Pharmacological and phytochemical evaluation of seven plants used for microbial disorders in South African traditional medicine. Thesis title: Pharmacological and phytochemical evaluation of seven plants used for microbial-related disorders in South African traditional medicine.
107 Figure 5.7: GC-MS chromatogram of a hot ethyl acetate subfraction obtained from methanol extracts of Protea caffra twigs…………. 109 Figure 5.9: GC-MS chromatogram of a methanol sub-fraction obtained from methanol extracts of Protea caffra twigs.……….
South African biodiversity and medical bio-prospecting
2 biqiltoota qoricha Afrikaa Kibbaa irraa argamuu danda’u kanneen akka Combretum edwardsii, Combretum krausii (CHUKWUJEKWU FI VAN STADEN, 2016), Centratherum punctatum (CHUKWUJEKWU et al., 2018), Merwilla plumbea (NCUBE et al., 2012) dabalatee , garuu waanta ta'e.
Aims and significance of the study
Literature review
Literature review on selected medicinal plants
- Bolusanthus speciosus Harms
- Botany and ethnomedicinal applications
- Ethnopharmacology and phytochemistry
- Cucumis myriocarpus Naud
- Botany and ethnomedicinal applications
- Ethnopharmacology and phytochemistry
- Ekebergia capensis Sparrm
- Botany and ethnomedicinal applications
- Ethnopharmacology and phytochemistry
- Prunus africana (Hook. F.) Kalkman
- Botany and ethnomedicinal applications
- Ethnopharmacology and phytochemistry
- Protea caffra Meisn
- Botany and ethnomedicinal applications
- Ethnopharmacology and phytochemistry
- Searsia lancea (L.f) Barkley
- Botany and ethnomedicinal applications
- Ethnopharmacology and phytochemistry
- Solanum panduriforme E. Mey
- Botany and ethnomedicinal applications
- Ethnopharmacology and phytochemistry
Afrikaa Kibbaatti mukti kun naannoo sanatti muka wisteria (Afaan Ingiliffaa) jedhamuun beekama; vanwykshout (kan Afrikaa); mogaba (Kaaba Sotoo); xurumbaa kan taphatu (Tswaana); gaara (Venda) fi gaara (Zulu) (ELISHA et al.,2015). Afrikaa Kibbaatti biqiltuun kun yeroo baay’ee abaaboo hadhaa’aa, kukkurama bosona (Ingiliffaa), kukkurama, abaaboo hadhaa’aa, kukkurama (Afrikaans) fi sendelenja (Zulu) jedhamuun beekama (HUTCHINGS et al., 1996).

Antimicrobial resistance and the essence of combination therapy
A few years ago, GANDHI et al.(2006) discovered Mycobacterium tuberculosis strains resistant to all known antibiotics in KwaZulu-Natal, South Africa, perhaps serving as early warning signs of an impending era of 'intractable bacterial infections'. Given that some medicinal plants such as Berberis fremontii have been reported to produce both bactericidal compounds and antibacterial resistance inhibitors (STERMITZ et al., 2000), understanding the intricate nature of combination therapies involving medicinal plant extracts will inevitably help to reach these two important research goals.
