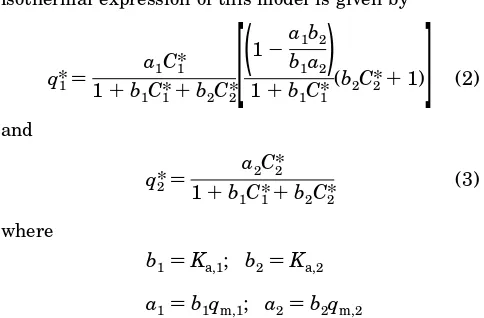Adsorption Behavior of Multicomponent Protein Mixtures
Containing
r
1-Proteinase Inhibitor with the Anion Exchanger,
2-(Diethylamino)ethyl-Spherodex
Gerard M. S Finette, Badlishah S. Baharin,
†Qi-Ming Mao, and Milton T. W. Hearn*
Centre for Bioprocess Technology, Department of Biochemistry and Molecular Biology, Monash University, Wellington Rd, Clayton, 3168 Victoria, Australia
The equilibrium binding behavior ofR1-proteinase inhibitor (R1-PI) in the presence of human serum albumin (HSA) has been determined in packed bed systems with the anion exchanger, 2-(diethylamino)ethyl (DEAE)-Spherodex. Experimental data derived for the individual proteins were compared with the corresponding data obtained from batch adsorption studies as well as studies in which mixtures of these two proteins were loaded at different concentration ratios onto columns of the same anion exchange adsorbent. The results confirm that R1-PI has a greater affinity for the anion exchanger, although competitive adsorption was observed as the inlet concentration of HSA was increased. Under these conditions, decreased binding capacities and lower dynamic adsorption rates were observed forR1-PI with the DEAE-Spherodex anion exchange adsorbent. The results are discussed in terms of the influence which various contaminants that occur in multicomponent mixtures of proteins from human plasma can have on the equilibrium binding characteristics of a target protein with weak or strong ion exchange adsorbents under conditions approaching concentration overload in preparative chromatographic systems. These investigations have also addressed, as the first part of an iterative approach for the simulation of the adsorption behavior of multicomponent mixtures of human plasma proteins with ion exchange and affinity chromatographic adsorbents, the ability of noncompetitive and competitive Lang-muirean models to simulate the adsorption of R1-PI in the presence of different concentrations of HSA to DEAE-Spherodex.
Introduction
Considerable advances have been achieved in recent years to increase the yield and availability of specific proteins from biological extracts through enhancement of the cellular expression levels of the corresponding genes by genetic engineering methods. Despite this progress, a fundamental issue still remains in product manufacture. In particular, the need exists to ensure that high recovery of bioactivity and high capacity of the specific target protein(s) are achieved with a selected adsorbent without sacrificing speed, productivity, and process economics. Typically, three or four stages of “downstream processing” are required to preconcentrate and fractionate the crude feed-stock into a suitable side-fraction containing the majority of the desired product. These methods of prefractionation and concentration are usually based on different molecular attributes of the target protein(s), allowing sequential exploitation of the differential solubility, state of aggregation, or partitioning behavior of the various components in the feed-stock or the simultaneous use of a combination of all three properties [Harris and Angal, 1989; Hearn and Anspach, 1990; Scopes, 1994].
Irrespective of the success of these initial preconcen-tration procedures, a stage is often reached particularly when the product has commercial value (e.g., in the food
or health industries), whereby the desired wild-type or recombinantly-derived protein has to be further purified from a still complex multicomponent mixture by specific procedures of adsorption chromatography. This situation is very evident with the process scale chromatographic purification of human plasma proteins. Often, the crude Cohn fractionated feed-stocks vary batch-by-batch in composition and relative abundances of a large array of compounds differing in molecular weights, physical, chemical, or biological properties and ligand-binding specificities. To ensure optimal adsorptive capture of the desired protein (or alternatively to maximize the capture of the contaminants and to allow the protein product to emerge in the breakthrough peak), it is necessary to characterize the influences that these contaminants have on the adsorption process. These unwanted components may include inter alia other proteins of very similar composition or three-dimensional structure, such as partially deamidated, phosphorylated, or glycosylated isoforms of the desired protein(s). The presence of these contaminants will impact on the adsorption behavior of the product of interest with the chosen adsorbent and thus on the economic productivity of the chromatographic procedure.
From practical considerations, ion exchange chromato-graphic procedures have come to occupy a central position in the fractionation of protein mixtures, typically permit-ting purification factors in the range of 10-100-fold [Yamamoto et al., 1988; Scopes, 1994; Levison et al., 1994]. The maximum effective dynamic adsorption ca-pacity (DAC) and the rate of dynamic adsorption (DAR)
* Author to whom correspondence should be addressed: email, milton.hearn@med.monash.edu.au.
†Present address: Department of Biotechnology, Universiti
Pertainian Malaysia, Serdang, Selangor, Malaysia.
265
Biotechnol. Prog.1997,13, 265−275
[Mao and Hearn, 1996] of a specific protein in a multi-component mixture for a specified ion exchange adsorbent will depend inter alia on the size, composition, and surface characteristics of the target protein, as well as the concentration of the target, the total protein content in the feed-stock, the viscosity and composition of the loading buffer/mixture, and the operating temperature. Because adsorbents of generic selectivity such as ion exchangers bind to proteins and other components en-compassing a wide range of molecular features via less discriminatory primary and secondary equilibrium proc-esses than those which occur with optimized biospecific affinity adsorbents, a larger range of contaminant sub-stances will be coadsorbed to different extents according to their affinities and abundances. With human plasma protein fractions, these contaminants, many of which will be present in relatively large and variable amounts in the feed-stock, can displace or be displaced by other components in the feed-stock, provided significant steric hindrance effects do not prevail. In this context, the physical properties of the adsorbent, including the pore volume and pore size have important practical conse-quences. It has been observed, for example, that albumin can displace large amounts of fibrinogen andγ-globulin that were already bound to polystyrene and rubber surfaces [Lee et al., 1974], although the extent of this displacement induced desorption was conditional on the pore size of the adsorbent.
In the present investigation, the impact of protein contaminants on the adsorption ofR1-proteinase inhibitor (R1-PI) to the anion exchange adsorbent DEAE-Spherodex has been examined. This study thus extends previous studies from this and other laboratories [Myerowitz et al., 1972; Murthy and Hercz, 1973; Musiani and Tomasi, 1976; Koj et al., 1978; Anspach et al., 1989; Finette et al., 1996a,b] on the purification of plasma proteins and other proteins from crude feed-stocks of mammalian origin, as well as the binding of individual proteins with different classes of coulombic, biomimetic, and biospecific affinity adsorbents derived from the same type of chro-matographic support materials under conditions ap-proaching dynamic overload and saturation of the ad-sorbent. In addition, the relevance of different Lang-muirean adsorption models in the simulation of the adsorption behavior with such multicomponent mixtures has been examined.
