Thanks are due to the Ethics, Law and Human Rights Working Group of the African AIDS Vaccine Program for partial financial support; Prof Douglas Wassenaar for his supervision, support and guidance, Ms Nicole Mamotte for her help in shaping this thesis, the Center for AIDS Program of Research in South Africa (CAPRISA) for giving me the opportunity to work as a researcher to start working at one of their research locations, and to all participants from the different research locations who participated in this study for their contribution to this research. This study explored the ethical, legal and human rights (ELH) dilemmas of researchers involved in preparing for and/or conducting HVTs in African countries.
Introduction
- Statement of the problem
- Objectives of the study
- Theoretical frameworks
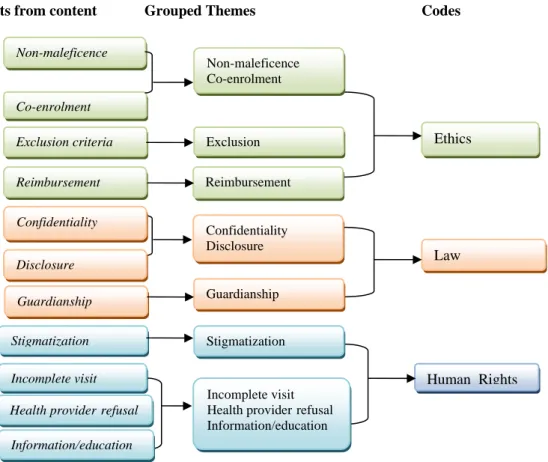
- Ethics
- Law
- Human Rights
- Questions the study seeks to answer
The appropriateness of conducting trials was examined in relation to the laws of each country, with the help of an inventory of African laws developed by the ELH working group of the UNAIDS AAVP (University of KwaZulu-Natal, 2009). . In relation to human rights, the study referred to the Universal Declaration of Human Rights (UN, 1948).
Literature review
HIV/AIDS in Africa
- Why is HIV/AIDS worse in Africa?
The spread of HIV/AIDS in Africa has been so rapid that before countries were even aware of the threat posed by this pandemic, it had deeply penetrated their communities (Poku & Whiteside, 2004). These patterns of sexual relationships are often a result of poverty and socio-economic inequality.
HIV/AIDS vaccine research in Africa
People at risk of HIV/AIDS are considered to be those in areas with high HIV/AIDS prevalence and incidence rates. A phase II trial can last two to three years (http://www.hvtn.org/science/phases.html.
Theoretical frameworks
- Ethics
- Legal frameworks
- Human rights
However, the country has ethical and legal structures, such as the Ministry of Health, that play a role in regulating the behavior of HVT in Botswana (Grant et al., 2005). Uganda also has statutory bodies such as the National Medicines Agency and the Uganda National Council for Science and Technology (UNCST) to regulate clinical trials (Grant et al., 2005).
ELH issues and barriers to the conduct of HVT in Africa
- Ethical issues and barriers
- Legal issue and barriers
- Human rights issues and barriers
- Resource related barriers
Identifying social value determinants for research is made more difficult by study priorities, which may change during the conduct of the study (Emanuel et al., 2004). First, regulatory bodies sometimes present conflicting interpretations of the law, and this affects the conduct of clinical trials (Armitage et al., 2008).
Related studies
Some researchers reported that they had concerns about the long-term physical impact of the study drug on the participants. Most participants reported having most of the infrastructure necessary for successful trial development. For example, in one of the HVTs in southern Africa, some trial participants did not do this.
Some of the researchers who participated in this study mentioned that they experienced concurrent sign-ups on their site. The results of this study show that most African HVT sites have most of the infrastructure necessary for the conduct of HVTs.
Literature review summary
Methodology
Aims
Research design and methodology
It is because of the strengths of quantitative and qualitative designs mentioned above that this study was conducted. The research seeks to leverage the strengths of both designs to provide an in-depth analysis and understanding of the research questions. The aim of the study was not only to generalize research findings to other contexts in Africa, but also to provide qualitative analyzes to provide contextual understanding.
