PHYLOGEOGRAPHY AND SPECIES LIMITS IN THE
PAPUAN WHITE SNAKE (
Micropechis ikaheka
)
(REPTILIA: SERPENTES: ELAPIDAE)
KELIOPAS KREY
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT LETTER
I hereby declare that dissertation with title: Phylogeography and species limits in the Papuan White Snake (Micropechis ikaheka) (Reptilia: Serpentes:
Elapidae) is the original result of my own research supervised by supervisory committee and has never been submitted in any form at any institution before. All information from other authors cited here are mentioned in the text and listed in the reference at the end part of the dissertation.
Bogor, January 2015
Keliopas Krey
SUMMARY
KELIOPAS KREY. Phylogeography and Species Limits in the Papuan White Snake (Micropechis ikaheka) (Reptilia: Serpentes: Elapidae). Supervised by
BAMBANG SURYOBROTO, ACHMAD FARAJALLAH, and DEDE SETIADI
Papua white snake, Micropechis ikaheka, has been distingushed into two
subspecies M.i. ikaheka and M.i. fasciatus. However, due to lack of data on their
morphology, distribution and ecology, both subspecies often ignored in scintific discussions and refers only byas , Micropechis ikaheka. This study was designed
to document the differences between M.i. ikaheka and M.i fasciatus by using
scales characteristics and reproductive organ or hemipenes. The study also examine, zoogeography, habitat, diet and cannibalism of both subspecies to better understanding the the ecology of one of the most venomous snakes in the world.
This study consists of three topics with method and also different in the size samples, respectively. The first study is the histology and microtopography of the skin were studied using paraffin method and Scanning Electron Microscope (SEM). The second is the hemipenis were examined by following Myers and Cadle (2003), and Zaher and Prudente (2003). The third is the abdominal surgery was carried out to document diet type and the investigate phenomenon of cannibalism in the snake. Specimens for this study came from the collection from Museum Zoologicum Bogoriense (MZB) and Universitas Negeri Papua’s Laboratory (LZU).
The research result also indicated that the markers of skin and hemipenis described the same structure and microornamentation in all M. ikaheka groups
being compared. Based specimens examined morphological descriptions do not s support the further differentiation in Micropechis. In short, two markers that used
in this study could not sufficiently indicate the differences in the level of species and subspecies of M. ikaheka. The study, however, were able to delineate
zoogeography and skin color patterns, which fall into four groups: M. i. ikaheka; M. i. fasciatus. M. i. ssp. “intermediate”; and M. i. ssp “black”. This study also
revealed that color variations did not overlap in their geographic distribution. It is therefore, it suggested that environmental factors may contribute to the skin color variations. It also assumed that habitat discontinuous and biogeography factors may also cause the loss of the connection between the original populations (vicarians) or follow colonization events (dispersal).
The examined of 22 stomach contents of M. ikaheka shows this species is
carnivorous, and prey on vertebrates such as amphibians, fish, reptiles, and Mammals. These snakes are opportunistic predators since they prey on all that can be captured, but mainly reptiles (61.5%), followed by mammals and fish (both 15.4%), and amphibians (7.7%). Although categorized as terrestrial animal, the diet study showed that M. ikaheka snakes often go into the water to hunt for fish
(the sample from Yapen island), including eel (the sample from Aru island). Based on prey items indentified, there is evidence that M. ikaheka is cannibal. At
cannibalism was due to competition between populations and means to control population.
RINGKASAN
KELIOPAS KREY. Filogeografi dan Batasan Spesies dalam Ular Putih Papua (Micropechis ikaheka) (Reptil: Serpentes: Elapidae). Dibimbing oleh BAMBANG
SURYOBROTO, ACHMAD FARAJALLAH, dan DEDE SETIADI
Ular putih Papua, Micropechis ikaheka, telah diperkenalkan menjadi dua
subspecies M.i. ikaheka dan M.i. fasciatus. Namun demikian, kedua subspesies
tersebut diabaikan dalam pembahasan-pembahasan ilmiah karena keterbatasan data morfologi, distribusi dan ekologi mereka sehingga hanya merujuk pada satu nama saja, Micropechis ikaheka. Penelitian ini mencoba menguraikan dan
membuktikan perbedaan intraspesies M. ikaheka lebih jauh dengan pendekatan
karakteristik kulit sisik dan alat reproduksi hemipenis. Selain itu, zoogeografi, habitat, diet dan kanibalisme juga dipelajari guna menambah pengetahuan ekologi tentang salah satu ular berbisa tinggi di dunia ini.
Penelitian ini terdiri dari tiga topik dengan metode masing-masing dan juga ukuran sampel yang berbeda. Penelitian pertama terkait Histologi dan microtopografi kulit, dipelajari menggunakan metode parafin dan Scaning Electron Microskop (SEM). Yang kedua adalah kajian hemipenis menggunakan
metode preparasi mengikuti Myers dan Cadle (2003), dan Zaher dan Prudente (2003). Yang terakhir adalah penelitian tentang diet dan fenomena kanibalisme berdasarkan isi perut. Seluruh sample M. ikaheka yang diamati dan diukur dalam
penelitian ini bersumber dari koleksi spesimen yang telah diformalin dan disimpan di MZB dan LZU.
Hasil penelitian menunjukkan bahwa keduanya, penanda kulit maupun hemipenis, menggambarkan struktur dan mikroornamentasi yang sama pada semua kelompok M. ikaheka yang diperbandingkan. Seluruh data dan deskripsi
morfologi yang diperoleh tidak menunjang diferensiasi takson Micropechis yang
telah diperkenalkan menjadi dua subspesies tersebut. Prinsipnya bahwa kedua penanda yang telah digunakan dalam penelitian ini tidak dapat membuktikan perbedaan level spesies maupun subspesies dalam M. ikaheka di Papua. Penelitian
ini, bagaimanapun, mampu menggambarkan zoogeografi dan pola warna kulit yang terbagi mejadi empat kelompok: M.i. ikaheka; M.i. fasciatus. M.i. ssp.
"Intermediate" ; dan M.i. ssp " Hitam". Penelitian ini juga mengungkapkan bahwa
variasi warna tidak tumpang tindih dalam distribusi geografis mereka. Oleh karena itu, meyakinkan bahwa faktor lingkungan dapat berkontribusi pada variasi warna kulit. Hal ini juga diasumsikan bahwa faktor diskontinyu habitat dan biogeografi juga dapat menyebabkan hilangnya hubungan antara populasi asli (vicarians) atau mengikuti kejadian kolonisasi (dispersal).
Hasil bedah 22 perut spesimen M. ikaheka menunjukkan bahwa ular
beracun ini merupakan karnifor sejati terhadap vertebrata amphibi, ikan, reptil, dan mamalia. Ular ini adalah pemangsa yang oportunistik, mereka memangsa semua yang dapat ditangkap, namun utamanya reptil (61.5%), diikuti oleh mamalia dan ikan (masing-masing 15.4%), dan amphibi (7.7%). Walaupun teresterial, petunjuk terbaru diperoleh dalam penelitian ini bahwa M. ikaheka
termasuk sidat (sampel dari pulau Aru). Berdasarkan identifikasi item mangsa, terdapat petunjuk bahwa M. ikaheka adalah kanibal. Pada saat ini, masih ada
catatan tentang alasan kanibalisme, tetapi diasumsikan bahwa kanibalisme ini karena persaingan antara populasi dan berarti untuk mengendalikan populasi.
Kata kunci: variasi geografis, ular putih, papua
Copyright©2015, Bogor Agricultural University
Copyright are protected by law
Prohibited to cite all or a part of this dissertation without referring to and mentioning the source. Citation permits to the purposes of education, research, scientific paper, report, or critism writing only; and it does not defame the name and honor of Bogor Agricultural University.
