F U L L P A P E R
PPS
www.rsc.org/pps
Photophysicochemical consequences of bovine serum albumin
binding to non-transition metal phthalocyanine sulfonates
Abimbola Ogunsipe and Tebello Nyokong*
Department of Chemistry, Rhodes University, Grahamstown, 6140, South Africa
Received 25th October 2004, Accepted 9th May 2005
First published as an Advance Article on the web 19th May 2005
The interactions between bovine serum albumin (BSA) and sulfonated metallophthalocyanine (MPc) complexes of aluminum (AlPcSmix), zinc (ZnPcSmix), silicon (SiPcSmix), germanium (GePcSmix) and tin (SnPcSmix) are studied using fluorescence quenching of BSA by MPc complexes. The fluorescence quantum yields of the non-aggregated MPc complexes (AlPcSmix, GePcSmixand SiPcSmix) decreased in the presence of BSA, but increased for the aggregated ZnPcSmixand SnPcSmixcomplexes. The BSA: MPc conjugates were less stable than the corresponding MPc complexes. The quenching constants were much higher for the non-aggregated complexes. The aggregated nature of the
complexes also affected the rate constants (kF,kIC,kISC) for the deactivation of the excited singlet state.
Introduction
The chemistry of phthalocyanines (Pcs) has continued to gain popularity in the last few decades because of its richness and the extensive applications of these compounds. The robust chemistry of Pcs is exploited in their use as photocatalysts. The existence of a transient but relatively long-lived excited state is vital in photocatalysis. Phthalocyanine complexes of Al(III),
Si(IV), Ge(IV), Sn(IV) and Zn(II) are favoured as good candidates for consideration in photocatalysis and photosensitization, due to the fact that these metal ions are diamagnetic and are expected to give relatively long-lived excited states. The excited singlet states of MPcs are short-lived (a few nanoseconds) and so this state is usually not considered for photocatalysis. The excited triplet states on the other hand have lifetimes in the micro-to milli-second range, which is long enough for a variety of quenching processes to compete favourably with phosphores-cence. Sulfonated aluminium phthalocyanine derivatives have been used successfully as photosensitisers in photodynamic therapy (PDT)1–3and there is an aggressive on-going effort to
develop other non-transition metal phthalocyanine sulfonates for the same purpose.
After injection into the blood stream, a PDT drug is prone to encounter serum proteins, which are known to constitute a major component of blood hence influences drug distribution. Bovine serum albumin (BSA) has been used widely4–6
for binding studies because it has been extensively characterized. The investigation of binding of porphyrin-like drugs with albumin is of interest and much energy has been invested into it.7–10
Serum albumins display effective drug delivery functions,7,11 therefore a study
of their binding to porphyrin-based drugs is of significance. Since the intrinsic fluorescence of proteins is usually quenched upon binding to tetrapyrrolic compounds,12this spectroscopic
behaviour provides a means of studying the interaction between these compounds and BSA. Interactions between BSA and MPc complexes are known13,14
and interaction between sulfonated ZnPc complex and BSA resulted in conformational change of BSA.15Fluorescence from the tryptophan residues in BSA has
been reported to be quenched in the presence of phthalocyanine derivatives containing Si, Zn or Al central metals.13,15,16In this
work, we report the binding of sulfonated phthalocyanine com-plexes of Al, Si, Ge, Sn and Zn to BSA; and the photophysical and photochemical consequences of the binding. The effect of central metal on these properties is explored.
Experimental
Materials
Aluminium (AlPcSmix), silicon (SiPcSmix), germanium (GePcSmix), tin (SnPcSmix) and zinc (ZnPcSmix) phthalocyanine com-plexes containing differently substituted sulfophthalocyanines were synthesised from the ClAlPc, (OH)2SiPc, (OH)2GePc (OH)2SnPc and ZnPc, respectively, using fuming sulfuric acid (30% SO3) according to the reported procedures for AlPcSmix.
17,18
The starting materials, (OH)2SiPc, (OH)2GePc and (OH)2SnPc, were prepared, purified and characterised according to litera-ture procedures.19
ZnPc and ClAlPc complexes were obtained from Sigma-Aldrich. The MPcSmix complexes are known
1
to contain a mixture of the di-, tri-, and tetra-sulfonated derivatives with an average of three sulfonate groups per molecule (Fig. 1). High pressure liquid chromatography was employed to characterize the mixture. BSA was purchased from FLUKA and phosphate buffer solution (PBS, pH 7.4) was used as solvent.
Fig. 1 Molecular structure of MPcSmix. M=Al, Si, Zn, Ge or Sn.
