214
Lesmana et al Med J IndonesCombination of interferon
alfa-2b and
ribavirin
in relapsed or
non-responding
chronic hepatitis
C
patients
following interferon therapy
Laurentius
A.
Iæsmana*,Unggul
Budihusodo-,Nurul Akbar*,
Ali
Sulaiman-,Sjaifoellah Noer*, Inge Ade Kristanti.,
Rianto
Setiabudy**Abstrak
Dua puluh enam pendeita dengan hepatitis kronik C (HKC) yang knmbuh atau tidnk berespons setelah terapi interferon (IFN) telah
dibeikan terapi iombinasi
IFi
atfa -2b 3 juta unit tiga kali seminggu dengan ribavirin (RIB) 800-1000 mg sehai selama 48 minggu' Dua puluh saiu (gl7o)dai
26 paiien dapat menyelesaikan studi initerdii
dari 12 pasien kambuh dan 9 tidakberespon sedangkan 5 pasiin keluar dari studi karenà eyek samping pada 3 pasien dan 2 lainnya tidak berhubungan dengan terapi. Pada kelompok kambuh 'responlengkap, kambuh dan respons perrnanen masing-masing didapatkan pada 9/12 (75Eù, 2/12 (I7Vo) dan 7/12 (587a) pasien. Sedangkni di'kelompok tidak beiespois perhirungan tersebut masing-masing didapatkan pada 3/9 (33vo),
I/9
(l (Eù, dan 2/9 (227o). Efek samping yang tersering ditemukan adalah gejala sepertiflu
didapatkan pada 18 pasien (86Vo). Terapi kombinasi IFI{ dan RIBi"p"t
*i*uiiikai
respons-perrnonen pada penderita HKC yang l<ambuh dan tidak berespon sementara terapi kombinasiini
lebih efektif untukyang kambuh daipadayang tidak berespons. (Meil J Inilones 2001; 10: 214-8)Abstract
Twenty six patients (pts) with chronic hepatitis C (CHC) who reLapsed or non-responded following.interferon (IFN) therapy were given'lFN â1fu-2b 3
itIIU
three times a weekfor 48 weel<s in combination with Ribavirin 800-1000 mg daily2I
(80,8Vù of the 26.pts -completedtie study
consisted of
t2
relapseri and 9 non-responders. Five pts dropped out due to drug adverse events in three pts and,on-drug related reason
in
the other tvvo. In the relapsed group complete resPonse, relapse and sustained response rates were obtainei in 9/12(Z5Vù, 2/2 (16,5%) and 7/12(58,3Vo) pts respectively. In the non-responding group, these figures were 3/9 (33'3Vo)' I/g(I1,1Eo), and 2/9(22,2Vo) pts, respectively. The most fiequent adverse event wasflu-like
syndrome, which was foundin 18
pts (85,7Eù. Combinationtheripy of IFN alfu-2b and riibavirin
may induce sustained virological responsein
relapsed and non' responding CHC patients. This combination therapy is more effectivefor
relapsers compared tofor
non'responders. (MedI
Indones 2001;I0:
214-8)Keywords
:
Chronic hepatitis C, combination therapy, interferon,ribaviin
Hepatitis C virus
(HCV)
is the mostcommon
causeof
post
transfu
s and
still
the most important
àtiology
of
ronic hepatitis
in
the
world.r
Although this
diseaseis
insidious
and themajority of
patients
do
not
develop
jaundice
at its
onset, about
807o
of
patients will progress
to
chronic hepatitis
and25Vo
of
this
lead
to
cirrhosis
and
hepatocellular
carcinoma.2r
Departmenf of Medicine Faculty of Medicine, IJniversityof
Indone s i a, J akarta, Indone sia**
Department of Clinical Pharmacology, Faculty
of
Medicine University of Indonesia, Jalarta, IndonesiaInterferon
(IFN)
alfa
is
the mostwidely
usedantiviral
agentfor
chronic hepatitis
C
(CHC) infection. Therapy
with IFN
alfa
will lead to
inhibition of HCV
replicat-ion
and amelioration
of
hepatic
necro-inflammatory
activity
in
about 5OVo of patientswith
chronic hepatitis
C. Unfortunately,
5OVoof
these responderswill
relapsewithin
6
months
of
discontinuing therapy.