PHYLOGEOGRAPHY AND SPECIES LIMITS
IN THE PAPUAN WHITE SNAKE (
Micropechis ikaheka
)
(REPTILIA: SERPENTES: ELAPIDAE)
KELIOPAS KREY
Dissertation
submitted in partial fulfillment of the requirements for a Doctoral Degree in Animal Biosience Major in Graduate School of Bogor Agricultural University
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
Examiners in the close examination: Dr. Ir. Mirza D. Kusrini Yance de Fretes, MES, PhD
Title : Phylogeography and Species Limits in the Papuan White Snake (Micropechis ikaheka) (Reptilia: Serpentes:
Elapidae)
Name : Keliopas Krey
Register Number : G362110051
Endorsed by, Supervisory committee:
Dr. Bambang Suryobroto Chairman
Dr. Ir. Achmad Farajallah, M.Si Prof. Dr. Ir. Dede Setiadi, MS
Member Member
Chair of Major in Animal Biosciences Dean of Graduate School
Dr. Ir. RR Dyah Perwitasari Dr. Ir. Dahrul Syah, M.Sc.Agr.
FOREWORD
This dissertation was developed from three topics articles that have submitted to scientific journals. The first topic entitled: Skin histology and microtopography of Papuan White Snake (Micropechis ikaheka) in relation to
their zoogeographical distribution, has been published on March 2013 in Hayati J Biosci 20(1): 7-14). The second topic entitled: The hemipenis of Micropechis ikaheka (Reptilia: Squamata: Elapidae) from Papua, Indonesia, with comments on
the phylogeography of the species, has been received in journal of herpetology. The third topic entitled: Micropechis ikaheka (Elapidae) in Papua, Indonesia: a
study of diet and cannibalism has been accepted in Herpetology Notes.
On July 15, 2011 the author enrolled into doctoral program student at Institut Pertanian Bogor (IPB) Graduate School, Majoring in Animal Bioscience, with financial support from BPPS (Beasiswa Pendidikan Pascasarjana).
I would express my sincere thank to Rector of IPB and the Dean of Graduate School; in particular to Dr. Bambang Suryobroto, Dr. Ir. Achmad Farajallah, M.Si, and Prof. Dr. Ir. Dede Setiadi, MS, who was served as Supervisory Committee.
This study was partially supported by the Ministry of National Education of Indonesia, Government of Papua and West Papua Province, and University of Papua. I thank all the supports which allow me to finish this study.
During my study, I have the most wonderful colleagues, associates, friends, and family, which. Without their supports and more contributions I may never finish this dissertation. I would take this opportunity sincerely thank:
1. Perhimpunan Herpetologi Indonesia (PHI), especially Dr. Mirza Kusrini, who invited in series of workshop, seminar and of course writing and the publishing of short news of Papuan snake in Warta Herpetofauna.
2. Colleague at the Conservation International Indonesia (CI), BP-Tangguh, PT. Ekologika Consultant, and PT. Freeport Indonesia for some accommodation and traveling during the field work in Papua.
3. Burhan Tjaturadi, and David Price for the willingness to provide some specimens of M. ikaheka.
4. Irvan Sidik who allowed me to access to the MZB collections and Dr. Sri Hartini for the assistance of the hemipenial line drawing on camera lucida at MZB Cibinong.
5. Tini Wahyuni who helps me to prepare of all of the equipment of the skin histology and hemipenial works in Laboratory of Micro Technique IPB. 6. Dr. Daisy Wowor and Ir. Ristiyanti Marwoto, M.Si, the researchers from
MZB who observed, photographed and documented the Micropechis-Anguilla interaction on Aru Island.
7. Prof. Eric Smith and Dr. Michael Harvey, who help in discussions and assistances for preparation methods on the snake hemipenial.
8. Mark O’Shea, who help in discussion and also send some of snake article 9. Devi Manuhua and Tri Setiadi who make the distribution map of M. ikaheka.
I am in debt for my family: Matias Marisan and her families, Martinus Krey, Markus Krey, Arnol Krey, Frans Krey, Hendrikus Krey, Evert Burwos, Noak Awi, Erwin Yenusi, and Ariel Taime. My dear wife, Valentina Mayabubun, and my three boy Yores, Mambri and Fanar are always together in Bogor.
Bogor, January 2015
TABLE OF CONTENTS
Structure of Snake HemipenialMATERIALS AND METHODS Field work
Skin Histology Preparation Hemipenial Preparation Diet Analysis
SKIN HISTOLOGY AND MICROTOPOGRAPHY OF PAPUAN WHITE SNAKE (Micropechis ikaheka) IN RELATION TO THEIR
ZOOGEOGRAPHICAL DISTRIBUTION Introduction
Materials and methods Results
Discussion
THE HEMIPENIS OF Micropechis ikaheka (REPTILIA:
SQUAMATA: ELAPIDAE) FROM PAPUA, INDONESIA, WITH COMMENTS ON THE PHYLOGEOGRAPHY OF THE SPECIES
Introduction
Materials and Methods Results
Discussion
Micropechis ikaheka (ELAPIDAE) IN PAPUA, INDONESIA: A
VII
VIII
GENERAL DISCUSSION
The Limitations of this Research and Future Study
Geographic Variation and Taxonomy Significant in M. ikaheka
Skin melanin and Sex Dimorphism of M. ikaheka
Diet and cannibalism phenomena with comment to conservation The contributions of snakes for conservation, and the local wisdom
GENERAL CONCLUSIONS AND SUGGESTION
Selected character variation in M. ikaheka
Identifiable prey items from gastrointestinal tract (GI) of M. ikaheka
Identified prey items in male, female and juvinile of M. ikaheka
Comparison prey items identified from gastrointestinal tract of M. ikaheka
11
Generalized epidermis of a squamate reptile
Diagram of the cross section of the snake integument
Sulcate (left) and asulcate (right) morphology illustrate of snakes hemipenis
Map of distribution of M. ikaheka in Papua, showing specific
zoogeographical
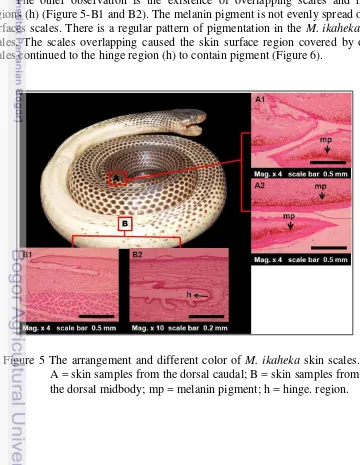
The arrangement and different color of M. ikaheka skin scales
The scales overlapping and pigmentation pattern of the dorsal caudal region
Photomicrographs of the dorsal midbody skin
8
SEM images illustrating the microstructure of the oberhautchen on the
dorsal outer scale surface of the mid-body (A,B) and caudal (C,D) scales SEM images illustrating the microtopography of the oberhautchen on the
ventral outer scale surface of the mid-body
Sulcate (A), asulcate (B) and lateral (C) view morphology of left hemipenis of M.i. ikaheka (LZU 28)
Sulcate (A) and asulcate (B) morphology illustrate of left hemipenis of
M.i. ikaheka (LZU 28)
Distribution of the seven (1-7) specimen of M. ikaheka used in this study
with geographic variation in color morph of the species in Papua
Prey items identified as a freshwater fish, Cyprinidae, eat by a juvenile of
M. ikaheka from Yapen Island
A juvenile M. ikaheka (length from tail to upper body without head: 400
mm) from the gastrointestinal tract of an adult
The cannibalism of M. ikaheka at Manokwari, West Papua Province
The direction of ingestion of M. ikaheka is head first
M. ikaheka (fasciatus form) found ingesting an eel, Anguilla sp., at
Warialau Island, North Aru
Geographical variation of M. ikaheka in Papua
Map of the distribution of M. ikaheka based on specimens now examined
and from the literature
Colour variation of the adult of M. ikaheka in Papua
The three distinct color patterns of the adult and juveniles of M. ikaheka
1
I GENERAL INTRODUCTION
Background
Papua is Indonesia‟s second largest island, encompassing 416.129 km2. Papua makes up the western half of the great sub continental island of New Guinea (Beehler 2007). This region is known to support a diverse range of marine and terrestrial ecosystems, as well as an array of endemic species. The habitat mostly consists of tropical rainforest, providing ample opportunity for a diversity of species to flourish.