Experimental Section
Materials. A 20% (w/v) solution of human serum albumin (HSA) and the R1-proteinase inhibitor (R1-PI) were provided by CSL Ltd. (Melbourne, Australia). The rabbit sera, anti-R1-proteinase inhibitor (A012, Lot 018) and anti-albumin (A001, Lot 049) for rocket immuno-electrophoresis, were purchased from Dako Corp. (Glos-trup, Denmark). The chromatographic support, DEAE-Spherodex LS adsorbent, was a gift from BioSepra AS (Villeneuve, La Garenne, France). The equilibration and loading buffer consisted of 0.025 M sodium acetate, pH 5.2. The latter buffer was prepared by mixing glacial acetic acid (Merck Australia Pty, Ltd., Kilsyth, Australia) with distilled water and adjusting the pH by the addition of 10 M NaOH. The elution buffer consisted of 0.02 M sodium acetate, pH 4.5, for HSA and 0.15 M sodium acetate, pH 4.5, forR1-PI. For all experiments examining the adsorption and elution processes, fresh buffers were made daily with degassed Milli-Q water and sterile filtered through 0.45µm Millipore filters (Millipore Corp., Bedford, MA).
The electrophoresis buffer was prepared from a stock solution containing 0.122 M barbitone (Merck Australia
Pty, Ltd., Kilsyth, Australia), 0.366 M Tris (Sigma Chemical Co., St Louis, MO), 0.0017 M calcium lactate (Hopkin & Williams Ltd., Essex, England), and 0.01 M sodium azide (Ajax Chemicals, Sydney, Australia). The Tris-barbitone running buffer, pH 8.7-8.8, was prepared by diluting the stock solution five times in distilled water. The remainder of the stock solution was kept at 4 °C until required. The running buffer was used for 2 consecutive days, after which it was replaced with fresh buffer. The gel-staining solution consisted of 0.05% (w/v) Coomassie Blue R250 (Sigma Chemical Co., St Louis, MO) in 25% (w/v) ethanol/10% (w/v) acetic acid and was kept in 500 mL flasks which were completely wrapped with alu-minium foil and stored at 4 °C. The destaining solution was a 25% ethanol/10% (w/v) acetic acid mixture. High-grade ethanol (90% proof) and acetic acid were purchased from Merck Australia Pty, Ltd. (Kilsyth, Australia).
Equipment. Batch Experimental Procedures. For the batch experiments, the experimental format was based on our previously described approach [Anspach et al., 1989] and involved a model 2238 UV spectrophotom-eter, a Microperplex peristaltic pump (model 2132), and a two-pen chart recorder (model 2210), all obtained from Pharmacia Biotech AB (Uppsala, Sweden). Uniform temperatures were ensured by the use of a thermostatted bath (model 2219 Multitemp-11) also purchased from Pharmacia Biotech AB (Uppsala, Sweden).
Packed Column Procedures. For experiments per-formed in packed chromatographic beds, the apparatus consisted of HR5/5 columns (dimensions 0.5 cm×5.0 cm) purchased from Pharmacia Biotech AB (Uppsala, Swe-den), connected to a Pharmacia FPLC LC-500 system programmed for automatic operation. A model-1 UV spectrophotometer set at a wavelength of 280 nm was used in conjunction with a chart recorder to monitor the effluent concentrations.
Electrophoretic Procedures. Horizontal electro-phoresis chambers were purchased from Pharmacia Biotech AB (Uppsala, Sweden) as part of a Multiphor II system and connected to a power pack (EPS 500/400). For electrophoretic experiments, the system was run at 90 V and 13 mA.
Methods. Procedures for the Batch Adsorption Experiments. A stock solution (5 mg/mL) of each protein was prepared from which solutions of different protein concentrations were made up to a final volume of 5 mL in plastic vials. Aliquots of the degassed ion exchange adsorbent (0.5 g) were added to these vials, and the solutions were then mixed overnight by means of a spinning wheel incubator at 25(0.1 °C, after which the adsorbent samples were centrifuged at 5000gin a ther-mostatted centrifuge (model KS-5200, Kubota, Boronia, Australia). After centrifugation, the supernatent was carefully removed from each plastic vial and stored in a fresh vial. Equilibration buffer (2 mL) was added to the gel pellet, and the mixture was briefly agitated and then centrifuged for 15 min at 5000g. The supernatent was carefully removed and added to the previously recovered supernatent, making a final volume of 7 mL. The protein content of this combined supernatent was determined by measurement at 280 nm using the model 2238 UV spectrophotometer in comparison with a standard curve of a highly purified sample of HSA orR1-PI. The amount of protein adsorbed was determined from the difference in concentration from the initial value added to the adsorption vial and the final value found in the super-natent.
Procedure for Rocket Immunoelectrophoresis.
dissolved in barbitone buffer (0.5 g in 500 mL) heated to 90 °C, with aliquots of 20 mL stored at 4 °C until required. Antibody-containing gels were prepared using glass plates (124 mm × 129 mm) to which Gel bond, purchased from Pharmacia Biotech AB (Uppsala, Swe-den) had been adhered. The agarose gel was warmed to 58 °C, and the respective amount of the HSA or anti-R1-PI serum added, with good mixing to ensure uniform distribution of the antiserum, and the mixture was spread on the Gel-bond-coated plates prewarmed to 60 °C. After the agarose solution had gelled, 22 wells (spaced 0.5 mm from each other) were cut using a punch (purchased from Pharmacia Biotech AB, Uppsala Swe-den). Immunoelectrophoresis of the test and standard protein samples was carried out for 16 h at 90 V and 12 mA or for 3 h at 205 V and 23-24 mA. Following washing with 0.1 M NaCl and then distilled water at 10 °C for 15 min, the gel plate was partially dried using filter paper for 15 min and air dried at 40 °C. When the gel plate was completely dried, the protein zones were stained for 5 min with the Coomassie Blue R250 staining solution. Low background was achieved by immersing the gel plate first in distilled water, then in the destaining solution, and finally in distilled water again.
Procedures for Chromatographic Experiments.
The Pharmacia HR 5/5 columns were packed with DEAE-Spherodex and connected to the FPLC which was pro-grammed to permit three experiments to be run sequen-tially with automatic data logging. The sequence for each experiment followed the following protocol: (i) equilibra-tion of the packed bed with loading buffer; (ii) loading of the protein solution until the column was saturated and a constant breakthrough curve was obtained; (iii) wash-ing of the column with equilibration buffer to remove unbound proteins; (iv) elution of the bound protein(s) with the appropriate elution buffer. During each of the ii-iv steps, the concentration of the protein in the effluent was monitored spectrophotometrically and the concentration changes were acquired with a data logger and displayed in real time as the effluent exited the column. Chro-matographic runs were initially performed with a single-component protein solution containingR1-PI (1 mg mL-1) or HSA (1 mg mL-1), and the breakthrough profile for each protein was then digitally extracted. Competitive adsorption was studied by loading the column with mixtures of both proteins at a concentration of 1 mg mL-1 forR1-PI and 1-20 mg mL-1for HSA. The ratio ofR
1-PI to HSA of 1:20 used here was selected to be in the same range as found in the Cohn fraction II+III supernatant [Myerowitz et al., 1972; Peterson and Sober, 1975] of human plasma.