Thus,
theoverall
sustainedalanine
response rate to alfa-IFN therapy isRibavirin
(RIB)
is a
synthetic nucleoside
analogueand has a broad spectrum
of in
vitro activity
againstboth
DNA
andRNA
viruses.Its
mechanism
of
actionis not entirely
clear,
but
it
may
act
through
depletion
Vol 10, No 4. October
-
December 2001Treatment
with
ribavirin
alone reduces serum
ALT,
combination
IFN
and
RIB
reduced relapse,
aslarge randomized
trials
have shown that combination
therapy
a
r sustained response compared
to
IFN
lo'rl
Improvement was
alsoobserved
in
terms
of
biochemical
and
histological
endpointsin
thosereceiving combination
therapy.Several
investigators
haveexamined this combination
therapy
for 6
months
for
thosepatients
who
relapsed afterresponding
toIFN treat
nt
orfor
thosewho did
not respond to [.lrJ.12'13'14In
this
open
clinical study, we
evaluate
the
efficacy
and safetyof
IFN alfa
-
2b
andRib
for
12 monthsin
relapsed
or
non-responding
CHC
patients
following
IFN
therapy.METHODS
This
was
an
open study
for
adult CHC
patients who
had relapsed or hadnot
respondedfollowing
aninitial
courseof
anyinterferon alfa
therapy using aminimum
of
3 MIUfor
aminirnum of
3months.
Responseto
aprior
therapy
is
defined
as
normal
serum A
documented
on
one
or
more
occasionduring
either
6weeks
prior
to
the end of
treatment
or
6
weeksfollowing
the endof
treatment.A
relapseis defined
as serum ALT > 1.5X
upperlimit
normal
(ULN) within
12
months
following
the end
of
the
initial
interferon
therapy.The
evidenceof CHC
was establishedby
theseropositivity
of
HCV-RNA
by
Roche
Amplicor
-PCR with
persistent
abnormal
liver
function
tests.5Patients
were
excluded
if
they
had
a
history
of
depression,
HIV
infection,
drug
abuse, evidence
of
decompensated
liver
disease anduncontrolled thyroid
abnormality.
The
patients consenting
the
study
were assignedto
receiveIntron
alfa 2b (Schering-Plough)
3MIU
administered
subcutaneously threetimes
a week(TTW)
for 48 weeks
in
combination
with
ribavirin
(RIB)
given concurrently
ateither
1000mg
daily (for
patients
< 75
kg of
body weight)
or
1200
mg daily
(for
patients>
75kg
of body weight)
in
divided
doses,for 48
weeks
as are-treatment
of
previously
relapsedor
non-responding
CHC
patients following
IFN
therapy. Dose
of
RIB
was reduced 507oif
hemoglobin
Combination of interferon q-2b and
ibavirin
in chronic hepatirts C215
level
decreasedbelow
10
g/dl
and dose
of IFN
was also reduced 50VoiÎ
white blood
cell
decreasedbelow
1.5X
10/1,granulocyte below
0.75X
10n andplatelet
below
50
X
10n.Adverse
events andlaboratory
testsincluding
hematology
and blood chemistry
wereperformed
at studyentry, monthly during
treatment, atend of
treatment,
every
3
months during follow-up
and at 6 months
of
follow-up.
Serum
HCV-RNA
was determined
at
study
entry,
atend
of
treatment,
and
at
6
months
of
follow-up in
majority
of
patients and results were
expressed
aspositive
and negative.
In
somepatients serum
HCV-RNA
was
also examined
every
3 months
during
treatment.Overall
response assessmentto combination
treatmentwas
determined
at
the
end
of
treatment and at
6months
following the
end
of
treatment.
Sustainedvirological
responders
were
those
with undetectable
HCV-RNA
at the endof
6 months observationperiod.
For either
ALT or
HCV RNA
response,
the
patient
could
either be
a
primary
non-responder
or
have respondedinitially with
breakthrough
at the endof
the treatment period.REST]LTS
Tbere were
twenty-six
subjects
who
could
beevaluated
in
this
study
twenty-one patients
havecompleted
the
treatment whereas
the
other
five
discontinued therapy.
Of
the
21 subjects
12 were
relapsers and 9 were non-responders.The
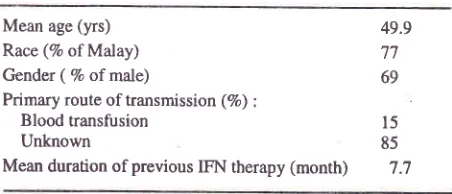
demographic dataof
26
patientswho
received re-treatment therapywith tFN
andRIB
is given
in
Table
1.The
majority of
patients were
males andMalay. In
most patients
the primary
mode
of
transmission
wasunknown
Table
l.