Eighty-three snake species have been recorded on Papua (Allison 2007), consisting of three percent of the worlds 2700 species (Taylor and O'Shea 2004). These eighty-three species are classified into seven families, including Acrochordidae (filesnakes), Boidae (boid), Pythonidae (pythons), Colubridae (tree snakes, water snakes and ground snakes), Elapidae (rear-fanged venomous snakes), and Typhlopidae (burrowing snakes). Within these families is the Cylindrophiidae, represented by Cylindrophis aruensis, which is endemic to the
Aru Islands (McDowell 1975; O'Shea 1996; Allison 2007). Five of the seven families (all except the Boidae and Cylindrophiidae) are represented in Australia and six of the families (all except the Boidae) occur in Southeast Asia. Most families appear to have had a long history in the Indo-Australian region (Allison 2007).
Only three hundred species of snakes in the world are dangerous to humans (Taylor and O'Shea 2004), one of which is Micropechis ikaheka (local name
"white snake"; English name "New Guinea or the small-eyed snake ikaheka") (O‟Shea 1996). This venomous snake is endemic to New Guinea and ranges from the lowlands up to about 1.500 m in the mountains. This species is widespread across the main island of New Guinea, as well as some of its smaller satellite islands (O'Shea 1996). M. ikaheka is a widespread and medically important
venomous snake in New Guinea. A better understanding of its taxonomy will underpin studies of venom variation, which may be of medical significance (Wuster W 25 January 2012, personal communication).
Until now, the most widely accepted hypotheses of M. ikaheka relationships
are based on analyses of external scale morphology. The species was described by Lesson in 1830, from a single specimen collected at Dorery (now known as Manokwari, West Papua Province, Indonesia) (Lesson 1830). In 1884 J.G. Fischer described M. i. fasciatus from the Aru Islands. The two subspecies, M. ikaheka ikaheka and M. ikaheka fasciatus (Fischer 1884) have been recognised by some
authors, but their distributional limits are unclear. M. i. fasciatus has been
considered an Aru endemic by some (e.g., Klemmer 1963), but others listed it from parts of Papua New Guinea (Loveridge 1948), restricting the M. i. ikaheka to
Papua. Due to lack of clear diagnostic characters, the subspecific designations have often been largely ignored (e.g., O'Shea 1996).
This dangerous snake, M. ikaheka, displays extreme geographic variation in
2
the main characteristics distinguishing populations in different areas of the distribution of M. ikaheka. This means that M. ikaheka exhibits prominent
geographical variation along the mainland of New Guinea as well as some of its smaller satellite islands in scale color. The black colored group is the new group found in northwestern Vogelkop Batanta and Waigeo islands. The yellow colored population is distributed in Vogelkop region starting from Bintuni, Manokwari, Sorong to the Salawati islands. Finally, the brown colored population is distributed from the Yapen islands to the mainland region from Waropen, Mamberamo, Jayawijaya, Mindiptana to Papua New Guinea. A hybrid color morph appears between the brown and the yellow populations in southwest Vogelkop, Bintuni. However, the taxonomic significance of this variation is not clear because the existing data do not distinguish between local adaptation, polymorphism or species-level differentiation.
Snakes serve an important role in nature, especially in a food chain. In Java, for example, snakes serve as predators controlling pest rodents that often attack rice plants (Whitten et al. 1999). However, human discrimination prevents snakes
from fully fulfilling this role in the ecosystem. On the other hand, carnivorous birds may prey on snakes in nature. However, so far there has been no information about predatory snakes in Papua. Some reports have indicated that a group of elapid snakes may be the main predators of other snake species in New Guinea (Shine and Keogh 1996; O'Shea 1994a), and, even more than that, they are cannibals (O'Shea 1994b).
Very little is known about this venomous species, despite some available data from Papua New Guinea (PNG). A number of papers and notes on the diet of
M. ikaheka have been published, but these were confined to the eastern half of
New Guinea, which comprises the independent country of PNG. In PNG the snake had been reported to prey on lizards, snakes, frogs, and small mammals (O'Shea 1994a, 1994b; O'Shea 1996; Shine and Keogh 1996).
Research Urgency
Although not well reported, there have been many cases of death of mothers and children, students, and workers in the plantation due to bites and exposure to venom spray by snakes in Papua, especially M. ikaheka. A 25 year
old man in Arso SP XI Jayapura was bitten by a male M. ikaheka (now deposited
at the British Museum of Natural History London, accession number BMNH 1994.525) although he recovered after spending two weeks in the hospital in Jayapura (Warrell et al. 1996). In another case, an elementary school student and
a mother in Manokwari died after 12 and 18 hours due to the venom of M. ikaheka (Krey observation). A young man on the island of Kar Kar only survived
for 6 hours after being bitten by M. ikaheka before he eventually died, and another
young man in Wewak also died after enduring 36 hours (Blasco and Hornabrook 1972). Finallly, a victim of M. ikaheka in Madang died 18 hours after the snake
attack (Hudson 1988).
The effect of M. ikaheka venom is very fatal and deadly because it
3
can also destroy blood cells (haemolytic) and poison muscle (myotoxic) (Hudson 1988; Warrell et al. 1996). Morphological features of this highly venomous
species are similar to the type of non-venomous Stegonotus spp. (O'Shea 1996),
which often confuses and endangers humans. In addition, the geographic variation in the skin color makes it difficult to distinguish whether the species is venomous or non-venomous, and the myth that says that the white snake venom (M. ikaheka)
is contained in its pointed tail, not in its bite, has also put humans in danger. Some indigenous Papuans in Vogelkop believe that the white snake has a mystical power that can cause rain if found or captured by humans.
Geographic variation in the skin color patterns of M. ikaheka in New
Guinea has never been fully understood. As a result, the taxonomic naming of the snakes has been neglected, and zoogeographical information is lacking, including information on certain endemic regions which are medically significant. Additionally, the possibility of hybrid zones and distribution overlap between populations of M. ikaheka in New Guinea is little understood. By comparing
morphological characters such as skin color and surface structure of oberthoucen
and the appearance of hemipenial morphology in conjunction with phylogeography, one can investigate interspecific differences. Understanding the biology of these snakes has implications for preventing bites to Papuans, and in the identification of species before medical treatment.
Pigment cells (chromatophores) scattered in the dermis layer of the skin,
exclusively on ectotherms, play a role in maintaining color constancy (Hildebrand and Goslow 2001). Adaptation to local conditions may be responsible for the variation of M. ikaheka skin color between populations, which may be maintained
by chromatophores. The distribution of chromatophores on the skin dermis has
the implication on the distribution of melanin colors by melanin cells (melanophores) that affect the variability of snake skin colors on the scales. Skin
histology, physiological phenomena and ecology have become important knowledge that can be learned in this study.
Micropechis ikaheka have been recorded to live on land (O'Shea, 1996),
very close to other terrestrial and or aquatic vertebrates. A study of the diet of M. ikaheka is important for our understanding of the inter and intraspecific
relationships of this species within the ecosystem. In addition, an increase in data availability regarding snakes species within Papua-Indonesia supports and strengthens the study of biology, ecology, evolution and natural history.
Aims of this Study This study will address the following aims:
1) Geographic variation on skin colour patterns of M. ikaheka in New Guinea is
not clearly known until now. This study aims to identify the distributional patterns, including endemic regions, hybrid zones or overlapping distributions among populations within M. ikaheka in Papua.
2) The dermis of M. ikaheka contains pigments implicated in the morphological
4
section of M. ikaheka skin, and its influence on maintaining melanin
distributions.