Results and Discussion
Equilibrium Models for Multicomponent Adsorp-tion Behavior of Proteins. The origins of different theoretical models which can be applied to describe the adsorption behavior of a single-component protein system with a chromatographic adsorbent have been detailed [Wankart, 1986; Hearn and Anspach, 1990; Bellot and Condoret, 1993; Finette et al., 1996; Nicoud and Seidel-Morgenstem, 1996] in recent separation science and chemical engineering literature. For multicomponent mixtures, both competitive and noncompetitive binding interactions can occur and, as a consequence, modifica-tion of these models based on single-component interac-tions is required. The simplest theoretical model which has been employed to describe the adsorption phenom-enon of a single component is based on the Langmuir equation. Application of this model to experimental data requires that the following assumptions to prevail: (i)
all binding sites are energetically equivalent and inde-pendent; (ii) no lateral or isodesmic interactions occur between the adsorbed molecules (i.e., further adsorption ceases once monolayer coverage has been achieved and independent binding of the proteins has occurred); (iii) the protein molecules are adsorbed onto a fixed number of well-localized sites in a single equivalent orientation; (iv) the binding is reversible. A variety of other models have also been proposed to accommodate the heterogene-ity of the binding sites present either at the surface of the adsorbent or at the surface of the adsorbed protein for a single-component system. These additional models include the noncompetitive and competitive biLangmuir isotherm [Graham, 1953], now widely used for the prediction of the elution bands of proteins in preparative zonal or displacement chromatography [Katti et al., 1990; Hossain and Do, 1996], the Freundlich isotherm or the composite Langmuir-Freundlich isotherm [Ruthven, 1984; Wei and Hearn, 1996], the Jovanovic isotherm [Huang and Horvath, 1987a,b], the Redlich-Peterson isotherm [McKay and Duri, 1989], the Temkin isotherm [Johnson and Arnold, 1995], the Fowler-Guggenheim isotherm [Bellot and Condoret, 1993], the S-shaped isotherm [Dowd and Yon, 1992], or combinations of these expressions as more complex representations of the competitive or noncompetitive binding of multiponent mixtures. The general forms of the more com-monly employed isothermal expressions are given in Table 1.
When an experimentally-derived isotherm for the binding of a single, low molecular weight component to an adsorbent has been reliably described over the major-ity of the concentration range by, for example, a Lang-muirean dependency, investigators have then shown a preference to extend the same theoretical treatment to describe the adsorption phenomena of the same com-pound when present as part of a multicomponent mixture [Jain and Snoeyink, 1973; Ruzgas et al., 1990; Srivastava and Tyagi, 1995]. Irrespective of the closeness of the numerical correlations which can be achieved between the experimental data and the predicted values derived from such isothermal approaches, elucidation of the adsorption process in terms of whether noncompetitive or competitive binding interactions occur between the target compound and other contaminants in the feed-stock represents a fundamental requirement for the chromatographic optimization and scale-up procedure if the predictive capabilities of the simulation model(s) are to take on any physical significance.
Because of their structural and conformational com-plexity, proteins represent a specially challenging case for the development of reliable adsorption models. As discussed elsewhere [Katti et al., 1990; Finette et al., 1996a; Hossain and Do, 1996; Mao and Hearn, 1996], iterative simulation approaches, involving the application of several different isothermal representations, allow, in terms of computational time and cost, an efficient strat-egy to determine more reliable values of the relevant adsorption parameters, such as q*, Kd, or the mass transfer constants (the latter often lumped into an apparent axial dispersion coefficient). Progressive refine-ment of the initially employed simulation model can then follow with the introduction of additional parameter terms which permit the simulation to more closely approximate the physical reality of the actual adsorption process. Noncompetitive or competitive Langmuirean models have been frequently used as the starting point for these representations of the adsorption behavior.
system. This model assumes that there is no competition between the target protein and other components in the mixture for the available adsorption sites. As a result, the adsorption characteristics of each component will be the same as if the other components were absent. The equation describing this extreme case of adsorption for a binary mixture can be given as
where
andKais the association constant,q*,qm, andC* refer to amount of adsorbed solute, the maximum capacity for the solute, and the concentration of the solute in the bulk solution while the subscripts 1 and 2 refer to the adsorbates 1 and 2, respectively.
In the context of ion exchange adsorption of proteins, this extreme case of noncompetitive adsorption is un-likely to occur even if the mixture comprises proteins of totally different molecular sizes, isoelectric points, surface electrostatic properties, or hydrophobic characteristics and hence different affinities for the adsorbent. Rather, this noncompetitive binding model can be anticipated to be more representative of situations where low molecular weight compounds such as fatty acid derivatives inde-pendently and noncompetitively bind to sites within the adsorbent not recognized by the protein.
Semicompetitive Langmuir Model. The semicom-petitive approach with multicomponent mixtures also assumes that the adsorption phenomena occurs with either no or only partial competition [Jain and Snoeyink, 1973]. However, in this case it is assumed that the sites are not equally available to all species, rather than being exclusively associated with one or other species. The
isothermal expression of this model is given by
and
where
andKais the association constant,q*,qm, andC* refer to amount of adsorbed solute, the maximum capacity for the solute, and the concentration of the solute in the bulk solution while the subscripts 1 and 2 refer to the adsorbates 1 and 2, respectively.
With protein systems derived from human plasma fractionation, this model can be anticipated to be ap-plicable for mixtures containing components of signifi-cantly different molecular sizes and chemical properties. Such circumstances may arise when low molecular weight peptide fragments (e.g., from proteolysis of the proteins) preferentially bind to immobilized ligands ac-cessible on inner porous surfaces which are not acac-cessible to the intact, larger protein species [Beelaram and Sadana, 1994; Mao and Hearn, 1996].
