Minor physical anomalies in children with autism
spectrum disorder
☆
Gabriele Tripi
a,⁎
, Sylvie Roux
b, Tatiana Canziani
a,c,
Frédérique Bonnet Brilhault
b, Catherine Barthélémy
b, Fabio Canziani
aaDepartment of Child Neuropsychiatry,
“Aiuto Materno”Hospital, University of Palermo, Via Lancia di Brolo 10 bis-90100 Palermo, Italy
bDepartment of Neurophysiological Explorations in Pedopsychiatry, INSERM U 619, Equipe n° 1, CHRU Bretonneau,
Bld. Tonnellé 37044 Tours, Cedex 1, France
cDidactic Area of Scientific English, Faculty of Medicine, University of Palermo, Via del Vespro, 129-90127 Palermo, Italy
Accepted 25 April 2007
Abstract
Aim:To investigate the rate and topological profile of minor physical anomalies (MPAs) (prenatal errors of morphogenesis) in a group of children with Autism Spectrum Disorder (ASD), in order to better set a temporal framing of embryological factors involved in the neurodevelopmental etiology.
Method:A new modified Waldrop scale and a mixed approach of computerized photogrammetry and classic anthroposcopy was used to detect the presence or absence of 41 MPAs in 24 children (mean age: 7 years; sex ratio: 22M:2F) with ASD and 24 healthy comparison subjects (mean age: 7 years; sex ratio: 19M:5F) selected with DSM IV and CARS.
Results:We found that children with ASD presenting MPAs (n= 23; 96%) had significantly higher rates of MPAs in four body areas (head, ears, mouth, hands); interestingly three of 41 MPAs best discriminated ASD groups from comparison subjects: abnormal head circumference, abnormal cephalic index, abnormal palate. Moreover, our results suggest that most MPAs occur predominantly after the first trimester of pregnancy.
Conclusions:These results support a prenatal neurodevelopmental model of the autism spectrum disorder.
© 2007 Elsevier Ireland Ltd. All rights reserved.
KEYWORDS
Autism spectrum disorder; Minor physical anomalies; Neurodevelopment
☆
The study was performed in accordance with basic principles of the Declaration of Helsinki (1); the hospital ethical committees of“Aiuto Materno”hospital (Palermo, Italy) and CHRU Bretonneau (Tours, France) have approved all the study procedures and all parents gave informed consent.
⁎ Corresponding author. Tel.: +390917035422, +393396965689.
E-mail address:gabrieletripi@hotmail.com(G. Tripi).
0378-3782/$ - see front matter © 2007 Elsevier Ireland Ltd. All rights reserved. doi:10.1016/j.earlhumdev.2007.04.005
a v a i l a b l e a t w w w . s c i e n c e d i r e c t . c o m
1. Introduction
Autism Spectrum Disorder (ASD) is a heterogeneous and wide spectrum [1] of neurobiological disorders [2–5] with a prevalence of 3 to 6/1000, and an approximate ratio of 3:1 of affected male to female[6].
Commonly in literature, autistic disorder (AD), pervasive developmental disorder-not otherwise specified (PDD-NOS), and Asperger syndrome (AS) are collectively referred to as ASD [7]; a wide phenotype characterizes this spectrum by impairments in three behavioural domains: social interac-tion; language, communication, and imaginative play; range of interests and activities[8].
Mental retardation[9], epilepsy[10], chromosomal and single gene disorders [11,12]and pre-, peri- and neonatal factors[13]are highly associated to this wide phenotype.
Furthermore, previous studies described different pat-terns of minor physical anomalies (MPAs) in association with ASD[14–19].
MPAs are mild errors of morphogenesis with a pre-natal origin (the first or the second trimester of pregnancy); their special relevance in psychiatry is determined by the common ectodermal embryonic origin with the central nervous system, representing external markers of abnormal brain development[14,20,21].
