ni/Al2O3. The high intensity diffraction peaks show the amount of crystalline components detected in the sample.
The higher the diffraction peak, the more crystalline phase of individual components is present in the sample. The diffraction peaks show the presence of corundum oxide and Co3O4 in the sample, which is consistent with the claim that the sample contains Co/Al2O3. The maximum intensity appears at the diffraction peak of corundum oxide and cobalt oxide, indicating that several crystalline phases of both components are detected in the sample.
The diffraction peaks indicate the presence of corundum, Co3O4 and NiO in the sample, which is consistent with the claim that the sample contains Co-Ni/Al2O3. The highest intensity appears at the diffraction peak of corundum oxide, nickel oxide and cobalt oxide, showing that more crystalline phases of all components in the sample are detected at the specified intensity. The diffraction peaks indicate the presence of corundum oxide, Co3O4AlNi and in the sample, which is consistent with the claim that the sample contains Co-Ni/Al2O3.
The diffraction peaks indicate the presence of corundum and NiCo2O4 in the sample, which is consistent with the claim that the sample contains Co-Ni/Al2O3. The highest intensity appears at both corundum oxide and nickel cobalt oxide diffraction peak, demonstrating that more crystalline phases of all components in the sample are detected at the specified intensity.
SEM RESULT
The larger particles are denoted as Al2O3 while the smaller particles are metal precursors, either cobalt or nickel.

EDX RESULT
- Sample 1
- Sample 1
- Sample 2
- Sample 4
- Sample 5
- Sample 6
The adsorption of liquid nitrogen starts at a relative pressure of 0.95 and stops at a relative pressure of 0.98. The desorption of liquid nitrogen starts at the relative pressure of 0.98, the same point for the completed adsorption. The curve continues to fall as the relative pressure decreases and stops at a relative pressure of 0.96.
Adsorption of liquid nitrogen starts at a relative pressure of 0.01, continues to increase and stops at a relative pressure of 0.99. Desorption of liquid nitrogen starts at a relative pressure of 0.99, at the same point of complete adsorption. The curve continues to decrease with decreasing relative pressure and stops at a relative pressure of 0.14.
The curve continues to decrease as the relative pressure decreases and intersects the absorption curve at the point 0.45, then it follows the adsorption curve to decrease and stop at the relative pressure of 0.14. Based on Figure 26, the adsorption/desorption curve for the 2.5%Co2.5%Ni/Al2O3 sample is quite similar in pattern. The curve continues to decrease as the relative pressure decreases and intersects with the adsorption curve at the 0.50 point, then continues to decrease and stops at the relative pressure of 0.14.
Adsorption of liquid nitrogen starts at a relative pressure of 0.01, continues to increase and stops at a relative pressure of 1.00. Desorption of liquid nitrogen begins at a relative pressure of 1.00, at the same point of complete adsorption. The curve continues to decrease as the relative pressure decreases and intercepts the adsorption curve at the 0.50 point and continues to decrease and intercepts the adsorption curve at the 0.25 point and then continues to decrease and stops at the 0.14 relative pressure.
The desorption of liquid nitrogen starts at the relative pressure of 0.99, the same point of the completed adsorption, continues to decrease and stops at the point of 0.01.
CONCLUSION
Recommendation
Steam reforming of acetic acid as a biomass-derived oxygenate: Bi-functional pathway for hydrogen formation over Pt/ZrO2 catalysts, Journal of Catalysis, 243, pp 263-269. Catalytic steam reforming of bio-oils for the production of hydrogen: effects of catalyst composition, Journal of Applied Catalysis A: General, 201 pp225–239. Yaman, (2003). Pyrolysis of biomass to produce fuel and chemical feedstocks, Journal of Energy Conversion and Management, 45, pp 651-671.
Chornet, (1999). Hydrogen from Biomass by Fast Pyrolysis/Catalytic Steam Reforming, Thesis, National Renewable Energy Laboratory, USA. Mirodatos, (2008). Continuous Hydrogen Production by Sequential Catalytic Cracking of Acetic Acid, Part I - Investigation of Reaction Conditions and Application in Two Parallel Reactors Operated in Cycles, Journal of Applied Catalysis A: General, 335 pp64–73. Lu, (2007). Investigation of the steam reforming of acetic acid to hydrogen over a Ni–Co metal catalyst, Journal of Molecular Catalysis A: Chemical, 261, p. 43–48.