Competitive Models. An alternative approach which can be used to describe the adsorption phenomena of a multicomponent system is to assume that there is total competition between target protein and other components in the mixture for adsorption to all the accessible sites of the immobilized ligand. The extension of the Lang-muir equation proposed by various investigators [Huang and Horvath, 1987a,b; Katti and Guiochon, 1988; Jacob-son et al., 1990; Katti et al., 1990; Ruzgas et al., 1990; Beelaram and Sadana, 1994; Srivastava and Tyagi, 1995; Table 1. Commonly Employed Theoretical Isotherm Models for the Adsorption of a Single Protein Component to an Immobilized Ligand Systema
Langmuir Isotherm
q*) qmC*
Kd+C*
Freudlich Isotherm
q*)Kκ
(C*)n
Langmuir-Freudlich Isotherm
q*) qm(C*)
n
K*d+(C*)
n
Temkin Isotherm
q*)qTln[1+KTC*]
Fowler-Guggenheim Isotherm
q*)(q
m-q*)C* exp(-âq*/qm)/Kd
Javanovic Isotherm
q*)q
m[1-exp(-θC*)]
Redlich-Petersen Isotherm
q*) qmC*
Kd+(C*)
n
S-shaped Isotherm
q*1) qm,1
+Kd,1C2
b1+b11
Kd,12C2
b11+q m,12C2
b12
and for multisite interaction
q*i)
qmC*i
1+
∑
j)1
N
Cjbj/K*d,j
q*2)
qm,2+Kd,2C2b2+b22
Kd,21C2b22+q m,21C1
b21
aLegend to symbols: C*, concentration of the protein in the bulk solution;C
jbj, concentration of the protein speciesjbinding to the
adorption sitebon the sorbent;q*, amount of protein bound at equilibrium to the adsorbent;qm, maximum capacity of the absorbent for the protein;qT, differential increase in the limiting capacity,qm, for protein adsorption with increasing binding affinity;KT, maximum
binding affinity constant;Kd, dissociation constant,Kd*, apparent dissociation constant;Kκ, an empirical constant;n, an empirical constant;
â, the interaction energy between two adsorbed molecules;θ, reciprocal of the dissociation constant,Kd; numerical subscripts (1, 11, 12,
and 22), correspond to different binding sites for the interaction of different molecules.
q*1)
a1C*1
1+bC*1 q*2)
a2C*2
1+bC*2 (1)
b1)Ka,1; b2)Ka,2
a1)b
1qm,1; a2)b2qm,2
q*1) a1C*1 1+b1C*1+b2C*2
[
(
1-a1b2b1a2
)
1+b1C*1(b2C*2+1)
]
(2)q*2) a2C*2
1+b1C*1+b2C*2 (3)
b1)K
a,1; b2)Ka,2
Mao and Hearn, 1996; Hortacsu and McCoy, 1996; Hossain and Do, 1996b] for the adsorption of two com-ponent mixtures can be expressed as
where the various terms are defined above and refer to adsorbates 1 and 2, respectively. The generalized ex-pression of this model for the adsorption of the various components within a multicomponent mixture takes the following form
where the termsa)bqmandb)Kaare defined above and the subscriptsiandjrefer to the adsorbatesiandj, respectively.
Although this generalized model has found application for multicomponent mixtures of organic compounds with substantial differences in molecular mass, charge, or hydrophobicity [Koj et al., 1978; Ruzgas et al., 1990], nevertheless this treatment assumes that the adsorbent has the same saturation capacity for all adsorbates present in the system. With most crude feed-stocks from biological extracts, this assumption will most likely not be physically realistic. Proteins and other components present in the feed-stock mixture are likely to have a range of different molecular sizes, and hence, these different substances will occupy different areas on the adsorbent surface. Moreover, during the adsorption process, proteins may undergo significant changes in their conformation [Tanford, 1968; Hortacsu and McCoy, 1996; Hossain and Do, 1996b]. These conformational changes will affect the binding capacities of the sorbent for the target protein and the other components present in the mixture. From a strictly physical perspective, this competitive Langmuir model is better suited to describing the adsorption of enantiomeric mixtures [Jacobson et al., 1990] rather than the adsorption of substances with widely different molecular and compositional properties. Nevertheless, the competitive Langmuir model remains one of the most commonly employed in the literature [Katti and Guiochon, 1988, 1990; Phillips et al., 1988] on the adsorption behavior of low molecular weight, biologically active substances and has as a consequence been examined in the present investigation as part of the comparative assessment of the binding behavior of R1 -PI in the presence of different concentrations of HSA.
Influence of Contaminants on the Equilibrium Adsorption of Proteins with Ion Exchange Ad-sorbents. Contaminants present in a crude feed-stock derived from the fractionation of human plasma can be classified into at least 10 categories. Five of these categories, mainly comprised of middle to high molecular weight substances, potentially represent the major con-tributors to the variation in the isothermal behavior of a target protein with a porous ion exchange adsorbent. The remaining five categories include the bulk water, low molecular weight detergents, and various salts or other so-called “cosolvents” which are added as purification aids for the inactivation of viruses or the removal of ag-gregated complexes during the initial steps of the Cohn fractionation of human plasma [Harris and Angal, 1989; Hearn and Anspach, 1990; Saha et al., 1992; Spangna and Pifferi, 1992; Thevenod et al., 1992; Jensen et al., 1995]. The influences of these latter contaminants relate
more to dilution and ionization effects and, hence, to the role of buffer capacity on volume overload of the adsorp-tion process when inappropriate buffer, pH, or sample dilution conditions are employed.
Superimposed on these effects, however, will be the consequences of competitive binding processes due to the remaining five categories of contaminants, each of which is able to affect the selectivity, recovery, and performance of a particular adsorbent target protein system. First, with crude feed-stocks derived from the cryoprecipitate/ supernatent and Cohn ethanol fractionation of human plasma, some contaminants will predominantly remain suspended in the feed-stock containing the target protein but will nevertheless cause significant changes in the dynamic adsorption properties of the adsorbent as well as the fluid dynamic properties of the packed or expanded bed. This first group of contaminants typically are represented by residual amounts of cell debris, protein aggregates, and protein-lipid or protein-polysaccharide complexes. These macromolecular contaminants will be physically too large to diffuse to any significant extent into the porous network of the adsorbent. Depending on whether the binding of this group of contaminants to the exterior surfaces of the porous adsorbent is reversible or irreversible, pore entry congestion and significantly reduction in the fluid flow characteristics of the adsorbent may result. This will lead to an elevation of the back pressure in packed beds of the adsorbent and particle agglomeration with expanded or fluidized bed systems [Draeger and Chase, 1991; Chase, 1994; Bjorklund and Hearn, 1996]. Also associated with the binding of these high molecular weight contaminants will be changes in the effective capacity and variation in the selectivity of the adsorbent for the desired target protein as a function of the loading conditions, due to loss of binding sites and modification of the surface chemistry of the adsorbent. Contaminants belonging to this group have traditionally been removed from the feed-stock by additional steps of centrifugation, membrane-mediated ultrafiltration, or specific salt and/or solvent fractionation procedures [Har-ris and Angal, 1989; Hearn and Anspach, 1990; Leterme and Boutry, 1993; Srivatava and Tyagi, 1995; Fayed et al., 1995; Ragoport et al., 1995]. In common with similar procedures used as removal processes with the other four major categories of contaminants discussed below, the price of these additional steps is often reduced yield of the desired protein.