According to the International Working Group report[22], which establishes a clear distinction between morphogenetic events developed during or after organogenesis, MPAs may be classified into “Minor Malformations (MM)” and “ Phenoge-netic Variants”(PV).
MM are qualitative defects of embryogenesis and arise during organogenesis; their classification is based on the principle of“all or none”, being true deviations from normal. In contrast, PV are phenogenetic quantitative defects of final morphogenesis arising after organogenesis and contin-uously modifying from the birth until sexual maturity; they morphologically represent the exact equivalent of the normal anthropometric variants[21–23].
The aim of the present study was to investigate the rate and topological profile of minor physical anomalies in a group of children with ASD, in order to better set a temporal framing of embryological factors involved in the neurodevelopmental etiology.
2. Material and methods
2.1. Subjects
The population of this study is composed by 24 subjects (22 M:2 F; mean age = 7 years; S.D. = 2.29) affected by ASD (18 subjects with AD, 2 subjects with AS , 4 subjects with PDD-NOS), and 24 healthy comparison subjects (19 M:5 F; mean age = 7 years, S.D. = 2.29). These subjects have been selected at the“Bretonneau Hospital-Department of Pedopsychiatry” in Tours (France) and at the “Aiuto Materno Hospital-Department of child neuropsychiatry” in Palermo (Italy). The study was performed in accordance with the Declaration of Helsinki; the two hospital ethical comities have approved all the study procedures and all parents gave informed consent. The population is composed by children of European origin and ethnically Caucasian, because normative physical
data have been well-defined in the Caucasian population
[24,25]. The ASD group has been selected only including subjects reflecting the symptomatic diagnostic criteria of Diagnostic and Statistical Manual of Mental Disorders-Fourth Edition (DSM-IV) [8] and the severity threshold of the Childhood Autism Rating Scale (CARS) (autism cut-off score: 29.5) [26]. Due to the clinical setting in which the CARS was conducted, CARS scores were not based solely on observation, but were based on a combination of observation of the child and information given by the parent(s) during interviews[27]. CARS scores were independently generated for all children by the same psychologist (T.C.) in the two departments. Sixteen of the ASD children had CARS total score ratings that fell in the mildly to moderately autistic range (30–36.5), and eight had ratings in the severe (N37.5) autistic range. Children with Rett Syndrome and childhood disintegrative disorder were excluded from the study. Potential ASD subjects were also excluded if found to have evidence of associated epileptic seizures, genetic or meta-bolic disorders (e.g.: tuberous sclerosis, neurofibromatosis, X fragile syndrome). Their exclusion has been based on a detailed clinical history, neurological and paediatric evalu-ation, metabolic tests, genetic and neuroradiological exams if necessary. As well as the ASD sample, the control group has been strictly selected in order to exclude from it all the subjects having family histories of neuropsychiatric and genetic pathologies.
2.2. Measures
2.2.1. Assessment of minor physical anomalies
MPAs were assessed for each subject using an extended and standardized scale consisting of 41 items, representing MPAs in six body areas (global head, eyes, ears, mouth, hands and feet) (see Appendix). The scale encompassed 19 items from the Waldrop scale[14]and 19 items based on other source[20].
We also included three items in accordance with the neuroectodermal origins of the anomalies they represent: cephalic index , philtrum length and interalar distance (nasal width).
The item of “cephalic index” has been added for the evidence in literature of subjects with ASD and brachyceph-aly or dolichocephbrachyceph-aly/plagiocephbrachyceph-aly [28,12], and because the only measurement of head circumference remains insufficient to qualify brain growth[29].
The item of “philtrum length” has been added for the evidence in literature of subjects with ASD and MPAs patterns including a short/long philtrum [30,9], while the item of “interalar distance”because this length gives information on midline brain anomalies[31].