3) All of the meristic and color pattern data available is not enough to define the differences between populations. This study attempts to re-diagnose this species based on hemipenal morphology, a study which has not been done since Lesson 184 years ago. Snake hemipenes show great variation in structural ornamentation among different species, and can be of great taxonomic importance.
4) Ecological knowledge of this highly venomous snake is very limited, despite available data from PNG. The collection at Laboratorium Zoology Unipa (LZU) and Museum Zoologicum Bogoriense (MZB) provide a unique opportunity to study the diet and cannibalism in M. ikaheka from the western
5
II LITERATURE REVIEW
The Micropechis species
Micropechis ikaheka, a monotypic species, is a highly venomous elapid
species native to New Guinea (Papua Indonesia and Papua New Guinea). Lesson first described it in 1830, from a single specimen collected at Dorery (Manokwari, West Papua Province, Indonesia) (Lesson 1830).
Micropechis ikaheka has a fairly stocky body with a relatively short tail.
The head is narrow and only slightly boarder and distinct from the neck, with very small eyes and a round pupil (O‟Shea 1996). Coloration of M. ikaheka indicates
variability by locally (O‟Shea 1996; Krey and Farajallah 2013). In PNG, the northern population exhibits different coloration from the southern. The northern population has a light to dark grey head and distinct from the yellow or cream scales; subcaudals 37-55, diveded; anal plate divided; 6 supralabials with 3th and 4 th contacting eye and temporolabial between 5 th and 6 th; loreal and subocular scaleabsent (Lesson 1830; Fischer 1884; Rooij 1917; O‟Shea 1996; Krey 2009).
Colour and Structure of Snake Skin
Reptilian scales are generally very colorful and organized in an attractive pattern. The colour of terrestrial reptiles can be diverse and their pigmentation complex, but less so in fossorial groups (Withers and O‟Shea 1993). The colours of snakes come from the pigments in the scales and from the way light reflects of the scales (Tylor and O'Shea 2004). Many elapids show great variation in the ground color (Shea et al. 1993), including the New Guinea elapid Micropechis ikaheka.
Every part of a snake‟s body is covered by a layer of skin scales. The scales,
like reptiles in general, grow from the top layer or the epidermis (Abdel-Aal et al.
2011), and usually made of a horny substance called keratin (Hildebrand and Goslow 2001). The skin plays a particularly important role in protecting them from desiccation, abrasion dehydration, ultraviolet radiation, and in providing an impermeable barrier to exogenous organism, including potential pathogens (Cooper 2006).
Like many vertebrates, reptilian skin has two principal layers i.e. the dermis and the epidermis. The deeper layer, the dermis, provides connective tissue with a rich supply of blood and lymphatic vessels, nerves and chromatophores (Cooper 2006), while the epidermis has no blood supply and forms the outer protective
6
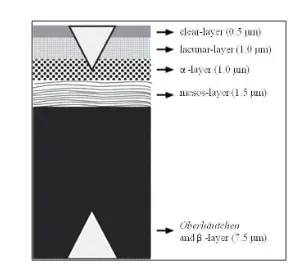
“stratum germinativum”, the deepest layer lining cells which have the capacity for rapid cell division, and the six layers which form each “epidermal generation”, the old and the new skin layers (Abdel and Mansori 2009; Abdel-Aal et al. 2011). The epidermis of snakes (Figure 2) now consists of six main layers
(Klein and Gorb 2012). From the outer scale surface towards the dermis are
known the “oberhautchen” layer, (β)-layer, mesos-layer, (α)−layer, lacunar layer
and the clear layer (Hildebrand and Goslow 2001; Abdel-Aal et al. 2011; Klein
and Gorb 2012).
Figure 2 Diagram of the cross section of snake integument. Source: Klein and Gorb (2012).
7
Structure of Snake Hemipenial
The structure of the male genitalia of snakes was first used for systematics by Edward Drinker Cope (Cope 1894) 120 years ago. Hemipenes are used regularly to study taxonomy, as new techniques of preparation are regularly proposed (Myers and Cadle 2003; Zaher and Prudente 2003). Recent papers have described hemipenial morphology from different snake species from many areas of the world (e.g. Keogh 1999; King et al. 2009; Jadin et al. 2010; Jadin and
Parkhill 2011; Smith et al. 2012).
The complete description of a hemipenis (Figure 3, Appendix 3) for systematic purposes includes recording information on its length, shape, condition of sulcus spermaticus, hooks, nature and pattern of ornamentation (spin, calyces, papillae) and type of apex. This technique follow Cope (1894), Dowling and Savage (1960) and the most useful and comprehensive for the Australia-New Guinean region snakes, Keogh (1999).
8
II MATERIALS AND METHODS
Field Work
Field work focused on the mainland of Papua and its satellite islands. All samples and information of M. ikaheka were obtained from throughout its
geographical distribution within Papua as well as Aru Island. A total of 47 specimens of M. ikaheka were partially collected in this research between 1967
-2012 (Appendix 4). The snake specimens were deposited in the Museum Zoologicum Bogoriense (MZB) LIPI in Cibinong, West Java and Zoology Laboratory University of Papua (LZU) in Manokwari, West Papua.
Observations of snakes were made day and night in a variety of habitats such as riverine, wetland, forest, and agricultural land. All individuals of M. ikaheka found in the field were captured by hand and preserved with 10%
formalin, and subsequently stored in 70% ethanol. Data was recorded, including locality, habitat, altitude and GPS coordinate.
Skin Histology Preparation
All materials used in this study are based on available formalin-preserved adult specimens in the collections of LZU. The paraffin method and Scan Electron Microscopy (SEM) were used for snake skin preparation. The paraffin method was used to study and analyze the internal structure of the snake skin layers. Only one specimen (assession number: SJR 07803 from Salawati Island) was used in this analyze as a model and the basic assumptions for the other color morph of M. ikaheka. The patterns of distribution of the pigments that affect skin color on M. ikaheka were also analyzed with a visual approach, although the paraffin method
was found to be ineffective. While SEM is useful in studying the outer layer
surface “oberthoutchen” texture of scales, microornamentation and also the
microtopography variation between the dorsal and ventral sides. Three specimens with assession number SJR 07721 from Batanta Island, SJR 08092 from Sentani, and LZU 30 from Bintuni was used in SEM analyze. All data obtained with this method were used to analyze differentiation between scales surface of M. ikaheka
in relation to their zoogeography in Papua.
Hemipenial Preparation
9
Hemipenial organs were prepared with the methods described in detail by Myers and Cadle (2003), and Zaher and Prudente (2003). We also made the morphological illustration of M. ikaheka hemipenis by using a camera lucida
(Nikon SMZ 800) stereo microscope. Hemipenial descriptions and terminology follows Cope (1894), Dowling and Savage (1960), and Keogh (1999).
Diet Analysis
Diets of Micropechis ikaheka are based on stomach contents of the
10
IV SKIN HISTOLOGY AND MICROTOPOGRAPHY OF
PAPUAN WHITE SNAKE (
Micropechis ikaheka
) IN
RELATION TO THEIR ZOOGEOGRAPHICAL
DISTRIBUTION
Introduction
A scaly, keratinized integument is one of the distinctive features of Reptilian, to make their skin airproof (Hildebrand and Goslow 2001). Snake scales, like other reptiles, grow from top layer or the epidermis (Abdel-Aal et al.
2011). In lepidosaurians (lizards, snakes, and sphenodontids) germinal layer of the epidermis spinosus-like keratinocytes alternate to hard (beta) and soft (alpha) layers (Toni et al. 2007; Chang et al. 2009). The whole skin of lepidosaurians is
covered by overlapping epidermal scales that protect them from abrasion and dehydration. The epidermis of lepidosaurs is of particular complexity and interest (Hildebrand and Goslow 2001), moreover with very colorful scales and organized in an attractive pattern. The color of terrestrial reptiles can be diverse and their pigmentation complex, marked with colorful patches.