Contaminants belonging to the four remaining groups usually consist of substances with average hydrodynamic diameters that are typically similar to or smaller than the diameter of the pores of the adsorbent. Thus, the different components which belong to each of these contaminant classes can diffuse inside the porous net-work to different extents. Depending on the chemical and physical properties of these contaminants, however, they will affect the binding capacity of the adsorbent in different ways. For example, contaminants with no structural similarity to the target protein, but which can collectively include partially degraded proteolytic frag-ments of other proteins, membrane associated protein fragments, DNA fragments, or lipopolysaccharides, can mask the primary, as well as any accessible secondary, binding sites on the sorbent in a manner analogous to the first group of pore-excluded contaminants discussed above. As a consequence, this second group of contami-nants, once they have diffused into the porous network, will affect the extent of adsorption of the target protein through steric hindrance, binding site depletion, and concentration dilution effects. If these contaminants interact with the adsorbent via specific processes (i.e., competitive interactions involving primary binding sites)
q*1)
a1C*1 1+b
1C*1+b2C*2 ; q*2)
a2C*2 1+b
1C*1+b2C*2
(4)
qi)
aiCi
1+
∑
j)1
N
bjCj
of low to intermediate affinity or via nonspecific processes (i.e., noncompetitive interactions with secondary binding sites), it may be possible to desorb many of these components by extensive washing of the adsorbent particles with a buffer of slightly weaker elutropic strength than required for the elution of the target protein [Koj et al., 1978; Mao and Hearn, 1996]. This washing step will allow these contaminants to diffuse from the porous network back into the bulk solution and thus to be swept from the adsorbent particle when either batch (stirred tank) or column configurations are em-ployed.
The third group of contaminants in partially fraction-ated human plasma protein preparations includes sub-stances that can be adsorbed nonspecifically with rela-tively high affinities onto the adsorbent via secondary binding sites (i.e., they bind via sites other than those directly involved in the primary selectivity of the ad-sorbent). As such, the binding behavior of this group of contaminants superficially will satisfy the criteria of noncompetitive or semicompetitive isotherms. De-pending on the type of adsorbent, the quality of its manufacture, the extent of chemical heterogeneity of the adsorbent surface, and whether attention has been given to the selection of the composition and pH of the loading buffer, the impact of this third group of contaminants can assume significant proportions in modifying the isothermal behavior of the target protein in a purification task. Adventitious binding of this group of contaminants will lead to progressive changes in the selectivity of the adsorbent as a function of loading time and cycle number. Often contaminants belonging to these three groups collectively constitute the largest mass of biomacro-molecular components in a crude feed-stock, although each component may be present in relatively low abun-dance which may vary between different batches of the feed-stock. Inappropriate or uncontrolled participation of these contaminants in these binding events can lead to severe feed-stock-dependent variations in the capacity and selectivity behavior of the adsorbent for the target protein as well as significant regeneration difficulties. In order to minimize the participation of these unfavorable binding effects, loading and wash buffers which are not fully compatible with the properties of the target protein may have to be used. As a consequence, decreased recoveries of the target protein may result [Yamamoto et al., 1988; Hearn and Anspach, 1990; Ruzgas et al., 1990; Levison et al., 1994].
Components represented by the fourth group of con-taminants directly compete with the target protein for the same adsorption sites of the adsorbent, and thus directly affect the binding capacity but not the selectivity. Often, these contaminants consist either of post-trans-lationally modified forms of the same target protein(s) or other proteins of totally different structure but with similar three dimensional surface regions which mimic in molecular properties the binding site characteristics of the protein of interest. Depending on their relative abundances, their affinities for the adsorbent, and their conformational stability profiles, the different members of this fourth group of contaminants can have very similar adsorption behavior very similar to that mani-fested by the target protein. This behavior will lead to very similar isothermal behavior and separation selec-tivities and thus represents a major optimization chal-lenge if the desired product is to be resolved in very high purity from the other similar binding components in the feed-stock (Mao and Hearn, 1996).
The combined influences of each of the above categories of contaminants will lead to reduced dynamic adsorption capacities (DAC) and reduced dynamic adsorption rates
(DAR) for the protein of interest, although enhancement of DAC in association with reduced DAR have also been observed in several circumstances, usually as a conse-quence of protein-protein multilayer phenomena. Thus, the adsorption capacity of individual proteins such as albumin, fibrinogen, and γ-globulin onto hydrophobic polymers has typically been found to be higher when the protein is presented as a single component rather than as part of a mixture of each protein [Lee et al., 1974]. Similar observations have been made with many other types of naked and chemically modified inorganic ad-sorbents (e.g., the observations of Beissinger and Leonard [1982] on the binding behavior with mixtures of albumin andγ-globulin incubated with quartz are representative). The opposite behavior has also, however, been noted, with greater binding capacities of target proteins observed in the presence of other proteins. Scopes [1994], for ex-ample, found that there was a 4-fold increase in the binding capacity of pyruvate kinase to CM-cellulose in the presence of lysozyme, while Johnston and Hearn [1996] have observed increased capacities with several plasma proteins and attributed this phenomenon to a so-called “multilayering roll-over” effect at the adsorbent surface.
Finally, a fifth group of contaminants within the feed-stock can act on the target protein itself, inducing degradation, proteolysis, and changes in the conforma-tion, thus affecting the binding capacity of the target protein with the adsorbent. In order to minimize the impact of this fifth group of contaminants, special precautions such as temperature control, the addition of enzyme inhibitors [Sponen et al., 1991] to the buffers/ eluents, or the use of preadsorption steps involving specific adsorbents bearing affinity ligands to capture these degradative contaminants are necessary.
Evaluation of the Experimental Equilibrium Ad-sorption Isotherms. As evident from the following results, the equilibrium binding and elution behavior of feed-stock mixtures containing HSA and R1-PI exhibit features characteristic of the competitive influences of some of the above five groups of contaminants. Experi-ments were initially carried out using the batch (finite bath) approach, and the results were then compared with experimental data obtained with packed beds. The experimental data from the batch adsorption experiments were processed in a similar way as reported previously [Anspach et al., 1989; Finette et al., 1996a,b]. Figure 1 shows the binding isotherm of HSA andR1-PI obtained from the experimental batch adsorption data acquired
with the ion exchange adsorbent, DEAE-Spherodex. Within the range of protein concentrations studied, the theoretical curves ofq* versusC* derived for HSA and R1-PI fitted the experimental data with good correlations (i.e., small residuals as assessed from nonlinear least squares fit analysis), particularly at the higher protein concentrations. This result indicates that the Langmuir model, which assumes that only one type of interaction takes place between both proteins and the functional dimethylamino groups immobilized onto the adsorbents, may provide a reasonable approximation for the adsorp-tion behavior of HSA and R1-PI to DEAE-Spherodex under these batch adsorption conditions. From Figure 1, it can be observed that the adsorption isotherm obtained for the equilibration binding of R1-PI onto DEAE-Spherodex was steeper at lower protein concen-trations compared to the corresponding experiments with HSA, indicating that DEAE-Spherodex has a greater affinity for R1-PI than for HSA. Consistent with this conclusion, the apparent dissociation constants (Kd) extracted from the plot ofC* versusC*/q* were equal to 1.7 × 10-7 M for R1-PI and 7.6 × 10-7 M for HSA, respectively (Table 2), while the maximum binding capacities qm of both proteins with the adsorbent were similar under these batch adsorption conditions.