Although the original Waldrop scale items were weighted 0,1 or 2 (according to the degree of deviation from the norm), items presented in our assessment were categorized as present (score 1) or absent (score 0) due to the complexity of categorizing a trait as more severe (weight 2) compared to mild (weight 1). A clear differentiation between MM and PV was done according to Trixler et al.[21](see Appendix).
distance, philtrum length, are borrowed from the works of Farkas et al. [25], while standards of examination are in accordance with Hall et al.[31], Trixler et al.[21]and Sivkov et al.[32].
2.2.2. Morphological examinations
Morphological examinations of the two groups were done by a mixed approach of computerized photogrammetry and classic anthroposcopy.
Photographs were obtained with a high-resolution digital camera. The measurements based on anthropometric land-marks and the photogrammetric examinations were carried out using an image analysis software (FlashCAD 2005®).
The standardized cranio-facial photographs were taken with the camera lens aligned with the subject's Frankfort horizontal plane1[31] and with an internal measure of scale (adhesive paper sticker) placed on the glabella landmark for the frontal view and on the condylion of the mandible for the profile view. The subject’s facial expression should be relaxed, not smiling, gently closed lips, eyes wide open, and no eyeglasses.
The hand photographs were taken with the camera lens perpendicular to the line that passes through the distal flexion crease at the wrist and the proximal flexion crease of the middle finger, while feet photographs with the camera lens perpendicular to the line that passes through the medial prominence of the first metatarso-phalangeal (MTP) joint and the lateral prominence of the fifth MTP joint of the foot (for the dorsal and plantar view).
The anthroposcopic approach was used for the items of “head circumference”(using a plastic tape measure),“soft ears”, “electric and fine hair”, “trichoglyphics”, and the items of oral hollow area (palate, tongue, uvula).
2.2.3. Statistical analysis
Descriptive statistical analyses were used to summarize the profile of the study population. All demographic data from autis-tic subjects were compared with controls using Student'st-test.
Nonparametric statistical analyses (Mann–Whitney U-test) were used to find significance difference between two groups on the means of MPAs total scores, of 6 MPAs body areas partial scores and the two partial scores of MPAs categories (minor malformation and phenogenetic variant).
Fisher's exact probability test, was used to determine which particular minor physical anomalies best discriminated ASD groups from comparison subjects. When this produced a significant difference, we conduced the same analyses in order to evaluate a co-occurrence of MPAs only in the ASD group, comparing the significantly higher anomalies with the extended scale items.
A statistical significance level was set atpb0.05 for all analyses.
3. Results
We examined the ability of our methodology to differentiate between ASD groups and control subjects. Concerning the ASD children with MPAs (n= 23; 96%), 6 subjects had more than
1Frankfort horizontal plane is defined by a horizontal line that
passes through the tragion, located at the notch above the cartilaginous projection in the front of the external auditory canal (tragus), and the lowest border of the bony orbital rim (orbitale).
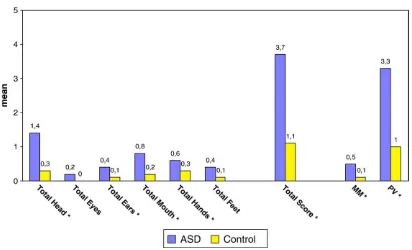
Figure 1 Scores for minor physical anomalies in ASD group and normal comparison children (⁎) =pb0.05.
Table 1 MPAs significantly more common in ASD group than in normal controls
MPAs Prevalence ASD (n= 24)
Control (n= 24)
pvalue
Head circumference 5 0 0.049
Cephalic index 7 1 0.047
High-steepled palate or narrow
5 anomalies, 17 had 1–5 MPAs, while 1 subject was without MPAs. In the control group no subject had more than 5 anomalies, 18 were with 1–5 MPAs, while 6 were without MPAs. Further, using Mann–WhitneyU-test, we found significant differences, between the two groups, on the means of scale total score (pb0,00001) as well as on means of MPAs partial scores in head (p= 0.000053), ears (p= 0.027), mouth (p= 0.001), and hands (p= 0.02) (Fig. 1). Minor physical anomalies in the eyes and feet were the only body areas in which we have not found significant differences.Fig. 1shows also a higher rate of differences, between the two groups, on the means of total MM score and PV score (respectively: p= 0.010,p= 0.00001).