Equipment
Absorption spectra were recorded on a Varian Cary 500 UV-Vis-NIR spectrophotometer. Fluorescence excitation and emission spectra were recorded on a Varian Eclipse spectrofluoremeter. Light intensities were measured with a POWER MAX5100 (Molelectron detector incorporated) power meter. Triplet life-times and yields were recorded on a flash photolysis system, with excitation pulses generated by a Nd:YAG laser (Quanta-Ray, 1.5 J/8 ns)-pumped dye laser (Lambda Physic FL 3002,
DOI
:10.1039/b416304d
Pyridine 1 dye in methanol). The analyzing beam source was from a Thermo Oriel xenon arc lamp, and photomultiplier tube was used as a detector. Signals were recorded with a two-channel digital real-time oscilloscope (Tektronix TDS 360). High pres-sure liquid chromatography (HPLC) was performed on a Quad-Gradient HPLC system, Agilent 1100 Series; fitted with an analytical column, l Bondapak C18 (390 × 3.00 mm) and connected to a variable wavelength UV-Vis detector (set atk=
365 nm). The mobile phase comprised of 50:50 methanol:water mixture, with a flow rate of 1 ml min−1and sample injection volume of 20ll.
Photodegradation methods
Photodegradation (Ud) quantum yield determinations were car-ried out using the experimental set-up and equations described in detail elsewhere.20–22
Typically, a 2 ml solution of MPcSmix (absorbance∼1.0 at the irradiation wavelength, the Q band) was irradiated using a General electric Quartz line lamp (300 W). A 600 nm glass cut off filter (Schott) and a water filter were used to filter off ultraviolet and infrared radiations respectively. An interference filter (Intor, 670 nm with a band width of 20 nm) was additionally placed in the light path before the sample. Light intensity used for the experiments was 4.82 ×
1016photons s−1cm−2.
Fluorescence quantum yields
Fluorescence quantum yields (UF) were determined by the comparative method23,24
whereF andFStdare the areas under the fluorescence curves of the MPcSmixand the reference, respectively.AandAStdare the absorbances of the sample and reference at the excitation wavelength respectively, andgandgStdare the refractive indices of solvents used for the sample (in water, g = 1.330) and
reference (in ether,g=1.352), respectively. Chlorophyllain ether (UF=0.32)25was employed as a reference. Both the sample and reference were excited at the same wavelength. The absorbance of the solutions at the excitation wavelength ranged between 0.04 and 0.05.
Triplet lifetimes and quantum yields
The deaerated solutions of the respective MPcSmix complexes were introduced into a 2 mm × 10 mm spectrophotometric cell and irradiated at the Q band with the laser system described above. Triplet quantum yields (UT) of the MPcSmix complexes were determined by the singlet depletion method.20
A comparative method20,26using zinc phthalocyanines
tetrasul-fonate (ZnPcS4) as standard was employed for the calculations, eqn. (2).
S andDAStdS are the changes in the singlet state absorbance of the MPcSmixcomplex and ZnPcS4, respectively.
esampleS ande Std
S are the singlet state extinction coefficients for the MPcSmix complex and ZnPcS4, respectively.UStdT is the triplet quantum yield for the standard ZnPcS4 in aqueous solution (UT=0.56).
27
Quantum yields of internal conversion (UIC) were obtained from eqn. (3), which assumes that only three processes (fluo-rescence, intersystem crossing and internal conversion), jointly deactivate the excited singlet state of an MPcSmixmolecule.
UIC=1−(UF+UT) (3)
MPcSmixbinding to bovine serum albumin
The binding of the MPcSmixcomplexes to BSA was studied by spectrofluorometry at room temperature (25◦C). A solution of
BSA in PBS 7.4 was titrated with increasing concentrations of the respective MPcSmix solution. BSA was excited at 280 nm and fluorescence recorded between 290 and 500 nm. The steady diminution in BSA fluorescence intensity with increase in MPcSmixconcentration was noted and used in the determination of the binding constants and the number of binding sites on BSA, according to eqn. (4).28–30
log
where F0 and F are the fluorescence intensities of BSA in the absence and presence of MPcSmix respectively; F∞, the fluorescence intensity of BSA saturated with MPcSmix;Kb, the binding constant;n, the number of binding sites on a BSA molecule; and [MPcSmix], the concentration of MPcSmix. Plots of log(F0−F)
(F−F∞)
against log[MPcSmix] would provide the values of
n(from slope) andKb(from the intercept).
For photophysical and photochemical studies, a molar ratio of 10 : 1 (BSA : MPcSmix, with [BSA]=7.5×10
−5M) was used. This ratio was used due to the abundance of proteins within most biological systems.
Determination of MPcSmixfluorescence lifetimes
MPcSmixfluorescence lifetimes were determined by monitoring the fluorescence intensity as a function of quencher con-centration. Here, two sets of data were obtained from: (i) quenching of BSA fluorescence by MPcSmixand (ii) quenching of MPcSmixfluorescence by BSA. For (i), the quenching of BSA by MPcSmix, the changes in BSA fluorescence intensity were related to MPcSmix concentrations by the Stern–Volmer relationship [eqn. (5)]:
BSAare the fluorescence intensities of BSA in the absence and presence of MPcSmixrespectively;kBSASV , the Stern– Volmer quenching constant; kQ, the bimolecular quenching constant; andsBSA
F , the fluorescence lifetime of BSA. s BSA determined from eqn. (6). For quenching of MPcSmixby BSA, similar eqns. (7) and (8) apply.