Demographic dataof
patients receiving re-treatment IFN and RIB.Mean age (yrs)
Race (7o of Malay) Gender (Vo of male)
Primary route of transmission (%) :
49.9 77 69
Blood
transfusion
15Unknown
85 [image:2.612.301.527.621.718.2]Vol 10. No 4. October
-
December 20A1 Combination of interferon a-2b and ribavirin in chronic hepatitisC
217INIERFERON
+
RIBAVIR.INFOLLOW
UP200
I80
160
140
120
àq
iæ
80
60
40
20
0
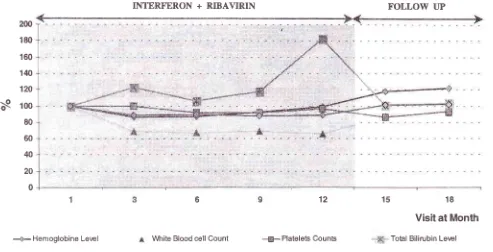
1
[image:3.612.40.533.90.342.2]-9-Hemoglobine Level
Figure 2. Hematological and their initial levels.
6
*
White Blood cell Count12
--g-Plal€lets Counts
Visit at Month
'Total Bilirubin Level 18 15
serum bilirubin changes during treatment and follow-up. Values are expressed as means of percentaqe
Discontinuation
of therapy occurred
in 5
(l9%o)of
26patients; three patients
because
of
adverse
events(severe weight loss, hyperthyroidism, and
psycho-logical
disturbance) andnon-drug
related reasonin
theother
two
patients (pneumonia and lossof
contact)."Flu-like"
symptoms (fatigue, fever, arthralgia, myalgia, and headache)were the most common
adverse events and were observedin
69Vo of patients. Mental symptomssuch
a5insomnia, sleepy, and psychiatric
disturbance were recordedin
l57o of patients. There were no singsof
specific adverse events
associated
with longer
re-treatment.
DISCUSSION
In
about 40-501o patientswith
chronic
hepatitisC, IFN
therapy can induce normalization
of
serum
alanineaminotransferase concentrations,
loss
of detectable
HCV RNA in
serurn andhistologic
improvement, but
the majority
will
relapse
after
cess
of
treat-ment.l5'16
Many
of
thesepatients
wiil
responseto re-treatment
with
but sustained response
isuncommon.'t'tt
A ,"
course
of
treatment
with
higher
doseof IFN or for
longer periods or both
maylead to
sustained responsein
20
to
5OVoof
patients, but these regimens aràcostly
andpoorly
tolerated.lT't8Several
initial
reports
have shown that
combination
IFN
andRIB is
more effective than re-treatment
with
IFN
alone
for chronic
hepatitis patients
who
relapsed or non-responded toprior IFN
therapy.l2'13'laThe
presentstudy
confirms
that combination
therapyof
Interferon alfa-2b
and ribavirin can
induce sustainedvirologic
responsein
relapsersand
in
non-responders
following
IFl.l
therapy. However,
thevirologic
and biochemical
responsesin relapsers
aregreater
than
in non-responders. These results
are in
agreement
with
previous studies.ll'la'le
The sustained
virologic
response
for
relapsers
andnon-responders
amounting 58.3Vo
and
22.2Vo,respectively,
in our
study
arehigher than
in
previous studiesusing the
samecombination treatment for
six months.l't''a'20 Thesediscrepancy
is probably
becauseof
thedifference
in
duration of
treatment.218
lzsrnana etal
"flulike"
andmental
symptomsreported in
98Vo and52Vo
of their
patients
greater thanin
our
study.Therefore,
we areof
theopinion
thatcombination
IFN
and
RIB
lor
12 months
might
be the re-treatment
of
choice
for
relapsed
or
non-responding
chronic
hepatitis C patientsfollowing
IFI,.I mono-therapy.Some
studies
have shown that during
combination
and
HCV
genotypeswere
not
measuredin this
studywe
can
not
postulate the probable
mechanismof
this
phenomenon.Our
study
hasrevealed
that
somepatients
developeddecrease
of
hemoglobin levels during
thecombination
treatment. Since
the
average
body
weight
of
our
patientsis
less comparedto that
of
patientsin
westerncountries, 600
to
800
mg ribavirin daily
might
besufficient.
In
conclusion,
the present data show thatcombination
IFN
and
RIB
for
12 months
is
safe and
effective in
inducing
sustained
virologic
responsein
relapsed or
non-respondingchronic hepatitis
C
patients. However,still low
responserate
in
non-respondersindicates
aneed
for
better therapeutic options.REFERENCES
l.
Booth JCL, Brown JL, Thomas FC. Tbe managementof
chronic hepatitis C infection Gut 1995; 37:449-54.2.
Tremolada F, Casarin C, Alberti A, Drago C, Tagger A,Ribero
M,
et al.
l.ong-term follow-upof
nonA,
non B(type C) post-transfusion hepatitis.I
Hepatol 1992: 16: 273-81.3.
Hoofnagle IF, Di Bisceglie AM. The treatment of chronic viral hepatitis. N Engl J Med 1007; 336:.347-56.4,
Patterson IL, Fernandez-Larson R. Molecular mechanismsof action of ribavirin. Rev Infect Dis 1990; 12:. 1139-46.