By using Fisher's exact probability test, 3 MPAs were found significantly more common in the ASD groups than in normal controls (Table 1). Of the 3 MPAs, 2 were abnormality of the head (abnormal head circumference, p= 0.049; abnormal cephalic index, p= 0.047) and 1 was abnormality of the mouth (abnormal palate,p= 0.007). All of them were PV.
Interestingly, in ASD group, all children with“abnormal head circumference”were macrocephalic (head circumfer-ence≥+1.5 SD), all children with“abnormal cephalic index”
were dolichocephalic (cephalic indexb75.9) and all children with“abnormal palate”have a high-steepled palate; there was no co-occurrence in the ASD group among these three anomalies as itself.
Further we found that, the 2 significant MPAs of head area (abnormal head circumference, abnormal cephalic index) significantly co-occurred respectively with 2 MPAs in hands area (brachydactyly and clinodactyly) (Table 2).
These analysis also shows intriguely that,“abnormal palate” (mouth) co-occurs with“abnormal nose length”(head) with a difference that approached significance (p=0.060) (Table 2).
No differences were observed between the autistic and control groups for demographic data. MPAs found statistically non-significant differences, which needs to be further investigated due to our small number of cases.
4. Discussion
The ASD patient group showed a much higher level of minor physical anomalies than the normal comparison subjects, corroborating the results from previous research[15–19].
The brain develops in a sequential and hierarchical way, then it is important to keep in mind that these minor physical anomalies are fossilized imprints of early disturbance in embryonic development and are unaltered by the subse-quent illness and its consequences [20]. Rather than examining the total occurrence of MPAs in ASD subjects vs
control, it seems more relevant to focus on specific ano-malies which are of particular relevance to the hypothetical neurodevelopmental failure of the disorder.
In the current study , significantly higher rates of minor physical anomalies were found nearly in all body regions except for eyes and feet.
Further in the head area, the higher rate of macrocephaly in our ASD group is in accordance with previous studies reporting increased head size in infancy in autism[19,33,34]. The results of head circumference, neuroimaging and post-mortem studies converge to show that the brain is abnormally large in some, but not all children with autism during post-natal development. The increased rate of head size during infancy is an intriguing finding because its meaning is uncertain in regard to brain development and course of the disorder[34].
Furthermore, the high prevalence of dolichocephaly (not related to macrocephaly) in our data is a feature, distin-guishing the ASD group from the normal controls.
Gray et al.[35]and Rajlakshmi et al. [36]have pointed out that cephalic index is a gestational age-dependent biometric parameter, in which intrinsic or extrinsic variations (unfavourable in pregnancy), may influence head shape between 14–28 weeks of gestation.
The results of Juul-Dam et al.[13]support previous findings reporting that there is a consistent association of unfavourable pre-, peri- and neonatal events in pregnancy, and autism.
These observations suggest that an abnormal cephalic index may characterize a subgroup of autism and, if replicated, will support the existence of disturbances between 14–28 weeks of gestation, which may correspond to a critical period for cortical development.
At present the literature data shows that no one pattern of head or brain growth is found in all children with autism; variability in head and brain size and rate and trajectory of head and brain growth appear to be part of autism, as we define it today[34].
The finding of a higher rate of MPAs in the mouth region is of special interest because high-steepled palate represents a microform of cleft palate, which is itself frequently associated with midline brain anomalies as in fetal alcohol syndrome or other neurodevelopmental disorders [21]. Furthermore, the data shows that high-steepled palate co-occur approaching significance, with“abnormal nasal width”. In our work all the ASD subjects with“abnormal nasal width”had“widening nose basis”. In literature previous studies have also related “widening nose basis”with midline brain anomalies[31].