FMPc
MPcare the fluorescence intensities of MPcS mix in the absence and presence of BSA respectively;kMPc
SV , the Stern– Volmer quenching constant; kQ, the bimolecular quenching constant; andsMPc
F , the fluorescence lifetime of MPcSmix. In both cases,kQis assumed to be the same. UsingkMPcSV from the plot generated by eqn. (7), andkQfrom eqn. (6), we can obtain the value ofsMPc
F from eqn. (8).
(kIC) and intersystem crossing (kISC) were obtained using eqns. (9a)–(9c):
kF=
UF
sF
(9a)
kIC=
UIC
sF
(9b)
kISC=
UT
sF
(9c)
Results and discussion
Effects of BSA binding on photophysical and photochemical properties of MPcSmixcomplexes
Fig. 2 shows the ground state electronic absorption spectra of MPcSmixmixtures in PBS 7.4. The Soret bands (not shown in Fig. 2) were observed between 339 and 349 nm for the MPcSmix complexes. The spectra of these complexes was the same in unbuffered water, that is in the absence of ions present in buffered solutions. Thus the ions in the buffered solution did not affect the aggregation behaviour of these complexes. AlPcSmix, SiPcSmix and GePcSmix are monomeric in nature, while ZnPcSmix and SnPcSmix are aggregated. These observations were recently31,32 confirmed by the addition of a surfactant, Triton X-100 to these complexes. AlPcSmix, SiPcSmix and GePcSmixgave no change in spectra, confirming lack of aggregation whereas ZnPcSmixand SnPcSmixgave marked enhancements of Q band intensity and a corresponding collapse of the peak around 630 nm, which is believed to be due to dimeric species. Aggregation in sulfonated MPc complexes is characterised by the presence of two main bands (instead of one for monomeric species) in the visible region. The lower energy band is due to the monomer and the higher energy band due to the aggregated species. The spectra for SnPcSmix and ZnPcSmix show more monomerization at the
Fig. 2 Ground state electronic absorption spectra of MPcSmixmixtures in (a) PBS 7.4 (concentrations:∼5×10−6M for AlPcSmixand SiPcSmix; 1.2 ×10−5 M for ZnPcSmixand SnPcSmix) and (b) unbuffered water (concentrations:∼4×10−7M for AlPcSmixand SiPcSmix; 8
×10−7M for ZnPcSmixand SnPcSmix).
lower concentrations used in Fig. 2b. The presence of multiple peaks in the Q band region of the un-aggregated SiPcSmix(shown in Fig. 2) and GePcSmix complexes, could be an indication of the presence of differently substituted components in the mixture. The degree of aggregation increases with lipophilicity, hence the prevalence of the less sulfonated fractions in solution is expected to increase aggregation. The prevalence of less sulfonated fractions in the aggregated ZnPcSmix and SnPcSmix complexes and more sulfonated fractions in GePcSmix, AlPcSmix and SiPcSmix was confirmed by HPLC, Figs. 3 and 4. From HPLC, it is expected that the most highly sulfonated (most soluble) would be the first to be eluted from the chromatographic column, and so give the lowest retention time and that the less sulfonated fractions as expected, give the highest retention times. Thus the HPLC signals with the lowest retention times (∼1 min) are assigned to the tetrasulfonated fractions using tetrasulfozinc phthalocyanine (ZnTSPc) as reference. ZnPcSmixand SnPcSmix gave similar traces (Fig. 3), with appreciable signals in the relatively high retention time regions of the HPLC traces, whereas GePcSmixAlPcSmixand SiPcSmixshowed signals at low retention times. These observations confirm the prevalence of the highly sulfonated fractions in AlPcSmix, SiPcSmixand GePcSmix, hence less aggregation, while in ZnPcSmixand SnPcSmix, the less sulfonated fractions are prevalent, and are more aggregated.
Fig. 3 HPLC trace for GePcSmix.
Fig. 4 HPLC trace for ZnPcSmix.
Addition of BSA to solutions of the MPcSmixspecies (Fig. 5) gave the same results as Triton X-100 addition, further con-firming the aggregated nature of ZnPcSmix and SnPcSmix. The monomerization effect of serum albumin on photosensitizers is well documented.33–35For the un-aggregated AlPcS
Fig. 5 UV/Vis absorption spectral change observed on addition of BSA to ZnPcSmixin PBS 7.4 (concentrations: ZnPcSmix=1.63×10−6M and BSA=1.63×10−5M).
a small bathochromic shift (∼2 nm) in the Q band position was observed on addition BSA, Fig. 6. This implies that a complex is actually being formed between the photosensitizer and the biopolymer. The lack of broadening or splitting in the Q band, suggests that upon conjugation with BSA, AlPcSmixis still not aggregated. A similar subtle change in Q band position was observed for SiPcSmix, but not for GePcSmix, ZnPcSmixand SnPcSmix. However some BSA–MPc conjugates are known not to show shifts in the Q band compared to MPc complex alone.13
The electronic absorption and fluorescence spectra of BSA in PBS 7.4 are shown in Fig. 7. BSA shows a strong absorption around 280 nm (loge=4.65) which is due to tryptophan and tyrosine residues.4,36
Fig. 6 UV/Vis spectral changes observed on addition of BSA to AlPcSmixin PBS 7.4. (concentrations: AlPcSmix =7.5× 10−6 M and BSA=7.5×10−5M).