The questionnaire that was used as a data collection method was a mixture of quantitative and qualitative items.
Procedures and data collection
The questionnaire, study cover letter and informed consent were sent by e-mail to trial sites as soon as local permission to conduct the study was granted. Both the study coordinators and sometimes PIs informed the research staff about the study and encouraged participation. To ensure confidentiality, the questionnaire and informed consent were sent by e-mail to the contact persons who then forwarded the two documents to their respective research teams.
Respondents can also complete the questionnaire at a time convenient for them and then email their responses back. Follow-up emails and phone calls were made to ensure that participants received the questionnaire.
Sampling
- Response rate
- Sample characteristics
- Distribution of the sample
- Funders/Sponsors
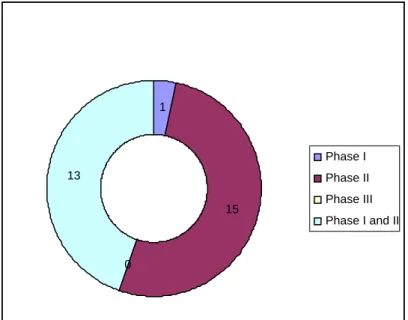
- Phase of trials conducted
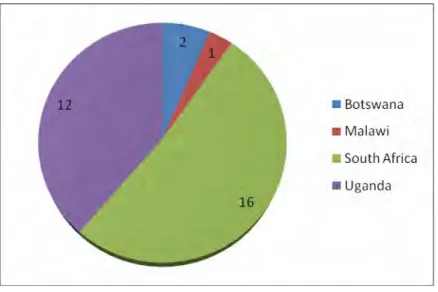
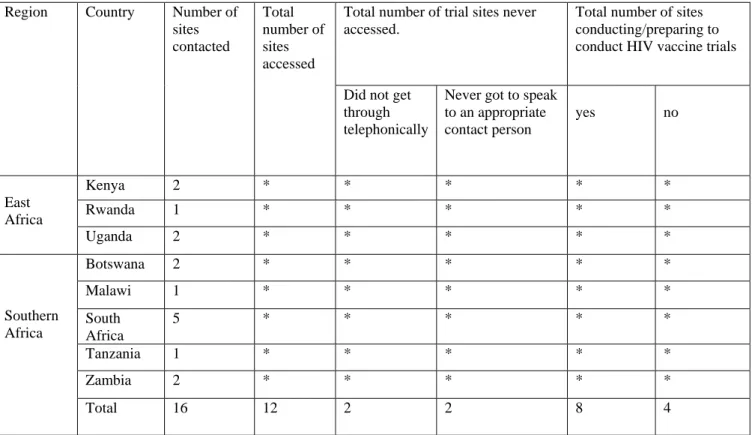
Snowball sampling was also used to enable identified contacts from study sites to identify other HVT sites in their area or country. Questionnaires were emailed to nine of the twelve HVT locations – who consented to conducting the study. Most respondents – approximately 52% (n=16) – in this study were from HVT sites in South Africa, followed by Uganda with approximately 39% (n=12), as illustrated in Figure 1.
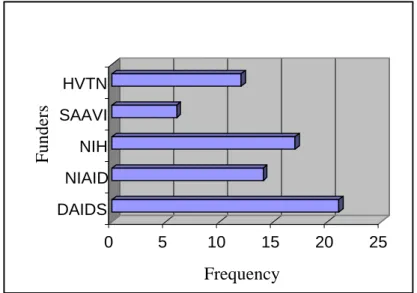
The low response rate of Botswana and Malawi is primarily attributed to the fact that the two countries had fewer HRT sites than South Africa and Uganda. The second most common funder of HRT sites preparing to perform and currently performing HRTs in this study was the US National Institutes of Health (NIH) indicated by 54.8% (n=17) of the researchers.