The co-occurrence between the statistically higher rate MPAs of head region with respectively, brachydactyly and clinodactyly is of special interest since previous studies also showed as, these anomalies are frequent features related to many chromosome abnormalities and autism [37,38]. In contrast, our data shows no statistically significance between the two subject groups for these anomalies as itself. To relate MPAs in head and hand regions we need further investigations.
Concerning the identification of a critical period for brain trauma by using a MPA scale with distinction between malformations and variants, our results suggest that most abnormalities occur predominantly after the first trimester of pregnancy; however, our data is insufficient to identify the exact period of developmental failure.
Table 2 Frequencies (percentages) for MPAs co-occurrence in ASD group
ASD group
pvalue Head circumference Brachydactyly 40% p= 0.036 Cephalic index Clinodactyly 86% p= 0.023 High-steepled palate
or narrow
The results observed in the current investigation appear not consistent with some previous studies which have attempted to determine whether specific MPAs are more frequent in autism. Walker [15] reported that his autism population had a significantly increased incidence of low-settled ear, adherent ear lobes, furrowed tongue, hyperte-lorism, 2–3 toe syndactyly. More recently Rodier et al.[18]
reported that posteriorly rotated ears, small feet and large hands occurred more frequently in children with autism.
This diversity of results, could suggest a morphological heterogeneity of ASD, representing another feature associ-ated to the wide clinical phenotype.
However the data of this study must be tempered against the methodological limitations. One limitation concerns our small number of cases; because of this, we have not calculated a cut-off score which optimally discriminate the patients from control subjects (maximizing sensitivity and specificity for ASD). Another notable limitation regards the lack of cognitive and language data in the two groups for the difficulties to find homogeneous criteria of neuropsycholog-ical assessment in the two hospitals. It is critneuropsycholog-ical that future investigations will include a higher number of subjects with a wide range of cognitive and communicative abilities. Although some remains sceptical regarding the etiological significance of neurodevelopmental indices such as MPAs, these stigmata occur more prominently in pervasive devel-opmental disorders [21]. Additionally, it is essential to examine the relationship of any MPA with clinical, neuropsy-chological, or neurobiological assessments with the goal of identifying and characterizing subgroups of individuals with ASD that may facilitate future investigations of the pathophysiology of this complex disorder and more impor-tantly help in the development of effective treatment strategies.
Appendix A
Minor physical anomalies
Items MM/PV Examination standards
Head
Index cephalic PV ≥±1.5 SD (euryon–euryon
line/glabella–opisthocranion line × 100)
Head circumference PV ≥±1.5 SD (glabella–
opisthocranion circumference) Electric and fine hair MM Hair soon awry and
unmanageable Trichoglyphics MM None, or 2/more
trichoglyphics
Fused eyebrows PV Apparent on inspection Prominent forehead MM Apparent on inspection Interalar distance
(nasal width)
PV ≥±1.5 SD (alare–alare line)
Anteverted nostrils PV Apparent on inspection Micrognathia PV Apparent on inspection
Eyes
Epicanthus PV Partial/total coverage of the lacrimal caruncle.
Eyes
Inner-canthal distance (hyper–
hypotelorism)
PV ≥±1.5 SD (endocanthion–
endocanthion line)
Heterochromia/ Brushfields spots
MM Unequal color of iris/yellow-grayish heterochromic spots in the outer third part of the iris Ptosis PV Apparent on inspection. Coloboma MM Apparent on inspection.