Fig. 8 shows the fluorescence emission spectra of AlPcSmix and ZnPcSmix (free and BSA-bound). For the un-aggregated species (AlPcSmix, SiPcSmixand GePcSmix), BSA binding resulted in reasonable fluorescence quenching which is manifested in de-crease in the fluorescence intensity (shown in Fig. 8 for AlPcSmix) and in fluorescence quantum yield (UF) values (Table 1). On the other hand, BSA binding resulted in increase in
Fig. 7 Ground state electronic absorption (i) and fluorescence emission (ii) spectra of BSA in PBS 7.4 (concentration=2×10−5M).
Fig. 8 Fluorescence emission spectra of MPcSmixin the presence and absence of BSA in PBS 7.4 (concentrations: AlPcSmixand ZnPcSmix= 3×10−6M; BSA=7.5×10−5M).
fluorescence intensity (Fig. 8) and inUFvalues for the aggregated ZnPcSmixand SnPcSmix, which further substantiates the fact that BSA monomerizes the aggregated species, hence the increase in fluorescence intensity rather than quenching. All the MPcSmix complexes quenched BSA as evident from the decrease in BSA fluorescence on titration with MPcSmix.
MPc photodegradation is believed to be a singlet oxygen-mediated process,37
thus its efficiency should be related to the rate of singlet oxygen generation, among other factors. Aggregation is also known31 to reduce photostability, and Table 1 shows
that aggregated complexes, ZnPcSmixand SnPcSmix, show larger degradation quantum yields. With the exception of GePcSmixfor which the value did not change (Table 1), MPcSmix photobleach-ing quantum yield (Ud) increased in the presence of BSA. We attribute this increase to the formation of active oxidative albumin species which could additionally photodegrade the MPcSmixcomplex.
Table 1 Photophysical and photochemical parameters of MPcSmixcomplexes in PBS 7.4. Values in brackets are in the presence of 10 molar proportions of BSA. [BSA]=7.5×10−5M
kQ/nm kF/nm UF sT/ls UT UIC 105/Ud UD
UOP
USnPcS OP
AlPcSmix 674 677 0.44V 2.93 0.44 0.12 0.40 0.42
(677) (683) (0.34) (0.59) (0.59)
SiPcSmix 678 682 0.34 2.90 0.45 0.21 0.71 0.49
(679) (682) (0.86) (0.30) (0.40)
GePcSmix 680 686 0.30 2.76 0.68 0.03 0.45 0.68
(680) (686) (0.24) (0.44) (0.57)
ZnPcSmix 673 677 0.16 2.95 0.53 0.31 3.65 0.45
(676) (681) (0.20) (17.1) (0.37)
SnPcSmix 688 699 0.05 2.52 0.59 0.36 1.59 0.42
Photosensitized oxidation of BSA
MPcSmixphotodegradation within the BSA–MPcSmixconjugate, was accompanied by substantial increase in intensity in the UV region of the spectrum (Fig. 9), implying that BSA photodegrades in the presence of singlet oxygen, generating products which absorb at about the same region as the original BSA absorbing components, hence the increase in the intensity in the UV region. Thus, BSA competes with the MPcSmixfor singlet oxygen. It is difficult to identify exactly which species is responsible for the increase in intensity in the UV region. Due to the relatively large concentration of BSA used in the experiments and the high rate constants for reaction of singlet oxygen with some amino acid side chains,38
it is logical to think that BSA would be a target for singlet oxygen. A series of endoperoxides and hydroperoxides have been indicated as products of amino acid side chain oxidations in proteins.38
In order to quantify the efficiency of BSA photooxidation, the increase in intensity in the UV region (at 305 or 330 nm) was used, and related to the photooxidation quantum yield (FOP), by the usual eqn. (10) employed for photodegradation studies:
UOP=
(At−A0)V/e
Iabst
(10)
whereAt andA0are the absorbances at 305 or 300 nm (both wavelength gave the same results) after irradiation fort s and before irradiation respectively; V, the reaction volume;e, the molar extinction coefficient of oxidation product; andIabs, the intensity of absorbed light in photon mol s−1.
Fig. 9 Spectral changes observed on photolysis of AlPcSmix in the presence of BSA. pH = 7.4. Irradiation wavelength at AlPcSmix complexes Q band maximum (674 nm). Light intensity used was 4.82× 1016 photons s−1 cm−1 (concentrations: AlPcSmix =6 ×10−6 M and BSA=6×10−5M). Irradiation time interval=1 min.