Reptilian skin has two principal layers i.e. the dermis and the epidermis. The epidermis of snakes now consists of six main layers (Klein and Gorb 2012). From the outer scale surface towards the dermis are known the “oberhautchen” layer, (β)-layer, mesos-layer, (α)−layer, lacunar layer and the clear layer (Hildebrand and Goslow 2001; Abdel-Aal et al. 2011; Klein and Gorb 2012).
A number of the larger elapids (venomous snake) show great variation in the ground color as shown as a New Guinea elapid Micropechis ikaheka. These
colour patterns are the main characteristics distinguishing populations of the distribution of M. ikaheka.
Micropechis ikaheka (local name "white snake"; English name "New
Guinea or the small-eyed snake ikaheka") is endemic to New Guinea ranges from lowlands to about 1.500 m in the mountains. The species is a venomous snake widespread across the mainland island of New Guinea as well as some of its smaller satellite islands (O'Shea 1996).
Two subspecies, M.i. ikaheka and M.i. fasciatus, have been recognised by
some authors, but their distributional limits are unclear. Due to lack of diagnostic characters, the subspecific designations have often been ignored (O'Shea 1996). For the further analysis on this research used species level. The most recent study by Krey (2009) on colour variation and zoogeography of the species in Papua found three distinct colour patterns i.e. black, brown and yellow. A unique opportunity to study the histology and microtophography skin scales of Papuan white snake (M. ikaheka) because there has been no basic research previous is
crucial in exploring our knowledge. Therefore, this works were aimed to study the internal histology of skin layer and the microtopography structure on the surface of scales of M. ikaheka. It is also related to the existence of the snake on different
11
Materials and Methods Study Specimen
Field studies focus on the mainland of Papua and its satellite islands.
Micropechis ikaheka was captured by hand and preserved in the field with 10%
formalin, subsequently were stored in 70% ethanol. Field observation of the snakes was carried out during day and night on some habitats such as along a river, swamp, forest, and plantations as well. All field data such as locations of the snake and their altitude were recorded.
Table 1 Study sites of M. ikaheka in Papua
Site number and
Locality Latitude Longitude
Biogeography
region Altitude (meter) Collector Black group
12
Specimens and locality data of M. ikaheka used in this study also consisted
of museum specimen collection from (i) Museum Zoology Bogoriense at Cibinong, Bogor; (ii) Zoology Laboratory of the State University of Papua at Manokwari; and (iii) the collections of several herpetologists. For the purpose of analysis, specimens of M. ikaheka were chosen as representatives for variations of
the existing groups. There are at least three groups-yellow, brown and black used in this study. Study sites have been surveyed as shown in Table 1.
Geographical location of M. ikaheka recorded with a Global Positioning System
(GPS). Most coordinates of latitude and longitude plus altitude were recorded using GPS map 60csx (Garmin Co). The mapping of several locations without recording the coordinates (site number 14, 15, 16, 22, 23, 24) was carried out based on village names from the record of the specimens collected. The Map Sources 6.9 and Arcview 3.3 software were used for mapping the snake existence on different zoogeographical locations in Papua.
Internal Skin Histology Preparation
The skin histology of the M. ikaheka was analyzed for comparison with
external surfaces of scales using paraffin method. All M. ikaheka specimens were
preserved in 10% formalin for laboratory processing. Only one specimen from yellow groups was used in this work as a model and the basic assumptions for the other group of the M. ikaheka. There are two characteristics of the snake skin
were studied, i.e. internal structure of the skin layer and pigmentation pattern. Two skin positions were choice for initial examination based on the color pattern of the M. ikaheka (yellow group). Position I is a representative for the
dorsal caudal region, while position II represents of the dorsal mid-body region. The skins were cut into + 0,5 cm pieces and fixed in FAAC (Formalin acetic acid calcium chloride) solution for + 24 hours. Fixative solution was removed from the tissues and washed several times with distilled water. The tissues were dehydrated in a graded series of ethanol: 30, 50, 70, 80, 95, and 100% each 15-30 minutes. After rarefaction in the xylene + 30 minutes, the tissues were infiltrated and embedded in paraffin.
Skins were cut (1 + 2 μm) with a rotary microtome in longitudinal planes. Several sections were deparaffined with xylene, dehydrate with ethanol, subsequently were stained with double haematoxylin-eosin.
Preparation of Snake Skin for Scanning Electron Microscope (SEM)
Three individuals of M. ikaheka representing for the black, brown, and
yellow groups were chosen for SEM observations. The SEM method was used to study the microtopography structure of the oberthoutchen layer surface between
the three groups of M. ikaheka. Skin characters between dorsal and ventral sides
of the snake body were observed were: surface texture of scales, microornamentation and microtopography variation.
13
positions was examined at different magnifications (X35-X1000) in topographical mode using scanning electron microscopy JEOL JSM 5310 LV. Several stages of preparation were taken before snake skin SEM observation i.e. cleaning, prefixation, fixation, dehydration, and drying. Dried samples were placed on the stub and then coated by Au using 5-8 mA ion current for 5 minutes, with 400-500 Å thickness using ion coater IB-2.
Results
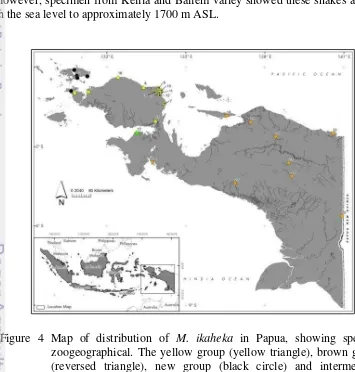
Distribution and Habitat of Micropechis ikaheka in Papua
Field observations in Papua and specimens study were carried out very interesting specific pattern distribution of M. ikaheka (Figure 4). Three groups of
the snake i.e. brown, yellow, and black have specific zoogeography in mainland of Papua and its satellite islands (such as Yapen Island, Batanta, and Waigeo Island). All altitude observations showed the species spread from 5 to 305 m ASL. However, specimen from Kelila and Baliem valley showed these snakes also live in the sea level to approximately 1700 m ASL.
Figure 4 Map of distribution of M. ikaheka in Papua, showing specific
14
Micropechis ikaheka that was found in the field observations confined to
terrestrial rainforest areas. The snakes also found in the monsoon areas and the swamps. M. ikaheka was a nocturnal and secretive semi-fussorial snakes which
inhabits leaf litter, loose soil or piles of decaying vegetation, husks of cocoa, coconut or palm oil. Several individuals were also observed in the hole of a fallen palm tree, under the tree buttresses and also in the rock crevice.
Internal Skin Histology Analysis
Position I: dorsal caudal region. The specimen M. ikaheka from yellow
group showed a unique distinct color of skin scales in different parts of the snake body (Figure 5). Paraffin method used in this work could not explain the arrangement of pigment cells (chromatophore) in detail. However, some general information about M. ikaheka skin coloration and pigmentation might be
described in these results. Dark colors on the entire surface of the dorsal caudal skin were due to the pigment (Figure 5-A1 and A2). The dark skin might contain melanophores, which only found within the dermis.
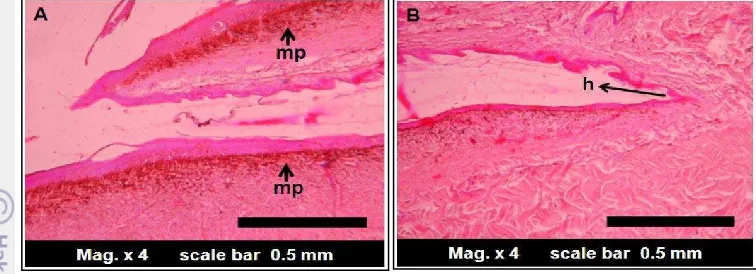
The other observation is the existence of overlapping scales and hinge regions (h) (Figure 5-B1 and B2). The melanin pigment is not evenly spread on all surfaces scales. There is a regular pattern of pigmentation in the M. ikaheka skin
scales. The scales overlapping caused the skin surface region covered by other scales continued to the hinge region (h) to contain pigment (Figure 6).