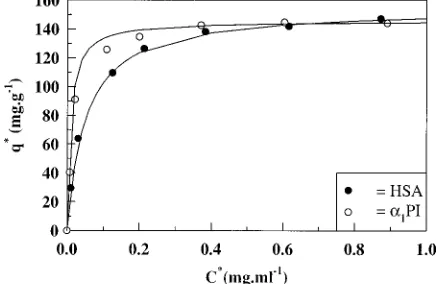
Single Component Adsorption. The shape and position of the breakthrough profile are the source of two important parameters that characterize the affinity and the binding capacity of an adsorbent for a protein. The single-component breakthrough curves for HSA andR1 -PI with packed beds of DEAE-Spherodex are shown in Figure 2. A high degree of asymmetry was observed in the breakthrough curve of R1-PI associated with an increase in the slope of the breakthrough profile at ca. 70% of bed saturation. At least three explanations can be advanced to account for the significant increase in slope of the breakthrough profile obtained from the adsorption ofR1-PI onto DEAE-Spherodex. First, it is possible that the highly purifiedR1-PI preparation was itself not a single component of unique composition and amino acid sequence, but rather a microheterogeneous mixture. Once some of theseR1-PI-related molecules had differentially bound to the functional groups on the adsorbent, these molecules could be displaced by other protein molecules present in the sample having greater
affinity for the immobilized functional groups. Second, theR1-PI preparation may contain a range of folded and partially unfolded species, with all of these species competing for the binding sites. Where specific folded or unfolded species have greater affinity for the ad-sorbent, these species will displace other folded or partially unfolded forms of lower affinity characteristics, leading to a change in the shape of the breakthrough profile during the initial stages of the loading process. Once all of the binding sites have been saturated with these specific conformational species of R1-PI, any re-maining species in the bulk solution will be detected at the column outlet, leading to a change in the shape of the breakthrough profile during the final stage of the loading process. Third, it is known [Myerowitz et al., 1972; Murthy and Hercz, 1973; Peterson and Sober, 1975; Musiani and Tomasi, 1976; Mast et al., 1992] thatR1-PI can exist in several proteolytic cleaved forms, each or all of which could have a greater affinity for the adsorbent. Assessment of theR1-PI preparations used in this inves-tigation by capillary electrophoresis and functional as-says has confirmed that the latter process constituted the major cause of the microheterogeneity seen with theR1 -PI breakthrough profile. The breakthrough profile of HSA also displayed a high degree of asymmetry particu-larly as bed saturation was approached at C/C0 ) 1. However, no corresponding sharp increase in the gradient of the breakthrough profile was observed.
From the breakthrough profiles, the amount of protein adsorbed onto the adsorbent was determined and the results are shown in Table 3. The capacity of the adsorbent for both proteins were similar, for example, 141 mg of HSA per gram of adsorbent and 142.8 mg of R1-PI per gram of adsorbent. These data with packed bed systems are within the same range as those obtained from the batch equilibrium binding experiments de-scribed above. The total amount of each respective protein collected during the washing stage, the first elution stage, and the second elution stage was also determined by rocket immunoelectrophoresis. The val-ues obtained from these quantitative immunoelectro-phoresis experiments were within the same range as those calculated from the integration of the area behind the breakthrough profiles of HSA andR1-PI, for example, 141 mg of HSA per gram of adsorbent was adsorbed compared to 140.8 mg per gram of adsorbent recovered following the washing and elution stages. The amount ofR1-PI (115 mgR1-PI per gram of adsorbent) collected at the second elution stage was ca. 80% of the amount of R1-PI adsorbed per gram of adsorbent. This difference between the amount of R1-PI recovered following the wash and elution steps and that loaded can be explained in terms of the strength of the elution buffer. The elution buffer employed in these experiments (0.15 M sodium acetate buffer, pH 4.5) was found from subsequent investigations to be unable to displace all theR1-PI bound to the adsorbent (as assessed from the immunoreactivity
Figure 2. Single-component breakthrough curves for the adsorption of HSA andR1-PI onto DEAE-Spherodex in packed beds of the same column dimensions containing the same amount of adsorbent. The flow rate employed was 1 mL min-1, and the protein solutions were loaded at a concentration of 1 mg mL-1.
Table 2. Equilibrium Parameters for the Adsorption of HSA andr1-PI onto DEAE-Spherodex Measured in Batch Experiments
proteins
qm
(mg g-1)
10-6qm
(mol L-1)
Kd
(mg mL-1)
10-7Kd
(M) HSA 153 2.3 0.05 7.6
R1-PI 145 2.7 0.009 1.7
Table 3. Amount of HSA andr1-PI Recovered During
Each Chromatographic Stage after Being Passed through a Packed Column Containing DEAE-Spherodex
HSA R1-PI concentration of protein at the inlet (mg mL-1) 1 1
amount adsorbed (mg g-1of adsorbent) 141 143
amount collected, washing (mg g-1of adsorbent) 36 9
amount collected, first elution (mg g-1
of adsorbent)
90 11
amount collected, second elution (mg g-1
of adsorbent)
15 115
total amount collected, washing plus elution (mg g-1of adsorbent)
from the rocket immunoelectrophoresis), and a higher ionic eluent was required to achieve this desorption.
From the rocket immunoelectrophoresis results with the protein collected after the washing and elution stages, it was evident that a proportionally greater amount of HSA was recovered after the washing process, indicating that the interaction between the HSA molecules and the secondary binding sites as well as some of the primary functional groups immobilized onto the adsorbent were weaker than the interaction between theR1-PI molecules and the same two classes of binding sites on the ad-sorbent.