Fig. 10 Determination of MPcSmix–BSA binding constant in PBS 7.4 (BSA concentration=2×10−5M).
Since the identity of the new species absorbing in the UV region is not known (hence the extinction coefficient is not known), eqn. (10) may not be used directly. We employed a
relative method described below. The rate of formation (R) of the oxidation products is given by eqn. (11):
R= (At
−A0)V/e
t (11)
Combining eqns. (10) and (11) gives eqn. (12a):
UOP=
R Iabs
(12a)
For another photosensitizer, we can write a similar eqn. (12b):
U′ OP=
R′
I′ abs
(12b)
Taking the ratio of eqn. (12b) over (12a), gives eqn. (13):
U′ OP
UOP
= R
′I abs
RI′ abs
(13)
Values of BSA photooxidation quantum yields were obtained relative to that in SnPcSmix, which is given an arbitrary value of 1.00. The relative values of FOP for all MPcSmix complexes studied are listed in Table 1. Higher values were observed for Al, Ge, and Sn complexes.
Interaction of MPcSmixwith BSA
The binding constants (Kb) obtained for MPcSmix binding to BSA, together with the binding stoichiometry of the complex formed were obtained using eqn. (4) (Fig. 10, for SiPcSmix); and the results are presented in Table 2 for [BSA]=2.0×10−5M and [MPc] ranging from 1.58×10−6to 1.58×10−5M. In addition for the aggregated SnPcSmixand ZnPcSmixlower concentrations ([BSA]=3.3×10−7M and [MPc]=1.67×10−7to 8.0×10−7M) were employed. The binding contant values were the same when unbuffered water was employed. Again showing that the salts in the buffered solutions do not affect the aggregation behaviour of these complexes. The values for the unaggregated Ge, Si and Al complexes are typical of MPc–albumin interactions in aqueous solutions,28and did not change on dilution to low concentrations
(∼10−7M) similar to those employed for SnPcS
mixand ZnPcSmix complexes (for binding and quenching constants shown in brackets, Table 2). The highest value of Kb was obtained for AlPcSmixwhich is particularly monomeric, followed by GePcSmix and SiPcSmix. The aggregated SnPcSmixand ZnPcSmixcomplexes gave the lowestKbvalues, but these values increased considerably (∼three-fold) at low concentrations as shown in Table 2. This shows that aggregation plays an important role in the binding. The involvement of a MPc dimer in BSA binding is indirect,
via dissociation into monomers. Consequently, Kb values for dimeric species are expected to depend largely on, and be limited by the inherent dimer dissociation constant. The variation of
Kb with the nature of the complex in Table 2 could also be a reflection of the relative affinity of BSA for the respective MPcSmix species. Since it is known that serum albumins have high affinities for negatively charged molecules,39the observed
larger values ofKbfor AlPcSmix, SiPcSmixand GePcSmixis a result of the predominance of highly sulfonated derivatives as shown by HPLC (Fig. 3). The number of binding sites on BSA (n) obtained from the experiments is∼1 (Table 2), which suggests a 1 : 1 stoichiometry for all the MPcSmix–BSA conjugates.
Fluorescence quenching analysis
Table 2 Quenching and binding data for MPcSmixcomplexes in PBS 7.4. Unless otherwise stated: [BSA]=2.0×10−5M and [MPc] ranges from 1.58×10−6to 1.58
×10−5M. Values in brackets are for [BSA]
=3.3×10−7M and [MPc] ranging from 1.67
×10−7to 8.0
×10−7M
Kb/10−6M−1 n kBSA SV/10
−4M−1 kMPc SV /10
−3M−1 k
Qa/10−12M−1s−1 sF/ns kF/10−7s−1 kISC/10−7s−1 kIC/10−7s−1
AlPcSmix 17.21 1.4 11.45 58.43 11.40 5.13 8.58 8.58 2.34
SiPcSmix 1.29 1.3 6.90 34.36 6.90 4.98 6.83 9.04 4.22
GePcSmix 0.81 1.1 4.54 18.48 4.54 4.07 7.37 16.71 0.74
ZnPcSmix 0.10 (0.34) 1.0 7.36 (6.72) 7.36 (6.72) 2.927 5.52 18.28 10.69
SnPcSmix 0.08 (0.21) 1.0 1.98 (3.19) 1.98 (3.19)
aValues determined from BSA quenching by MPcSmix.