Antibacterial screening
Introduction
Materials and methods
- Plant collection
- Source of chemicals
- Extraction of plant for biological activities
- Antigonococcal activity
- Preparations of gonococcal stocks
- Disk-diffusion bioassay
- Antigonococcal microdilution bioassay
- Antibacterial activity
- Bacterial stock preparation
- Microdilution bioassay
- Antibacterial synergy
- Checkerboard titration method
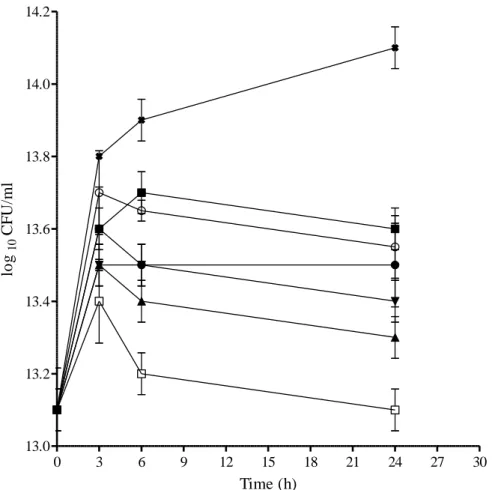
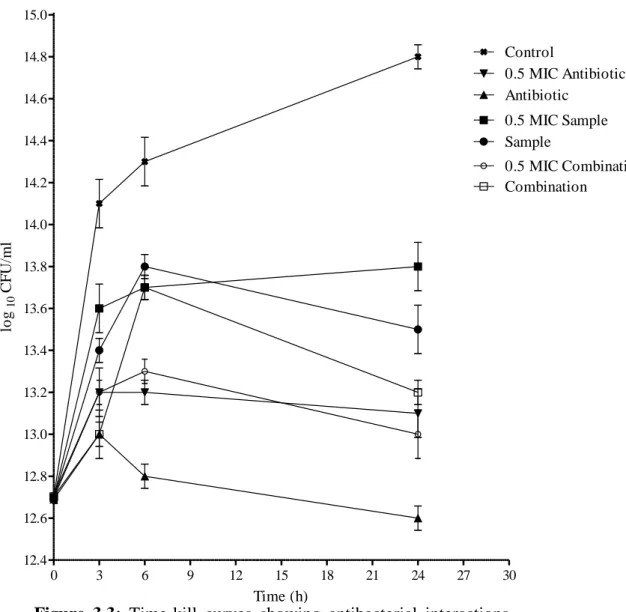
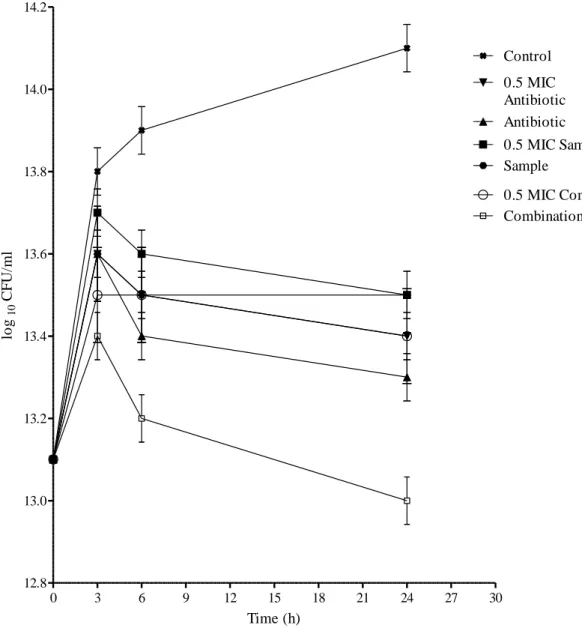
- Time-kill bioassay
A colony of each strain was inoculated into 5 ml of sterile MH broth and incubated overnight at 37 oC in an orbital shaking water bath (50 rpm). Five hundred microliters of the test bacterial strain was then added to give a final inoculum of approximately 5 x 105 cfu/ml. The inoculum was then incubated for 90 min at 37 oC in an orbital shaking water bath (50 rpm).
Results and discussion
- Antigonococcal activity
- Antibacterial activity
- Antibacterial synergy
Instead of using the usual herbal preparations from plants such as Callilepis salicifolia, Jatropha zeyheri, Cotyledon orbiculata, Catharanthus roseus and Senna italica to treat gonorrhea (ERASMUS et al., 2012), herbalists can now alternatively use the leaves of S. Since season and climate have an impact on the chemical profiles of most plants (KADU et al., 2012), it is imperative that further studies be conducted to determine whether the antibacterial properties exhibited by B. In the present investigation , 50% of the plant extracts examined demonstrated significant activities against gram-positive E .
Gram-negative bacterial strains have thick hydrophilic peptidoglycan layers that hinder the influx of most, especially hydrophobic, antibacterial agents into their cells (REECE et al., 2011). Cooking, mixing different herbs, as well as mixing herbs with non-plant material during the preparation of traditional medicines often activates and/or increases the bioavailability of phytocompounds in the extract (CANO AND . VOLPATO, 2004; AZIZAH et al., 2009). PETERSEN et al. (2006) reported that such exaggerations are often a direct consequence of interactions between bacteriostatics and bactericides in combination therapies.
After observing large inconsistencies in results obtained from the same checkerboard treatments, RAND et al. (1993) recommended the use of at least five repetitions per each checkerboard test and further suggested that greater than 80% agreement between repetitions is required for accuracy. While the checkerboard test primarily assesses the bacteriostatic nature of antibacterial agents, the time-kill test determines the rate and extent at which these agents kill the test bacterial strains (MUNDY et al., 2016). This is largely because the time-kill kinetic graphs give a more vivid picture of changes in bacterial populations after exposure to antibiotics over time and have the added advantage of showing us whether the antibacterial effects are concentration- or time-dependent (PFALLER et al. . , 2004).