Ears
Low seated ears PV A straight line is drawn from the cutaneous auditory meatus (porion) to meet a profile line (glabella–labial superius) above/below the upper edge of the nasal ala Adherent earlobes MM Partly/total adherence or
total absence (rather rare). Asymmetric ears PV Normal morphology but
difference in the size and protrusion from head. If one ear is low seated this is counted as a asymmetry as well on this item
Soft ears PV Soft and pliable ears that do not spring back into place Malformed ears MM Incomplete/absence
development of helix, scapha, antihelix, tragus or anotia (rather rare) Auricular skin-tag MM Apparent on inspection
Mouth
Philtrum length PV ≥±1.5 SD (sellion–labial
superius)
Thin upper lip PV Apparent on inspection High-steepled palate
or narrow
PV The roof has an acute angle rather than an arch or a narrow flat area across the top Furrowed tongue MM One or more deep furrows not
along the center line of the tongue
Tongue with smooth-rough spots
MM Localized thickening of the surface of the tongue. Make sure this is not due to elevated papillae caused by recent consumption of certain food Cleft lip MM Apparent on inspection Cleft uvula MM Apparent on inspection
Hands
Clinodactyly PV Deflection exceeding 8° Simian/Sydney
crease
MM A single uninterrupted palmar crease from the radial to the ulnar border/proximal transverse crease extends to the ulnar border of the hand.
(continued on next page)
Hands
Brachydactyly PV Apparent on inspection Tapering fingers PV Apparent on inspection Overlapping fingers PV Apparent on inspection Micronychia (nail
hypoplasia)
PV Small or almost absent fingernails
hyperconvex fingernails
PV Apparent on inspection
Feet
Third toe PV Third toe is same length/ longer than second.
Syndactyly MM Partial or total fusion of toes Gap between first
and second toe
PV The distance between the first and the second toes is the same/exceeds the width of the second toe
Overlapping toes PV Apparent on inspection Retarded toes PV Apparent on inspection Deep sole crease MM The sole crease extends to
the area between the origin of first and second toes Hyperconvex
toe-nails
PV Apparent on inspection
References
[1] Wing L. The autistic spectrum. Lancet 1997;350:1761–6. [2] Lelord G, Adrien JL, Barthélémy C, Bruneau N, Dansart P, Garreau
B, et al. Further clinical evaluations elicited by functional biological investigations in childhood autism (article in French). Encephale 1998;24:541–9.
[3] Akshoonoff N, Pierce K, Courchesne E. The neurobiological basis of autism from a developmental perspective. Dev Psychopat 2002;14:613–34.
[4] Courchesne E, Redcey E, Kennedy DP. The autistic brain: birth through adulthood. Curr Opin Neurol 2004;17:489–96. [5] Berthoz A, Andres C, Barthélémy C, Rogé B. L' Autisme. De
la recherche à la pratique. Paris: Ed. Odile Jacob publisher; 2005.
[6] Muhle R, Trentacoste SV, Rapin I. The genetics of autism. Pediatrics 2004;13:472–86.
[7] Bertrand J, Mars A, Boyle C, Bove F, Yeargin-Allsopp M, Decoufle P. Prevalence of autism in a United States population: the Brick Township, New Jersey, investigation. Pediatrics 2005;108: 1155–61.
[8] American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, DSM IV. 4th ed. Washington DC: American Psychiatric Association publisher; 1994.
[9] Gillberg C. The neurobiology of infantile autism. J Child Psychol Psychiatry 1988;29:257–66.
[10] Minshew NJ. Indices of neuronal function in autism: clini-cal and biologic implications. Pediatrics 1991;87:774–80 [suppl.].
[11] Cook EH. Genetics of autism. MRDD Research Reviews, vol. 4; 1998. p. 113–20.
[12] Cohen C, Pichard N, Tordjman S, Baumann C, Burglen L, Excoffier E, et al. Specific genetic disorder and autism: clinical contribu-tion towards their identificacontribu-tion. J Autism Dev Disord 2005;35: 103–6.
[13] Juul-Dam N, Townsend J, Courchesne E. Prenatal, perinatal, and neonatal factors in autism, pervasive developmental
Appendix A (continued) disorder—not otherwise specified, and the general population. Pediatrics 2001;107:63–8.
[14] Waldrop MF, Pederson FA, Bell RQ. Minor physical anomalies and behaviour in preschool children. Child Dev 1968;39: 391–400.
[15] Walker HA. Incidence of minor physical anomaly in autism. J Autism Child Schizophrenia 1977;7:165–76.