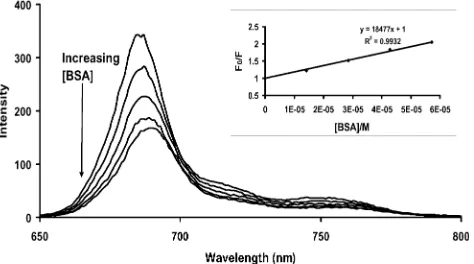
complex) quenching. The Stern–Volmer analysis of fluorescence data was used to discern the actual quenching mechanism. Figs. 11 and 12 show the quenching of BSA and SiPcSmixin PBS 7.4, respectively by each other. The slopes of the plots shown as inserts gave kBSA
SV andk MPc
SV , respectively. Fig. 12 shows the quenching behaviour of MPcSmix typical of the un-aggregated complexes (Al, Si and Ge complexes). As discussed above for the aggregated Zn and Sn complexes, there was an increase in fluorescence intensity (rather than quenching) upon addition of BSA, hence thekMPc
SV values could not be determined, whereas all MPcSmixcomplexes could quench BSA withkBSASV values shown in Table 2.
Fig. 11 Fluorescence emission spectral changes for BSA on addition of increasing concentrations of SiPcSmix. Inset: Stern–Volmer plot for SiPcSmix quenching of BSA. (Concentrations: BSA = 2× 10−5 M; SiPcSmixvaries from 1.67×10−6M to 1.5
×10−5M.)
Fig. 12 Fluorescence emission spectral changes of GePcSmixon addi-tion of increasing concentraaddi-tions of BSA. Inset: Stern–Volmer plot for BSA quenching of GePcSmix. (Concentrations: GePcSmix=4×10−6M; BSA varies from 1.4×10−5M to 5.7×10−5M.)
Values ofkQ were determined from eqn. (6) for quenching of BSA by MPc and are listed in Table 2. These values are of the order of 1012 M−1 s−1, Table 2. The acceptable value of kQ for dynamic quenching according to Einstein– Smoluchowski approximation40 at room temperature is of the
order of 1010M−1s−1. The high values ofk
Qobserved in Table 2, suggests that the fluorescence quenching of BSA by MPcSmixis not initiated by dynamic quenching, but by static quenching, and the kBSA
SV andk MPc
SV values in eqns. (6) and (8) are due to
static quenching. These static quenching constants (kBSA SV and
kMPc
SV ; Table 2) for BSA and the MPcSmixcomplexes reveal the relative degrees of interaction between BSA and the MPcSmix complexes. Both constants are highest for AlPcSmix. From these values, it could be inferred that AlPcSmixpossesses the highest degree of interaction with BSA while SnPcSmixhas the lowest. The observed lower values ofkBSA
SV for ZnPcSmix and SnPcSmix are not unconnected with their aggregation, and the relatively higher values for the former could imply the lower aggregation tendency of ZnPcSmix compared to SnPcSmix. The high kBSASV andkMPc
SV values for AlPcSmixcompared to the other monomeric complexes (SiPcSmixand GePcSmix), suggests that the latter two have lower affinities for BSA than the former.
Fluorescence lifetimes of the MPcSmix complexes (sMPcF , Ta-ble 2) were calculated fromkMPc
SV andkQvalues using eqn. (8), and the values are quite close to the typical MPc fluorescence lifetimes.19,28The procedure described here for calculation ofsMPc F could however not be employed in the case of the aggregated ZnPcSmixand SnPcSmix, whose fluorescence intensities increased on BSA addition, hence thekBSA
SV values could not be calculated. Among the three: AlPcSmix, SiPcSmixand GePcSmixwhich are monomeric, fluorescence lifetime decreases as the atomic mass of central metal ion increases, most probably due to an enhanced intersystem crossing. The literature value for ZnPcSmix
27
is notably less than those calculated for AlPcSmix, SiPcSmix and GePcSmix(Table 2) which is attributable to the aggregated nature of the complex.
Kinetic data
The rate constants for the excited singlet state deactivation processes (kF,kICandkISC) were calculated [using eqn. (9a)–(9c)] and are listed in Table 2. The values could not be obtained for SnPcSmixwhosesFvalue was not available; but among the three unaggregated species, AlPcSmixshowed the highest values ofkF, while GePcSmix showed the lowest. Comparison of ZnPcSmix with the rest is not appropriate due to its aggregated nature. The values ofkISC, as expected, increased with the mass of the central metal ion, which is a manifestation of the heavy atom effect that promotes intersystem crossing. As expected, ZnPcSmix gave the highest value ofkIC, which is again readily attributed to aggregation. GePcSmixgave the lowest value ofkIC, indicating that this species is perhaps the most photoactive in the list. The superior photoactivity of this species is also shown in Table 1, where its values ofUF,UTandUDare consistently high.
Conclusions
properties of the mixtures were altered in the presence of BSA. This work also provides an approximate but simple route to the determination of fluorescence lifetimes of MPc complexes, using steady-state measurements.
Acknowledgements
This work has been supported by the National Research Foundation of South Africa as well as Rhodes University. AO thanks ICSC world laboratory and Mellon foundation for scholarships.
References
1 N. A. Kuznetsova, N. S. Gretsova, V. M. Derkacheva, O. L. Kaliya and E. A. Luk’yanets, Sulfonated phthalocyanines: aggregation and singlet oxygen quantum yield in aqueous solutions,J. Porphyrins Phthalocyanines, 2003,7, 147–154.