Conclusions
Phenolic profiles and mutagenic properties of selected plants
- Introduction
- Materials and methods
- Plant collection and preparation of samples
- Source of chemicals
- Phenolic composition of selected plants
- Mutagenic properties of selected medicinal plant
- Results and discussion
- Phenolic compositions of selected medicinal plants
- Mutagenic properties of selected medicinal plant species
- Conclusions
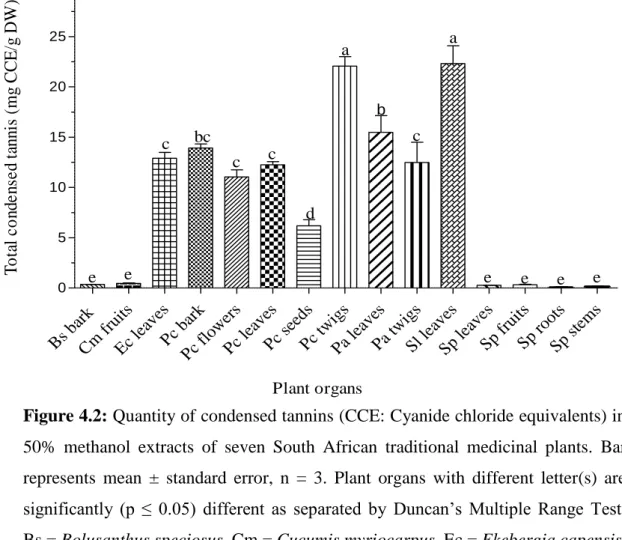
As described by MAKKAR et al. (2000), the Folin C test was used to determine the total phenolic content in each plant organ tested. The concentration of condensed tannins (proanthocyanidins) in each test plant organ was determined using the butanol-hydrochloric acid test (MAKKAR et al., 2000). The amount of flavonoids in each plant organ was determined using the aluminum chloride test as previously described by MAKKAR et al.
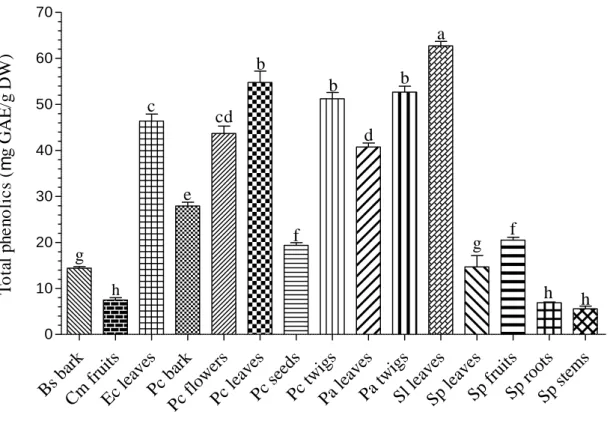
In the present study, different quantitative phytochemical analysis techniques were used to determine the amount of phenolic compounds present in each tested plant organ. The antibacterial properties of phenolic compounds are essentially attributable to the numerous hydroxyl and other functional groups they possess that often bind to the intra- and extracellular components of bacterial cells (COWAN, 1999; BORGES et al., 2013). It should be noted that almost all phenolic compounds screened in the present study were present in both medicinal plants (Figures 4.1-4.3 and Tables 4.1-4.2).
However, as some medicinal plant species are inherently poisonous (TAYLOR et al., 2003), the safety of traditional herbal medicines remains a serious cause for concern. The absence of legislative guidelines regarding the manufacture, prescription and administration of herbal remedies in folk medicine (FENNELL et al., 2004) exposes patients to the risks of medicinal plant poisoning. Often cases of phytopoisoning are poorly documented and as such the actual mortality due to medicinal plants remains largely unknown (MACE et al., 1998).
Identification of putative antibacterial compounds in Searsia lancea and
Introduction
77 such as thin layer chromatography (TLC) is often used to further purify the fractions obtained from column chromatography (ATANASOV et al., 2015). Consequently, in vitro and in vivo pharmacological tests are often necessary to determine the efficacy and toxicity of the isolated compounds. In addition, the data presented in Chapter 4 (Figures 4.1-4.3 and Tables 4.1-4.2) indicate that both plants contain a wide range of phenolic compounds that have antibacterial properties.