Figure 5 The arrangement and different color of M. ikaheka skin scales.
15
Position II: dorsal midbody region. Approximately five layers were identified in microscope observation (Figure 7). These were the oberhautchen, the
beta (β -layer, the mesos layer, the alpha (α)-layer, and the dermis. The β and α
layer consisted of cells which become keratinized with the production of two types of keratin (β and α keratin). The oberhautchen did not show smooth
characteristics, followed the inner scale surface and hinge region (h) that composed of thin beta-layer.
Figure 6 The scales overlapping and pigmentation pattern of the dorsal caudal region. mp = melanin pigment.
16
Microtopography Structure of M. ikaheka Skin Scales
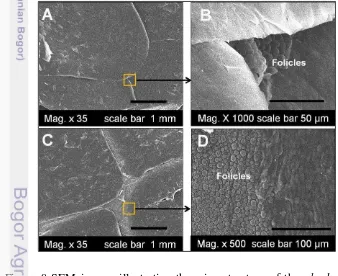
Position I and II: dorsal midbody and caudal region. Based on SEM analysis of the oberhautchen on the dorsal outer scale surface of the mid-body and caudal
of M. ikaheka were smooth and consisted none of microornamentation of pits/
pores and ridges (Figure 8-A and C). These structures were common to all of specimen investigated in this work. However, dorsal scales with magnification of x1000 and x500 showed many follicles on the entire surface of the boundary scales (Figure 8-B and D).
The SEM observation of the dorsal scales surfaces also showed overlapping scales, which is common in the reptile taxon. The dorsal midbody scales were larger than those of dorsal caudal scales. Scales boundaries of the dorsal midbody scales were covered by overlapping of large scales.
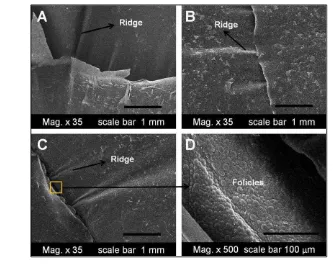
Position III: ventral midbody region. In general, the ventral mid-body scales of M. ikaheka were larger than other body scales. Several ventral scales of the
snake have specialized ridge-like microornamentation (Figure 9-A, B, and C). There were at least two ridges on each ventral scales with irregular arrangement. As shown by the dorsal scales, the ventral boundary between scales showed many follicles as well (see Figure 9-D). However, the follicles on the ventral scale lay between the two scales, in contrast with the follicles on dorsal scales that were located at the base of the scales.
Figure 8 SEM images illustrating the microstructure of the oberhautchen
17
Discussion
Zoogeographical Distribution of M. ikaheka in Papua
According to the most generally accepted classification (O‟Shea 1996), the yellow population is the subspecies of M.i. ikaheka with the distribution
throughout Vogelkop region, including Salawati Island in north-western Papua. The brown group with dorsal banding pattern (M.i fasciatus) inhabits Yapen
Island in northern Papua, and most of the New Guinea mainland: northern, southern and central region to Papua New Guinea. Surprisingly, an intermediate color appears between both groups (sub-species) in southwest Vogelkop, Tanah Merah, lowland forest of Bintuni, plus a new group (black group) from Batanta and Waigeo Island (Krey 2009). The intermediate individual might indicated the Bintuni as the hybrid zone of M.i. ikaheka from the Vogelkop region with M.i. fasciatus from the other regions.
Coloration of M. ikaheka indicated a variable locally. This study has no
sufficient data to explain the variation in color pattern of the M. ikaheka in Papua.
However, it explained the possibility of the current population of M. ikaheka
founded following colonization events (dispersals) or the occurrence connections between formerly populations (vicarians). Yapen Island is a small fraction of the Van Rees Mountains on the mainland of New Guinea (Polhemus and Allen 2007), which led to the fauna include M. ikaheka on the island joined with the northern
coast of Papua. Discontinuous local biogeography by Sagawin Strait that Figure 9 SEM images illustrating the microtopography of the oberhautchen on
18
separates the Batanta and Salawati Island (approximately 5 km) result a fraction vicarian for paradise bird species (Paradisaea minor and Cicinnurus magnivicus)
where both species does not exist in Batanta and also Waigeo Island (Beehler 2007).
General Description of the Skin Scales
Scales of various shapes and sizes cover the skin of Micropechis ikaheka.
The number, arrangement, size and shape of the scales vary greatly from one species to the other, however they are genetically fixed for each species (Abdel-Aal et al. 2011). In this study, scales microtopography of all of M. ikaheka
specimen was similar. However, corresponding with their geographic distribution, they differ in color scales sharply. Therefore, the scales and color patterns might provide simple and accessible recognition of characteristics to classify M. i.ikaheka and M. i. fasciatus in New Guinea.
Similarity of the microtopography scale surface and there is no microornamentation in all specimen examined in this work, due to the snake occupy the same terrestrial habitat and use the same substrate as well (O‟Shea 1996). However, Gower (2003) found six characters of microornamentation variation in 27 species of fossorial uropeltidae snake, such as cell shape, cell borders, pores/pits, cell length, denticulations and their length, denticulation length: cell length. Moreover, Klein and Gorb (2012) found difference in epidermis thickness, scale size and shape of the microstructure of the four snake species [Lampropeltis getula californiae (terrestrial), Epicrates cenchria cenchria
(generalist), Morelia viridis (arboreal), and Gongylophis colubrinus
(sand-burrowing)] correlated with a specific adaptation to factors such as habitat, substrate and locomotion.
Disparity between scales boundary and scales region (Figure 8) might be related to the scales contact intensity with substrate surface. Scales boundary might contain slightly thin keratin for relaxation maintenance when the snake moves. In addition to protecting the body, scales also aid in locomotion, assist retain moisture, and alter the surface characteristics (Abdel-Aal et al. 2011).
The ridge found in ventral scales might assist M. ikaheka when they moved
on the surface of the substrate or channel. Gower (2003) found regular ridges in some uropeltid snake and suggests that the ridges microornamentation might offer performance advantages for life in soil. However, the functional significance of
M. ikaheka ridges is difficult to ascertain. There are no data on the relative
locomotors mechanics among M. ikaheka as yet.
Melanin pigments were identified in dorsal body with dark color (Figure 5 and 6). These findings suggested that of skin color was due to melanin richness. The staining method needed to distinguish the color of the different types of melanin in M. ikaheka in the future studies. Although this study only used one
sample of the yellow group, one can predicted that the entire surface of the dark skin of the snake contained abundant melanin pigment. Kikuchi and Pfenning (2012) found black tissue in the skin of non-venomous scarlet kingsnake
Lampropeltis elapsoides contained only melanophores, which were mostly large
19
The green python Morelia viridis is commonly yellow or red in juveniles
but green in adult. The pythons are regionally variable in colour patterns found throughout the lowland rainforest of New Guinea and adjacent far northeastern Australia (Rawlings and Donnellan 2003). Gibson and Fals (1979) found that a melanic morph of a temperate zone snake maintained a higher body temperature when exposed to solar radiations than the lighter, striped morph snake. Therefore, one can conclude that colors are response with respect to age, seasons, and other environment factors. In this study, further ecology study of M. ikaheka is needed
to explain the environmental factor drives color differentiation in M. ikaheka
pattern.