Instrument
- Content
- Validity of the instrument
- Reliability of the instrument
To increase the reliability of the data collection instrument, a questionnaire was used instead of an interview and other face-to-face methods of data collection. Second, the use of both open and closed items increased the reliability of the questionnaire. Using only open-ended questions can lead to laziness and unwillingness to complete questions on the part of respondents, while using only closed-ended items can result in a lack of authenticity in responses (Cohen et al., 2007).
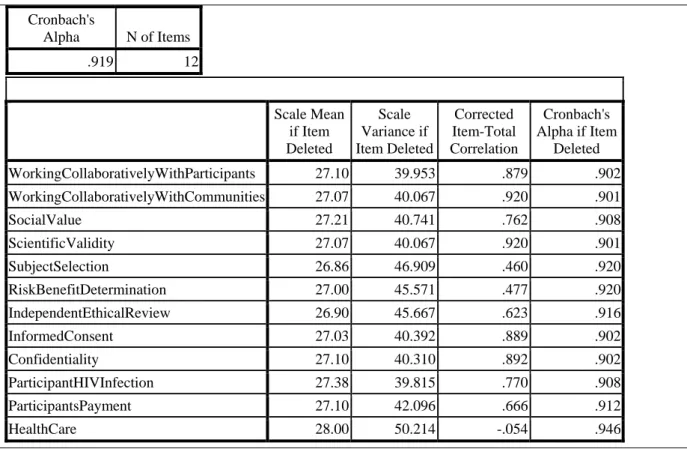
The questionnaire was reliable in identifying the ethical, legal and human rights challenges facing researchers in implementing HVT in African countries. Items 7A to 7L of the questionnaire measured these challenges and reliability calculations yielded a Cronbach's alpha of .919 as shown in Table 1 below in the SPSS output.
Analysis of Data
- Analytic technique of qualitative data
- Informed consent procedure
- Procedures to ensure confidentiality
- Ensuring a favourable risk-benefit ratio
Although not randomly selected, this study included researchers from different parts of Africa and this hopefully increased the external validity of the results of this study. All of these themes reflect some of the ethical issues in HVT, as shown in the literature review. One of the essential aspects of research ethics is the informed consent (IC) process.
It is therefore important for the researcher to weigh the costs of the study against the potential benefits. As a result, some of the concerns involved in this study were in the form of time the researchers needed to commit to completing the questionnaire.
Results
- ELH dilemmas and challenges
- Ethics challenges
- Law dilemmas and challenges
- Needs and material resources
- Identified needs
- Infrastructure
- Material resources used to solve ELH problems at site
- Guidelines and frameworks used to solve ELH dilemmas
- Summary of results (Main findings)
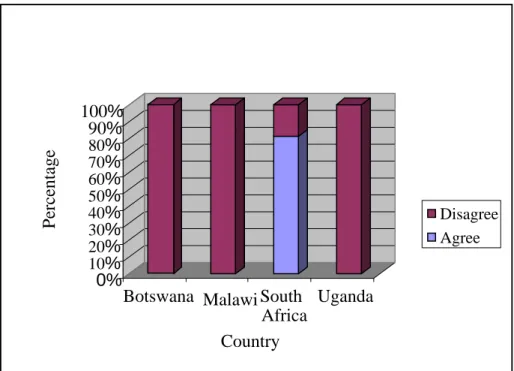
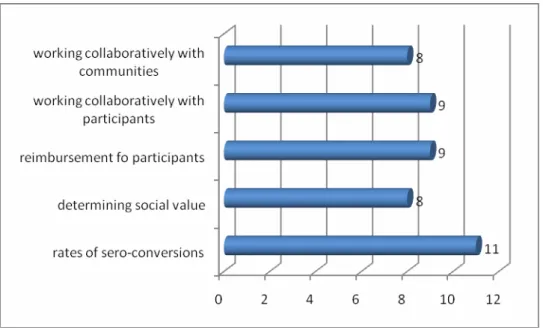
Just over half of the researchers (n=16) who participated in this study indicated that they believed that many participants were motivated by. Two ethical challenges emerged as a result of the reimbursement of trial participants: Participants' lack of understanding of the informed consent documents and co-enrolment. Half [50%] (n=8) of the researchers from Sites in South Africa reported that it was a challenge to work in collaboration with communities as depicted in figure 12 below.