2 R. Edrei, V. Gottfried, J. E. Van Lier and S. Kimel, Sulfonated phthalocyanines: photophysical properties,in vitrocell uptake and structure-activity relationships,J. Porphyrins Phthalocyanines, 1998,
2, 191–199.
3 J. D. Spikes, Phtalocyanines as photosensitizers in biological systems and for the photodynamic therapy of tumors,Photochem. Photobiol., 1986,43, 691–699.
4 D. Silva, C. M. Cortez and S. R. W. Louro, Quenching of the intrinsic fluorescence of bovine serum albumin by chlorpromazine and hemin,
Braz. J. Med. Biol. Res., 2004,37, 963–968.
5 E. L. Gelamo and M. Tabak, Spectroscopic studies on the interaction of bovine (BSA) and human (HSA) serum albumins with ionic surfactants,Spectrochim. Acta, 2000,56, 2255–2271.
6 G. Ming, J. W. Zou, P. G. Yi, Z. C. Shang, G. X. Hu and Q. S. Yu, Binding interaction of gatifloxacin with bovine serum albumin,Anal. Sci., 2004,20, 465–470.
7 A. K. Bordbar, A. Eslami and S. Tangestaninejad, Spectral investi-gations of the solution properties of 5,10,15,20-tetrakis(4-N -benzyl-pyridyl)porphyrin (TBzPyP) and its interaction with human serum albumin (HSA),J. Porphyrins Phthalocyanines, 2002,6, 225–232. 8 A. K. Bordbar, S. Tangestaninejad and A. Eslami, Optical absorption
spectroscopy study on the interaction of 5,10,15,20-tetrakis(4-N -benzyl-pyridyl)porphyrin with human serum albumin,J. Biochem., Mol. Biol. Biophys., 2001,5, 143–152.
9 T. T. Tominaga, V. E. Yushmanov, I. E. Borissevitch, H. Imasato and M. Tabak, Aggregation phenomena in the complexes of iron tetraphenylporphine sulfonate with bovine serum albumin,J. Inorg. Biochem., 1997,65, 235–244.
10 I. E. Borissevitch, T. T. Tominaga, H. Imasoto and M. Tabak, Fluorescence and optical absorption study of interaction of two water soluble porphyrins with bovine serum albumin. The role of albumin and porphyrin aggregation,J. Lumin., 1996,69, 65–76.
11 D. C. Carter and J. X. Ho, Structure of serum albumin,Adv. Protein Chem., 1994,45, 153–203.
12 B. M. Aveline, T. Hasan and R. W. Redmond, The effects of aggregation, protein binding, and cellular incorporation on the photophysical properties of benzoporphyrin derivative monoacid ring A (BPDMA),J. Photochem. Photobiol. B: Biol., 1995,30, 161– 169.
13 J.-D. Huang, S. Wang, P.-C. Lo, W.-P. Fong, W.-H. Ko and D. K. P. Ng, Halogenated silicon(IV) phthalocyanines with axial poly(ethylene glycol) chains. Synthesis, spectroscopy properties, com-plexation with bovine albumin andin vitrophotodynamic activities,
New J. Chem., 2004,28, 348–354.
14 X.-L. Chen, D.-H. Li, Q.-Z. Zhu, H.-H. Yang, H. Zheng, Z.-H. Wang and J.-G. Xu, Determination of proteins at nanogram levels by resonance light-scattering technique with tetra-substituted sulfonated aluminium phthalocyanine,Talanta, 2001,53, 1205–1210. 15 B. Xie, J. Huang, J. Xue, N. Chen and J. Huang, Combining interaction of amphiphilic phthalocyanine zinc with bovine serum albumin,Fenxi Huaxue, 2003,31, 1159–1163.
16 I. Rosenthal, V. Y. Shafirovich, N. E. Geacintov, E. Ben Hur and B. Horowitz, The photochemical properties of fluoroaluminum phthalocyanine,Photochem. Photobiol., 1994,60, 215–220. 17 K. Ozoemena, N. Kuznetsova and T. Nyokong, Comparative
photosensitised transformation of polychlorophenols with different sulfonated metallophthalocyanine complexes in aqueous medium,
J. Mol. Cat. A: Chem., 2001,176, 29–40.
18 M. Ambroz, A. Beeby, A. J. McRobert, M. S. C. Simpson, R. K. Svensen and D. Phillips, Preparative, analytical and fluorescence
spectroscopic studies of sulfonated aluminium phthalocyanine pho-tosensitizers,J. Photochem. Photobiol. B: Biol., 1991,9, 87–95. 19 C. W. Dirk, T. Inabe, K. F. Schoch, Jr. and T. J. Marks, Cofacial
assembly of partially oxidized metallamacrocycles as an approach to controlling lattice architecture in low-dimensional molecular solids. Chemical and architectural properties of the “face-to-face” polymers [M(phthalocyaninato)O]n, where M=Si, Ge, and Sn,J. Am. Chem. Soc., 1983,105, 1539–1550.