In microscope investigation the skin of the snake were covered by keratinocytes. There are two main types of intermediate filament proteins in vertebrate keratinocytes: alpha (α) and beta (β) keratins. The epidermal appendages of reptiles and birds are characterized by the presence of both alpha (α) and beta (β) type keratin proteins (Sawyer et al. 2000). Micropechis ikaheka
have both α and β-keratins. These cells are thus being transformed into a hard protective layer called oberhautchen which constitutes the strongest outermost
layer (Chang et al. 2009; Abdel-Aal et al. 2011). The α -keratogenic tissue
provides a barrier to water loss while an overlying β-keratogenic layer provides mechanical stiffness to the skin (Licht and Bannett 1972; Chang et al. 2009). The
β-keratins are also responsible for the mechanical resistance of scales in reptiles (Toni and Alibardi 2007). Although β -keratin is important but the thickness of the layer is different in every scales region.
Overlapping scales and production of β-keratins provide strong protection (Wu et al. 2004). β-keratins are specific proteins utilized to make hard structures
including scales, scutes, and claws (beak and feathers in birds) (Toni et al. 2007).
In this study, β-keratin observed at the underside scale is thinner than the top surface (Figure 5-B2 and Figure 6) even much thinner on the hinge region (h). The β-layer arrangements of M. ikaheka are similar to the American anole Anolis carolinensis. Maderson et al. (1998) found the β-layer of the A. carolinensis was
the thinnest, close to the sense organ and in the inner scale surface and hinge regions. The hinged region (h) that exists between scales should be flexible to assist the snakes when they moved across substrate.
This snake skin histology study showed that the thin hinged region can be the entry point for ectoparasites mite or other pathogens invade the host. (Gloydius halys halys). In the slive snakes of M. ikaheka we found some of mite
20
V THE HEMIPENIS OF
Micropechis ikaheka
(REPTILIA:
SQUAMATA: ELAPIDAE) FROM PAPUA, INDONESIA,
WITH COMMENTS ON THE PHYLOGEOGRAPHY OF
THE SPECIES
Introduction
The Papuan white snake or New Guinea small-eyed snake, Micropechis ikaheka, is an endemic species to New Guinea and some of its small satellite
islands (Karkar, Walis, Myosnom, Batanta, Waigeo, Yapen). This venomous snake ranges in elevation from sea level to about 1500 m (Slater 1968; Hudson 1988; O'Shea 1996) and was first described by Lesson in 1830, from a single specimen collected at Dorery (now known as Manokwari, West Papua Province, Indonesia) in former Dutch New Guinea (Lesson 1830).
Two subspecies, Micropechis i. ikaheka and M. i. fasciatus (Fischer 1884),
have been historically recognized but their distributional limits are unclear. Due to lack of clear diagnostic characters, the subspecific designations have often been largely ignored (e.g., O'Shea 1996). Characters of color between both subspecies place M. i. ikaheka (Yellow) throughout the Vogelkop Peninsula in Western New
Guinea (Papua, Indonesia), and M. i. fasciatus (Brown) extending from Yapen
Island, offshore northwestern Papua, and through most of New Guinea, including its type locality, the Aru Islands (Krey 2009). The species inhabits dense coastal
swamp and rain forests (Slater 1968). An intermediate color morph (Yellow-Brown) was found between the two sub-species, a single adult male from the southwestern base of the Vogelkop Peninsula (Tanah Merah village, Bintuni Regency, Sumuri District). Also, a new population, black in body color, was found at Batanta and Waigeo Islands (Krey 2009), off the western shore of the Vogelkop Peninsula.
A recent study of the scale microtopography of M. ikaheka by Krey and
Farajallah (2013) found similarity in the microstructure of specimens belonging to the two currently recognized subspecies; the scale surface cannot be used to distinguish among these snakes. Herein we examine hemipeneal morphology among different populations of M. ikaheka in Papua.
The intromitent organs of squamate reptiles, the hemipenes, show great variation in structural ornamentation among different species. These ornamental structures can be of great taxonomic importance, defining some of the main groups of Hydrophiid Elapid snakes (Keogh 1999), and have been used since early attempts to classify the major groups of snakes (Cope 1894). Recent papers have described hemipenial morphology from different snake species from many areas of the world (e.g. Keogh 1999; King et al. 2009; Jadin et al. 2010; Jadin and
Parkhill 2011; Smith et al. 2012). Hemipenial characters are particulary useful in
evolutionary studies because they are closely associated with species differentiation and reproductive behaviors (King et al. 2009; Jadin et al. 2010).
21
features of this copulatory organs differ among recognized populations of M. ikaheka in Papua.
Materials and Methods Study Specimen
A total of seven specimens of Micropechis ikaheka were examined (Table
1). All of these are formalin-preserved adult specimens in the collections of the Zoology Laboratory, Papua University (LZU) in Manokwari, West Papua, and the Museum Zoologicum Bogoriense (MZB) in Cibinong, West Java.
Hemipenial Preparation
At the present work hemipenial organs were prepared with the methods described in detail by Myers and Cadle (2003), and Zaher and Prudente (2003). Partially everted left hemipenis were removed from specimens, softened in 2% KOH for six hours, fully everted with petroleum jelly stained with blue candle wax dye, tied off, and returned to 70% ethanol for permanent storage (Jadin and Smith 2010; Harvey et al. 2012). We also made the morphological illustrate of M. ikaheka hemipenis by using a camera lucida (Nikon SMZ 800) stereo microscope.
Hemipenial descriptions and terminology follows Cope (1894), Dowling and Savage (1960), and Keogh (1999).
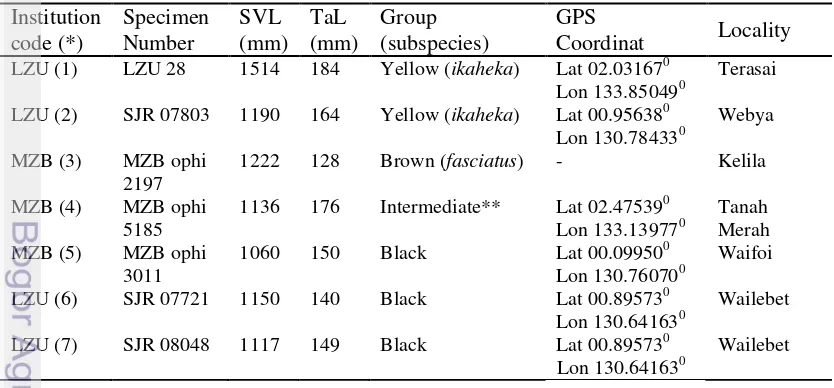
Table 2. Specimen materials of M. ikaheka used in this study
Institution
5185 1136 176 Intermediate** Lat 02.47539
0
Lon 133.139770 Tanah Merah
MZB (5) MZB ophi
22
Results
Description and Variation of Hemipenes of Micropechis ikaheka
The everted left hemipenis of seven M. ikaheka were examined. We found
similar structure, shape, dimensions and ornamentation in all of the organs, irrespective of provenance or color. Herein we describe the hemipenes of a specimen assignable to the type species (i.e., M. i. ikaheka) and based on the left
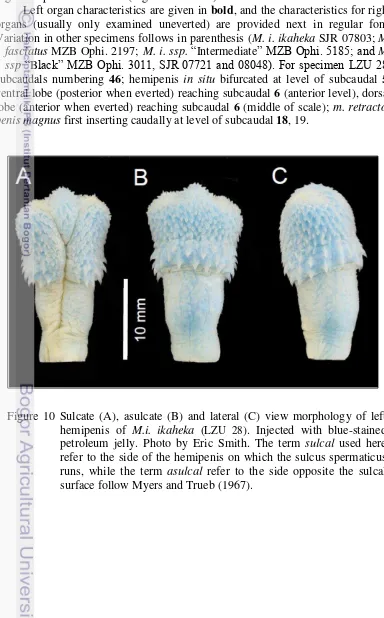
organ of specimen LZU 28 (Figure 10 and 11).