5.
Richard O, Anderson J, Schvarcs R, Weiland O. Ribavirintreatment for chronic hepatitis C. Lance!
l99l;
337: 1058-61.6.
Di
BisceglieAM,
Sindo M, Fong TL, Fried MW, Swain MG, Bergasa N, et al.A
pilot study of Ribavirin therapy for chronic hepatitis C. Hepatology 1992; 16:&9-53.
7.
ChemelloL,
CavalletoL,
BernadinelloE,
Guido M,Pontisso P, Alberti
A.
The effectof
interferon alfa andribavirin
combination therapyin
native patients with chronic hepatitis C. J Hepatol 1995;23:8-12.8.
LayMY,
Kao JH, Yang PM, Wang JT, Chen PJ' ChanKW,€!
al. Lnng-term efficacy of ribavirin plus interferonMed
I
Indonesll.
alfa
in
the
treatmentof
chronic hepatitisC.
Gastro-enterology 1996;l I : 1307 -12.Reichard
O,
NorkransG, Fryden
A,
Braconier J, SonnerborgA,
WeilandO.
Randomized double-blind, placebo-controlledtrial
of
interferon alpha 2bwith
andwithout ribavirin
for
chronic hepatitisC.
Lancet 1998;351:83-6.
Poynard T, Marcellin P, Læe S, Niederau C, Minuk GS,
Ideo G, et al. Randomized trial of interferon alfa-2b and
ribavirin for 48 weeks
or
for 24 weeks versus interferon alfa-2b plus placebofor 48
weeksfor
the treatment ofchronic infection with hepatitis C virus. Lancet 1998; 352:
1426-32.
Mchuthison JG, Gordon SC, Schiff
ER,
Shiffman ML,Lee
VM,
RustgrVK,
et al. Interferon alfa-2b alone or incombination with ribavirin as initial treatment for chronic hepatitis C. N Engl J Med 1998; 339:1485-92.
Schvarcz
R,
Yun
ZB,
SonnerborgA, V/eiland
O.Combined treatment with interferon alfa-2b and ribavirin
for
chronic hepatitisC
in
patientswith
a previousnon-response or non-sustained response to interferon alone. J
Med
Virol
1995; 46: 43-7.Brillanti
S, GarsonJ,
Foli M,
Whitby
K,
Deaville R,Masci C, et al.
A
pilot study of combination therapy withribavirin
plus
interferon
alpha
for
alpha-interferon resistant chronic hepatitis C. Gastroenterology 1994; lO7:8t2-17.
Bellobuono A, Mondazzi L, Tempini S, Silini E, Vicari F,
Ideo
G.
Ribavirin and
interferonalpha
combination therapy vs interferon alpha alonein
the retreatmentof
chronic hepatitis
C:
a randomized clinicaltrial. J
ViralHepat 1997;4: 185-91.
15.
Davis Gl,BalardLA,
Schiff ER, Linsay K,Bodenheimer HC Jr, Perrillo RP, et al. Treatment of chronic hepatitis C with recombinant interferon alfa: a multicenter randomized,controlled trial. N Engl J Med 1989; 321: 1501-6.
16,
Poynard T, Leroy V, Cohard M, Thevenot T, Mathurin P,Opolon P, et al. Meta-analysis
of
interferon randomized trials in the treatment of viral bepatitis C: effects of dose and duration. Hepatology 1996 ; 24: 778-89.17.
Alberti A, Chemello L, Noventa F, Cavaletto L, De SalvoG. Therapy of hepatitis-C: re-treatment with alpha interferon.
Hepatology 1997 ; 26 (Suppl
l):
37 5-1425.18.
KeeffeEB,
HollingerFB,
Consensus Intereron Study Group. Therapy ofhepatitis C: consensus interferon trails. Hepatology 1997;26: Suppll;
l0lS-107S.19.
Davis GL, Esteban-Mur R, RustgiV,
Hoefs J, Gordon SC, Trepo C, et al. lnterferon alfa-2b aloneor
in
com-bination
with
ribavirinfor
the treatmentof
relapseof
chronic hepatitis C. N Engl J Med 1998; 339:1493-99.
20.
HeathcoteEIL,
Keeffe EB, Læe SS, Feinman SV, Tong MJ, Reddy KR, et al. Re-treatment of chronic hepatitis C with consensus interferon. Hepatology 1998;27: 1136-43.21.
Hoffmann RM, Berg T, Teuber G, LeifeldL,
Jung MC,Spengler U, et al. Interferon -antibodies and breakthrough
phenomenon during ribaviriMnterferon-alpha combination
therapy and interferon-alpha monotherapy
of
patientswith chronic hepatitis C. J Gastroenterol 1999; 37: 715'
23. 9.
l0
12.
l3