Table 3 shows that the amount of HSA collected after a two-stage elution procedure was greater in the first elution fraction, while the amount ofR1-PI collected was greater in the second elution fraction. When these two proteins were loaded separately onto the column, 63% of the total amount of HSA loaded was recovered at the first elution step (i.e., with the eluent 0.02 M acetate buffer solution pH 4.5) and 86% of the total amount of R1-PI loaded was recovered from the second elution step (i.e., with the eluent 0.15 M acetate buffer solution pH 4.5). As shown in Table 3, only 8% of the R1-PI was also recovered from the first elution step. The fact that little of the R1-PI was recovered from the first elution step again qualitatively confirms that the association constant for the interaction betweenR1-PI and DEAE-Spherodex was greater than that with HSA. The amount of HSA collected at the second elution stage with the stronger elution buffer (with the eluent 0.15 M acetate buffer solution pH 4.5) was 7.3 mg per gram of adsorbent, indicating that in the range of concentration studied some lateral interaction involving adsorbed HSA dimers or secondary binding to of HSA monomers other classes of ligand binding sites could have occurred. In other investigations [Oddie et al., 1996], we have shown that the interaction of HSA or BSA dimers with positively charged functional groups immobilized as strong anion exchange adsorbents involves smallerKdvalues than the corresponding interactions between the monomer and the same functional groups immobilized onto the adsorbent. As a result, the buffer used to elute monomeric HSA from the column (0.02 M sodium acetate buffer, pH 4.5) would not have been strong enough to displace HSA dimeric species. These data lead to the conclusion that further purification step(s) would have to be performed to remove HSA from the recovered second elution stage fractions containingR1-PI, when mixtures of HSA andR1-PI are coloaded onto a column containing DEAE-Spherodex.
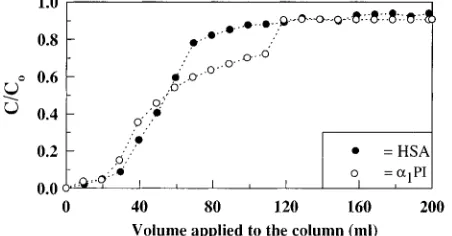
Two-Component Mixtures. The normalized con-centration profiles for HSA and R1-PI when a solution containing a mixture of each protein at a concentration of 1 mg mL-1was loaded onto a packed bed of DEAE-Spherodex are shown in Figure 3. Several important observations can be made from these results. First, it was evident that the shape of the breakthrough profile generated from the loading of an equivalent mass of HSA andR1-PI during the initial stage of loading were similar to the breakthrough profiles observed when each of these proteins was individually loaded onto the same column. Up to 80% saturation, the breakthrough profile ofR1-PI in the double-component mixture was very similar to that exhibited by R1-PI alone. The breakthrough profile generated by rocket immunoelectrophoresis for the ad-sorption of HSA when present as a mixture was steeper than the profile for the protein alone, indicating that HSA was displaced byR1-PI. An increase in the slope of the breakthrough profile of R1-PI was also observed at ca. 80% of bed saturation, indicating that after displacing HSA from some of the sites present on the adsorbentR1 -PI itself was being displaced by another component(s) in
the mixture. This displacement trend is not without precedent [Subramanian and Cramer, 1989; Hearn and Anspach, 1990; Johnston and Hearn, 1996] and indicates that contaminant protein(s) can bind competitively in the presence of R1-PI to the same functional groups im-mobilized onto the adsorbent, thus reducing the number of binding sites available forR1-PI adsorption.
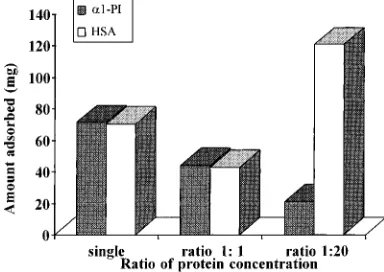
In subsequent overload experiments, 1:1 and 1:20 HSA/ R1-PI mixtures were loaded onto packed beds, and rocket immunoelectrophoresis was also performed to quantify the amount of HSA and R1-PI recovered after each loading, washing, and elution stage. The results are shown in Tables 4 and 5. In these experiments, the total amount of protein recovered after the loading, washing and elution stages was 226 mg forR1-PI and 233 mg for HSA respectively, normalized per milliliter of adsorbent. The total amount of HSA recovered from the loading and washing steps alone was equal to 215 mg (i.e., 92% of the total amount of HSA loaded onto the column). These results demonstrate that a larger amount of HSA was recovered in the combined breakthrough and wash frac-tions while the amount of R1-PI adsorbed was reduced when an equivalent mass of HSA andR1-PI was loaded onto the column. When expressed in terms of milligram of protein per gram of adsorbent, the amount of R1-PI adsorbed, determined from the integration of the break-through profile, was 144 mg per gram of adsorbent when R1-PI was loaded alone onto the column packed with DEAE-Spherodex. This amount was reduced to 88 mg per gram of adsorbent when a mixture containing an equivalent mass of HSA and R1-PI was loaded in the column. Similarly, in the presence ofR1-PI at the same inlet concentration, the amount of HSA adsorbed was reduced to 54 mg per gram of adsorbent, compared to 141 mg per gram of adsorbent when the HSA alone was loaded onto the column packed with the same amount of adsorbent.
Figure 3. Breakthrough profiles for the adsorption of HSA and
The experimental adsorption values of HSA andR1-PI were compared with the corresponding values derived from the noncompetitive model and the fully competitive model (eqs 1 and 4). These two models were chosen because they represented the broadest description of the adsorption phenomena of a two-component mixture. As noted above, the noncompetitive Langmuir model as-sumes that no competition occurs for the available adsorption sites between the two proteins while the fully competitive model assumes that both proteins are freely able to compete for the same number of binding sites on the DEAE-Spherodex. With both models, it was assumed that equilibrium was reached in the column and the inlet concentration of respective proteins (C1andC2) could be substituted forC1* andC2* in eqs 1 and 4, respectively. The amount of each protein bound to the adsorbent in the packed column experiments and the calculated values from these two respective models are shown in Table 6. It can be observed that the experimentally determined value of 54 mg of HSA adsorbed per gram of adsorbent from the 1:1 mixture was not accurately predicted by the competitive or noncompetitive models, although the noncompetitive model provided a value similar to that experimental observed for the batch and column adsorp-tion experiments with HSA alone. Similarly, the experi-mentally determined value of 88 mg per gram of ad-sorbent forR1-PI was not predicted by either the fully competitive or the noncompetitive Langmuir models.
These discrepancies between the experimental and pre-dicted values calculated from either model of multicom-ponent Langmuir-type adsorption indicate that the ad-sorption processes withR1-PI-HSA mixtures with DEAE-Spherodex are more complex than either model can describe (i.e., additional secondary interactions must occur during the interaction of the protein with the ion exchanger).