20 A. Ogunsipe, J. Y. Chen and T. Nyokong, Photophysical and photochemical studies of zinc(II) phthalocyanine derivatives-effects
of substituents and solvents,New J. Chem., 2004,7, 822–827. 21 I. Seotsanyana-Mokhosi, N. Kuznetsova and T. Nyokong,
Photo-chemical studies of tetra-2,3-pyridinoporphyrazines,J. Photochem. Photobiol., 2001,140, 215–222.
22 A. Ogunsipe and T. Nyokong, Effects of substituents and solvents on the photochemical properties of zinc phthalocyanine complexes and their protonated derivatives,J. Mol. Struct., 2004,689, 89–97. 23 S. Fery-Forgues and D. Lavabre, Are fluorescence quantum yields
so tricky to measure? A demonstration using familiar stationery products,J. Chem. Educ., 1999,76, 1260–1264.
24 J. Fu, X. Y. Li, D. K. P. Ng and C. Wu, Encapsulation of Phthalo-cyanines in Biodegradable Poly(sebacic anhydride) Nanoparticles,
Langmuir, 2002,18, 3843–3847.
25 A. Montalban, H. Meunier, R. Ostler, A. Barrett, B. Hoffman and G. Rumbles, Photoperoxidation of a Diamino Zinc Porphyrazine to theseco-Zinc Porphyrazine: Suicide or Murder,J. Phys. Chem. A, 1999,103, 4352–4358.
26 J. H. Brannon and D. Magde, Picosecond laser photophysics, group 3A phthalocyanines,J. Am. Chem. Soc., 1980,102, 62–65.
27 A. Harriman and M. C. Richoux, Attempted photoreduction of hydrogen using sulfophthalocyanines as chromophores for three-component systems,J. Chem. Soc., Faraday Trans. 2, 1980,76, 1618– 1626.
28 S. M. T. Nunes, F. S. Sguilla and A. C. Tedesco, Photophysical studies of zinc phthalocyanine and chloroaluminum phthalocyanine incorporated into liposome in the presence of additives,Braz. J. Med. Biol. Res., 2004,37, 273–284.
29 S. Lehrer and G. D. Fashman, The fluorescence of lysozyme and lysozyme substrate complexes, Biochem. Biophys. Res. Commun., 1966,23, 133–138.
30 D. M. Chipman, V. Grisaro and N. Shanon, The binding of oligosac-charides containing N-acetylglucosamine and N-acetylmaramic acid to lysozyme,J. Biol. Chem., 1967,242, 4388–4394.
31 A. Ogunsipe and T. Nyokong, Effects of central metal on the photophysical and photochemical properties of non-transition metal sulfophthalocyanines,J. Porphyrins Phthalocyanines, 2005,9, 121– 129.
32 A. Ogunsipe and T. Nyokong, Photophysical and photochemical studies of sulfonated non-transition metal phthalocyanines in aque-ous and non-aqueaque-ous media,J. Photochem. Photobiol. A: Chem., DOI: 10.1016/J.Photochem. 2005.03.001.
33 K. Tabata, K. Fukushima, K. Oda and I. Okura, Selective aggrega-tion of zinc phthalocyanine in the skin,J. Porphyrins Phthalocyanines, 2000,4, 278–284.
34 D. J. Ball, S. R. Wood, D. I. Vernon, J. Griffiths, T. M. Dubbleman and S. B. Brown, The characteristics of three substituted zinc phthalocyanines of differing charge for use in photodynamic therapy. A comparative study of their aggregation and photosensitizing ability in relation tomTHPC and polyhaematoporphyrin,J. Photochem. Photobiol. B: Biol., 1998,45, 28–35.
35 G. Valduga, E. Reddi and G. Jori, Spectroscopic studies on zinc(II) phthalocyanine in homogeneous and microheterogeneous systems,
J. Inorg. Biochem., 1987,29, 59–65.
36 V. Levi and F. L. G. Flecha, Labelling of proteins with fluorescent probes, photophysical characterization of dansylated bovine serum albumin,Biochem. Mol. Biol. Educ., 2003,31, 333–336.
37 G. Schnurpfeil, A. K. Sobbi, W. Spiller, H. Kliesch and D. W ¨ohrle, Photo-oxidative stability and its correlation with semi-empirical MO calculations of various tetraazaporphyrin derivatives in solution,
J. Porphyrins Phthalocyanines, 1997,1, 159–167.
38 M. J. Davies, Reactive species formed on proteins exposed to singlet oxygen,Photochem. Photobiol. Sci., 2004,3, 17–25.
39 A. I. Filyasova, I. A. Kudelina and A. V. Feofanov, A spectroscopic study of the interaction of tetrasulfonated aluminum phthalocya-nines with human serum albumin,J. Mol. Struct., 2001,565–566, 173–176.