Left organ characteristics are given in bold, and the characteristics for right organs (usually only examined uneverted) are provided next in regular font. Variation in other specimens follows in parenthesis (M. i. ikaheka SJR 07803; M. i. fasciatus MZB Ophi. 2197; M. i. ssp. “Intermediate” MZB Ophi. 5185; and M.
i. ssp “Black” MZB Ophi. 3011, SJR 07721 and 08048). For specimen LZU 28:
subcaudals numbering 46; hemipenis in situ bifurcated at level of subcaudal 5; ventral lobe (posterior when everted) reaching subcaudal 6 (anterior level), dorsal lobe (anterior when everted) reaching subcaudal 6 (middle of scale); m. retractor penis magnus first inserting caudally at level of subcaudal 18, 19.
Figure 10 Sulcate (A), asulcate (B) and lateral (C) view morphology of left hemipenis of M.i. ikaheka (LZU 28). Injected with blue-stained petroleum jelly. Photo by Eric Smith. The term sulcal used here
refer to the side of the hemipenis on which the sulcus spermaticus runs, while the term asulcal refer to the side opposite the sulcal
23
The left hemipenis was everted, removed and prepared. The organ is relatively simple in ornamentation, with hooks, spines and calyces; it is only slightly bilobed, symmetrical, and centrolineal. The organ is about 26.15 mm (21.14, 20.32, 20.75, 13.64, 20.19, 17.32) long from base of cloaca and including pedicel; sulcus spermaticus dividing at about 17.22 mm (16.07, 16.91, 16.66, 11.05, 14.33, 10.79) from base. Organ length about 14.21% (12.89, 15.87, 11.78, 9.09, 14.42, 11.62) of the 184 mm (164, 128, 176, 150, 140, 149) of tail length. The sulcus extends from the median side of the organ, divides at 6.91 mm (5.84, 7.12, 5.14, 4.93, 5.61, 5.48) from the apex of the organ, and extends to the medial side of the apical tip of each lobe. Distal surfaces are relatively round and with only small papillae. Folds that define the sulcus spermaticus, before bifurcation,
are somewhat thin posteriorly but, somewhat thicker anteriorly (dextrally) and at the base. The crotch area directly above the point of sulcus bifurcation expands to
the level of the slightly developed lobes, is convex and is covered in small spicules, few in comparison to other areas of the main body of the organ. Numerous small spines, from about mid-base to level of hooks, demarcate the distal portion of the base, laterally and on the sulcate and asulcate surfaces. With 35 (30, 30, 37, 37, 37, 31) hooks surrounding the organ. A total of 21 (21, 23, 15, 14, 24, 18) spines border the sulcus spermaticus on the anterior edge (dextral),
H SS AP
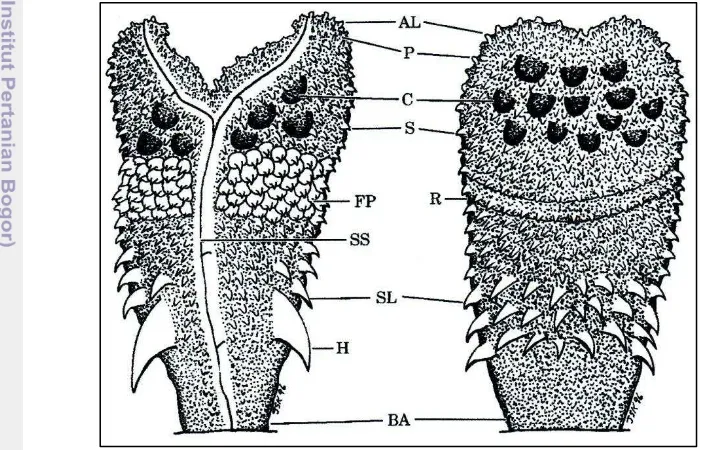
S Sc
Figure 11 Sulcate (A) and asulcate (B) morphology illustrate of left hemipenis of M.i. ikaheka (LZU 28). The line drawing illustrate views with camera lucida, showing sulcus spermaticus (SS), hooks (H), spicules (Sc), spines (S), small spines (Ss), and apical lobe (AP). Illustrated by Keliopas Krey.
A
B
24 3.49]) are relatively small; they surround and demarcate the distal edge of the base and are arranged forming a band at mid-organ level. Hook length was measured from the tip to the proximal part of the base. Largest hooks proximal to sulcus spermaticus, largest 2.3 mm (1.52, 2.47, 1.45, 1.76, 1.54, 2.20) in lenght. The hooks are relatively straight and directed towards the base and sides of the organ, somewhat laterally. The distal halves of the lobes are slightly papillated or spinulated, with small calycular areas on the asulcate side, three or four, between tips and medial expanded crotch area, slightly below the lobes and opposite to the sulcal terminus, with only a few minute spines. Calyces are only slightly scalloped. The body of the hemipenis is devoid of melanocytes.
Discussion
Until recently, the hemipenial structure of intraspecific level on M. ikaheka
was not available. The hemipenial morphology of 64 terrestrial Australian elapids was described by Keogh (1999). He describes little intraspecific variation within the species and specimens at his disposition. Our study provides the first description of hemipenial variation (Figure 10 and 11) at intraspecific level in
Micropechis ikaheka, a snake belonging to the Australian Elapid radiation
investigated by Keogh (1999). We examined hemipenial characteristics for the four color morphs of M. ikaheka in Papua, Indonesia, some corresponding to
currently recognized subspecies, namely, Yellow (M. i. ikaheka), Brown-striped
(M. i. fasciatus), Black, and Intermediate (Yellow-Brown).
There are three traditionally shape character of snakes hemipenial i.e. single, bilobed, and divided, defined by Dowling and Savage (1960). The hemipenis of all seven specimens of M. ikaheka we examined were bilobed and with the sulcus spermaticus bifurcating near the crotch continuing to the apical lobes like a”Y”.
The ornamental structures of the hemipenis of M. ikaheka shown a great
basic knowledge for defining taxonomic of the groups of M. ikaheka in Papua,
Indonesia. We found variation in hemipenial proportions (length of organ and position of sulcus spermaticus) and number of hooks around the organ and spines
bordering the sulcus spermaticus (Table 3). Although our sample size is small and
there is considerable variation in proportions it is difficult to associate these with any of the current subspecies or color morphs. The same is true for the hook and spine counts. Extremes in variation are almost invariable encompassed by those of the better-sampled color morphs. In table three we ordered the color morphs left to right as they occur in Papua, west to east, and the ranges of variation do not even appear to show gradients in distribution, for example, black M. ikaheka,
25
Peninsula (with both low an high counts). The intermediate and the brown (M. i
fasciatus) morphs contain high and low counts, respectively, both within the
ranges of the first two morphs.
Color variation has been described by previous authors (O‟Shea 1996; Rooij 1917; Krey and Farajallah 2013) in the context of zoogeographical distribution in mainland New Guinea some of its smaller satellite islands. Although there were striking difference in color variations on skin scales and zoogeographical (Krey and Farajallah 2013), Micropechis ikaheka has shown no differentiation on
hemipenial structure between group examined in this study. Therefore, the hemipenial characteristic cannot provide a significant differentiation to distinctive specific or subspecific limits on Micropechis in Papua.
Geographic variation in color is evident but we find little overlap in the distribution of the different color groups (Figure 12). Micropechis i. fasciatus is
found widespread across the mainland to the eastern part of the island, in New Guinea, and including Yapen island in the north (Krey and Farajallah 2013) and Aru island in the south (Slater 1968). Micropechis i. ikaheka is limited to the
Vogelkop peninsula, while M. i. Black is found only on Waigeo and Batanta
islands (Krey and Farajallah 2013). Evolutionary processes act on M. ikaheka and
color variants have arisen in different populations, from the east to west in New Guinea. This color variation might be related to environmental factors. Further and coming investigation, including molecular sequencing of different populations, will try to elucidate the nature of variation in these fascinating snakes.
Table 3 Selected character variation in M. ikaheka
26
Figure 12 Distribution of geographic variants of M. ikaheka in Papua,