Furthermore, little is currently known about the antibacterial compounds in both plants. Based on this premise, the present study aimed to identify the antibacterial compounds from the leaves of S.
Material and methods
- Identification of putative antibacterial compounds in Searsia lancea
- General
- Plant collection and extraction
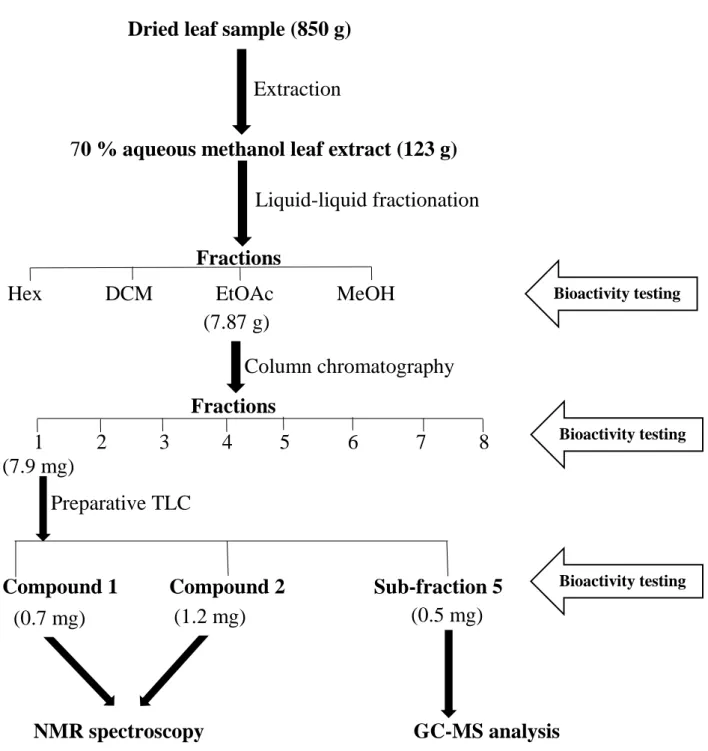
- Liquid-liquid fractionation of the crude plant extract
- Column chromatography
- Preparative Thin Layer Chromatography
- Gas Chromatography-Mass Spectroscopy (GC-MS)
- Identification of putative antibacterial compounds in Protea caffra
- Plant collection and extraction
- Liquid-to-liquid fractionation and GC-MS analysis
The resulting fractions were separately concentrated to dryness in vacuo to give four solvent fractions: hexane (Hex), dichloromethane (DCM), ethyl acetate (EtOAc), and MeOH. All four fractions were screened for antibacterial activity against a panel of seven bacterial strains (E. faecalis, MDR E. coli, K. pneumoniae, MDR K. pneumoniae, S. aureus, penicillin-resistant S. aureus and N. gonorrhoeae) as described in section 3.2.5.2. Fractions with similar chemical profiles were pooled and subjected to antibacterial susceptibility tests as described in Section 3.2.5.2.
The fraction (7.9 mg) was loaded onto a silica glass TLC plate and developed using a DCM/EtOAc (1:1) solvent system. DCM = Dichloromethane, EtOAc = Ethyl acetate, GC-MS = Gas Chromatograph Mass Spectroscopy, Hex = Hexane, MeOH = Methanol, NMR = Nuclear Magnetic Resonance, TLC = Thin Layer Chromatography. GC-MS analysis was performed at the School of Chemical and Physical Sciences, University of KwaZulu-Natal, Pietermaritzburg using a Shimadzu QP-2010 SE gas chromatography coupled to (an Agilent) 5973 Mass Selective detector and controlled by Agilent Chemstation -software.
Three microliters of the sample was injected into the column with the injector temperature set at 250 oC. The compounds were identified by direct comparison of the mass spectrum of the analyte at a specific retention time with that of reference standards found in the National Institute of Standards and Technology (NIST) library. The four fractions were screened for antibacterial activity against a panel of eight bacterial strains (E. faecalis, E. coli, MDR E. coli, K. pneumoniae, MDR K. pneumoniae, S. aureus, penicillin-resistant S. aureus and N gonorrhoeae) as described in Section 3.2.5.2.