None of the South African researchers ranked the UNAIDS guidelines as helpful; instead they ranked and rated the SOP as very useful. Fifty percent [50%] of the study coordinators rated the GCP as very useful, but the other half did not rank the GCP as useful.
Discussion
- ELH challenge
- Ethics dilemmas
- Legal challenges
- Human rights challenges
- Needs and resources
- Guidelinesand frameworks
- Theoretical frameworks
Researchers in South Africa previously indicated that participants' infection with HIV during the study is one of the challenges they face. One of the challenges researchers faced at their trial sites was protecting trial participants from potential harm. Second, one of the obligations of researchers is to guarantee the autonomy of participants and the exercise of their free will (Emanuel et al., 2005).
As a result of illiteracy, some participants have difficulty understanding the IC and other aspects of the study (Lindegger et al., 2006). The framework was also successful in identifying some of the challenges researchers face when conducting HVTs in African countries.
Limitations of the study
The use of the Universal Declaration of Human Rights was helpful in affirming that human rights are universal and therefore scholars from different parts of Africa had almost similar human rights concerns. Some of the reasons for this were that they are too busy on their site. In some cases, contacts promised to make their respective PIs aware of the study, but after several attempts at feedback, they still had not done so.
As a result of the aforementioned limitations related to a small sample size, researchers from other parts of Africa - for example West and Central Africa - were not represented. Researchers from these underrepresented regions may have different ELH challenges which – this study may not have identified – may also need to be investigated.
Conclusions and recommendations
- New knowledge
- Conclusions
- Recommendations
- Recommendations for further research
This study also indicates that there is a need to educate and update the research staff on relevant and available ethical and legal frameworks that inform the conduct of HRTs in their respective countries. Researchers in HRTs in African countries have reported experiencing ethical, legal and human rights challenges when conducting HRTs at their sites. Other ethical challenges researchers have faced include determining social value of the research, paying participants, collaborating with participants and communities, and explaining trial closure to participants.
Promote refresher training for research staff on various guidelines that inform the implementation of HVT, such as the UNAIDS (2007) guidelines on host community engagement. Most of the researchers involved in this study were often unaware of some of the relevant guidelines available to inform the implementation of HVT.
Guidelines for good practice in conducting clinical trials with human participants in South Africa. Experience in conducting multiple community-based HIV prevention trials among women in KwaZulu-Natal, South Africa. HIV vaccine research South Africa's ethical framework and its ability to promote the well-being of trial participants.
Ethical and legal challenges in enrolling adolescents in medical research in South Africa: implications for HIV vaccine trials. Commentary: Obligations for participants harmed during the N-9 multi-center vaginal microbicide trial in South Africa: Was the N-9 trial ethical.
Appendices
Dilemmas of ethics, law and human rights, needs and resources of researchers in HVT in Africa Informed consent. ETHICAL, LEGAL AND HUMAN RIGHTS DILEMMA, RESEARCHER NEEDS AND RESOURCES IN HIV VACCINE TRIALS IN AFRICA. ETHICAL, LEGAL AND HUMAN RIGHTS DILEMMA, THE NEED AND RESOURCES OF RESEARCHERS IN HIV VACCINE TRIALS IN AFRICA.
This study aims to explore ethical, legal and human rights dilemmas and needs. The purpose of this study is to explore the ethical and legal dilemmas and dilemmas and needs of researchers and the availability of the necessary material resources and infrastructure for the ethical conduct of successful HIV vaccine trials in African countries.
Cover letter
Informed consent
Questionnaire
Letter to contact person
Letter asking for permission
Identified HVT sites
Email log
Telephone log