[16] Campbell M, Geller B, Small AM, Petti TA, Ferris SH. Minor physical anomalies in young psychotic children. Am J Psychiatry 1978;5:573–5.
[17] Gualtieri CT, Adams A, Shen CD, Loiselle D. Minor physical anomalies in alcoholic and schizophrenic adults and hyper-active and autistic children. Am J Psychiatry 1982;139: 640–2.
[18] Rodier PM, Bryson SE, Welch JP. Minor malformations and physical measurements in autism: data from Nova Scotia. Teratology 1997;55:319–25.
[19] Miles JH, Hillman R. Value of a clinical morphology examination in autism. Am J Med Genet 2000;91:245–53.
[20] Ismail B, Cantor-Graae E, McNeil TF. Minor physical anomalies in schizophrenic patients and their siblings. Am J Psychiatry 1998;155:1695–702.
[21] Trixler M, Tenyi T, Csabi G, Szabo R. Minor physical anomalies in schizophrenia and bipolar affective disorders. Schizophr Res 2001;52:195–201.
[22] Spranger J, Berirschke K, Hall JG, Lenz W, Lowry RB, Opitz JM, et al. Errors of morphogenesis: concepts and terms. J Pediatry 1982;100:160–5.
[23] Opitz JM. Heterogeneity and minor anomalies. Am J Med Genet 2000;92:373–5.
[24] Feingold M, Bossert WH. Normal values for selected physical parameters: an aid to syndrome delineation. BD:OAS X, vol. 13; 1974. p. 1–15.
[25] Farkas LG, Munro IR, Kolar JC. Anthropometric facial propor-tions in medicine, Springfield. 1987; Ed. Charles Thomas publisher Ltd. USA.
[26] Schopler E, Reichler RJ, Renner BR. The childhood autism rating scale (CARS). 1988 Ed. Western Psychological Services. Los Angeles. California.
[27] Teal M, Wiebe M. A validity analysis of selected instruments used to assess autism. J Autism Dev Disord 1986;16:485–94. [28] Lauritsen M, Mors O, Mortensen PB, Ewald H. Infantile autism
and associated autosomal chromosome abnormalities: a regis-ter-based study and a literature survey. J Child Psychol Psychiatry 1999;40:335–45.
[29] Gosselin J, Cahagan S, Amiel-Tyson C. The Amiel–Tyson neurological assessment at term: conceptual and methodolog-ical continuity in the course of follow-up. MRDD Research Reviews, vol. 11; 2005. p. 34–51.
[30] Orstavik KH, Stromme P, Ek J, Torvik A, Skjeldal OH. Macrocephaly, epilepsy, autism, dysmorphic features, and mental retardation in two sisters: a new autosomal recessive syndrome? J Med Genet 1997;34:849–51.
[31] Hall GH, Froster UG, Allanson JE. Handbook of normal physi-cal measurements. 1989 Ed.Oxford Mediphysi-cal Publications. USA.
[32] Sivkov ST, Akabaliev VH. Minor physical anomalies in healthy subjects: internal consistency of the Waldrop physical anomaly scale. Am J Hum Biol 2003;15:61–7.
[33] Gillberg C. Head circumference in autism, Asperger syndrome, and ADHD: a comparative study. Dev Med Child Neurol 2002;44: 296–300.
[34] Lainhart E. Advances in autism neuroimaging research for the clinician and geneticist. Am J Med Genet C Semin Med Genet 2006;142C:33–9.
[36] Rajlakshmi Ch, Shyamo SM, Bidhumukhi Devi, Chandramani Singh L. Cephalic index of foetuses of Manipuri population—a baseline study. Anat Soc India 2001;50(1):8–10.
[37] Ingram JL, Stodgell JC, Hyman SL. Discovery of allelic variants of HOXA1 and HOXB1: genetic susceptibility to autism spectrum disorders. Teratology 2000;62:340–93.