Results and discussion
- Identification of putative antibacterial compounds in Searsia lancea
- NMR assignments
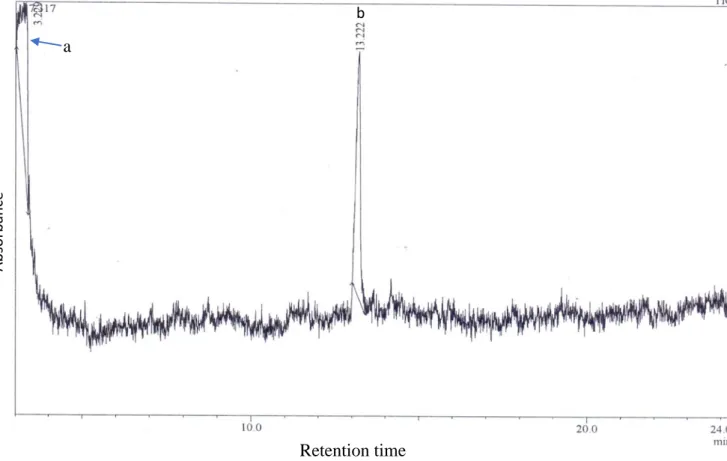
- GC-MS analysis of a bioactive Searsia lancea methanol leaf extract sub-fraction
- Identification of putative antibacterial compounds in Protea caffra
Eicosane was previously detected in Rhus vernicifera (JAVIDNIA et al., 2008) and Rhus succedanea (HUIPING, 1987), while 1-nonadecanol was found in Rhus coriaria (BAHAR AND ALTUG, 2009). As shown with 1-nonadecanol, 1-tetracosanol (Figure 5.5) was also shown to have broad-spectrum antibacterial activities (MAHMOOD et al., 2014). 98 particularly actinomycetes, have also been reported to produce 1-tetracosanol (VENUGOPAL et al., 2014), probably as part of their natural defense mechanism.
1-nonadecanol and eicosane are long-chain fatty acids whose antibacterial mechanisms are thought to be related to their strong oxygen radical scavenging properties (YASA et al., 2009). 1-Adamantyl heterocycle is often incorporated into anti-infective molecules to improve their efficacy (HASSAN et al., 2010). Several potent antimicrobial and antiviral agents such as rimantadine (MANCHAND et al., 1990), oxadiazole (EL-EMAM et al., 2004), isoxazole (MAKAROVA et al., 2002) and thiadiazole (KRITSANIDA et al., 2002) all 1-adamantanyl derivatives.
1-Heptacosanol, another compound detected in the cold ethyl acetate fraction, is a fatty alcohol present in plants (KOAY et al., 2013), seaweed (MURUGAN AND IYER, 2014) and cuttlefish, Sepiella inermis (RAVICHANDIRAN et al., 2013). It also has several potential therapeutic uses, given that it has been discovered in medicinal plant extracts with antioxidant (MURUGAN AND IYER, 2014; . AL-ABD et al., 2015), nematocidal (SULTANA et al., 2010) and antidiabetic properties. (UNNIKRISHNAN et al.). al., 2014) properties. Oxalyl chloride is a synthetic compound used in oxidative processes involved in the production of antibiotics, pesticides, herbicides and other organic products (ABUBAKER AND WANDRUSZKA, 1991; MAO et al., 2004).
Conclusions
Two of the detected compounds, 1-adamantanecarboxylic acid and levoglucosan, are often incorporated into antimicrobial moieties either as carbon skeletons or to improve the effectiveness of the parent drug. However, further studies are warranted to determine the specific bioactive molecules responsible for the antibacterial properties of P.
GENERAL CONCLUSIONS
Traditional Healers and Lay People: A Qualitative and Quantitative Approach to Local Knowledge of Medicinal Plants in Muda (Mozambique). The efficacy and safety of nine South African medicinal plants in controlling the Bacillus anthracis Sterne vaccine strain. Antimicrobial properties and phenolic content of medicinal plants used by the Venda people for conditions related to sexually transmitted diseases.
-inflammatory and mutagenic evaluation of medicinal plants used by Venda people against venereal and related diseases. Pharmacological properties and protein binding capacity of phenolic extracts of some Venda medicinal plants used against cough and fever. Antimicrobial activity and probable mechanisms of action of medicinal plants in Kenya: Withania somnifera, Warbugia ugandensis, Prunus africana and Plectrunthus barbatus. and its 3-galactoside from Rhus lancea.
Use of ethnoveterinary medicinal plants in cattle by Setswana-speaking people in the Madikwe area of the North West Province of South Africa.