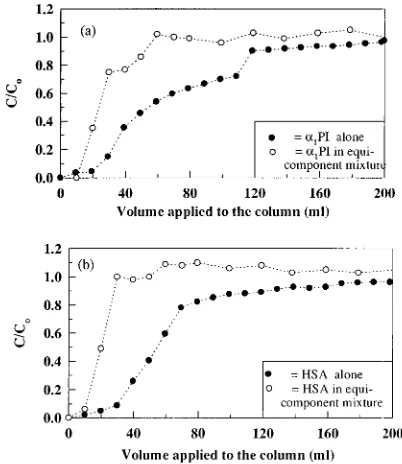
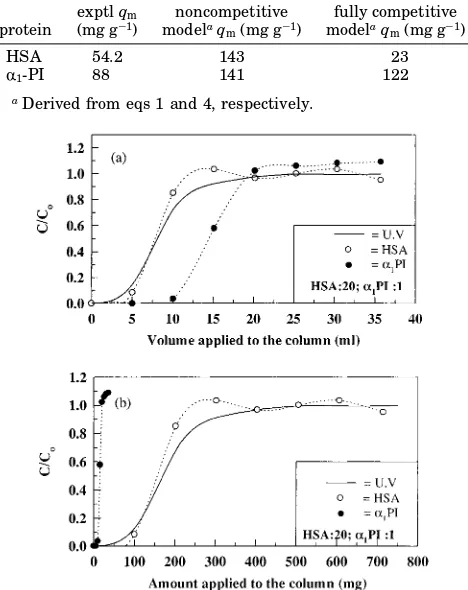
The effects of this more complex competitive binding were clearly demonstrated when HSA at a higher con-centration thanR1-PI was loaded together with the latter protein onto the column. Figure 4 shows the normalized concentration profiles generated when a solution contain-ing a mixture ofR1-PI and HSA (in the ratio of 1:20) was loaded onto the column. Figure 4a illustrates the break-through profile ofR1-PI (plotted as the volume of protein solution loaded versus C/C0) was shifted to the larger volume values. However, when the amount of the specific protein applied to the column (volume applied× inlet concentration) was plotted versusC/C0(Figure 4b), the opposite trend was observed for each respective protein, with the breakthrough profile of R1-PI shifted to lower values.
Figure 5 shows as a histogram the amount of R1-PI adsorbed when (a) a single component, (b) a 1:1 mass mixture, and (c) a 20:1 mixture of HSA andR1-PI was loaded respectively onto a column packed with DEAE-Spherodex. As evident from Figure 5, the amount ofR1 -PI adsorbed decreased when the competitive contaminant Table 4. Amount of the Respective Proteins Recovered
after Each Chromatographic Stage Determined from Rocket Immunoelectrophoresis
ratio of inletR1-PI
to inlet HSA (1:1) R1-PI (mg) HSA (mg) summation of pooled
loading sample+
washing sample+
elution sample
225.96 232.48
R1-PI HSA
recovery of respective protein
amount (mg)
recovery (%)a
amount (mg)
recovery (%)
ratio ofR1-PI to HSA in each fraction washing process 1.5 0.7 5.4 2.3 22%R1-PI
78% HSA first elution 4.5 2 16.1 6.9 22%R1-PI
78% HSA second elution 35.4 16 4.9 2.1 88.%R1-PI
12% HSA third elution 2.6 1 0.7 0.3 79%R1-PI
21% HSA
aThe % recovery was defined as the percentage of each protein
loaded onto the column and collected in each chromatographic step.
Table 5. Amount of the Respective Proteins Recovered after Each Chromatographic Stage Determined from Rocket Immunoelectrophoresis
ratio of inletR1-PI
to inlet HSA (1:20) R1-PI (mg) HSA (mg)
summation of pooled loading sample+
washing sample+
elution sample
36.98 719.5
R1PI HSA
recovery of respective protein
amount (mg)
recovery (%)
amount (mg)
recovery (%)
ratio ofR1-PI to HSA in each fraction washing process 0.9 2.4 55 7.7 2%R1-PI
98% HSA first elution 3.9 10.4 46 6.4 8%R1-PI
92% HSA second elution 14.5 39.1 16.6 2.3 47%R1-PI
53% HSA third elution 1.8 1.8 2.9 0.4 38%R1-PI
62% HSA
Table 6. Measurement of theqmEquilibrium Parameter
for the Adsorption of HSA andr1-PI onto DEAE-Spherodex in Packed Columns Using a 1:1 Mixture of the Two Proteins
protein
exptlqm
(mg g-1)
noncompetitive modelaqm(mg g-1)
fully competitive modelaqm(mg g-1)
HSA 54.2 143 23
R1-PI 88 141 122
aDerived from eqs 1 and 4, respectively.
(HSA) was present in the solution. Competitive binding was clearly demonstrated by the greater amount of HSA adsorbed onto DEAE-Spherodex when the ratio of HSA toR1-PI was increased from 1:1 to 20:1. These observa-tions are in accordance with the participation of a competitive binding model. Since more HSA molecules are present, these molecules can compete withR1-PI for the binding sites, despite the fact thatR1-PI has a greater affinity for the adsorbent. Contrary to the result when a solution ofR1-PI alone was loaded onto the column, the relativeR1-PI yield (defined as the percentage ofR1-PI loaded onto the column and recovered in the second elution) was greater when a solution containing the mixture of HSA (20 mg mL-1) andR
1-PI (1 mg mL-1) was loaded (i.e., 40% ofR1-PI was recovered compared to 16% when a solution containing a mixture of HSA andR1-PI each having a concentration of 1 mg mL-1 was em-ployed). The percentage recovery of HSA was un-affected by changes in the inlet concentration, with 92% of HSA recovered when a 1:1 mixture was loaded onto the column compared to 91% when the 20:1 mixture was loaded.
From rocket immunoelectrophoresis and SDS-PAGE results, it was evident that the purity of R1-PI in the recovered fractions was greater when a solution contain-ing a mixture of HSA andR1-PI each having a concentra-tion of 1 mg mL-1 was loaded onto the column. The purity ofR1-PI was 90% compared to 50% in the presence of a higher concentration of HSA. It is possible that lateral interaction involving HSA molecules can occur at these higher protein concentrations. The interaction between the HSA dimers and oligomers with the ad-sorbent being stronger than the interaction between the HSA monomer and the adsorbent would result in the HSA dimer not being desorbed by first elution (wash) buffer as readily and hence will partially co-elute with theR1-PI at the second elution step.
Conclusions
The experimental results show that the Langmuir model could be used to predict with reasonable accuracy (i.e., with small least squares residuals) the adsorption of HSA orR1-PI onto the ion exchanger DEAE-Spherodex from single-component solutions. Similar observations have been made for other proteins with various other types of adsorbents by previous investigators [Subrama-nian and Cramer, 1989; Anspach et al., 1990; Katti and Guiochon, 1990; Katti et al., 1990; Ming and Howell, 1994; Hortacsu and McCoy, 1996; Mao and Hearn, 1996]. The maximum capacity extracted from the semireciprocal
plot was similar to the experimental values for both proteins with the affinity of DEAE-Spherodex forR1-PI approximately six times greater than the affinity of the same adsorbent for HSA. The frontal analysis results on the q* values for these two proteins at an inlet concentration of 1 mg mL-1paralleled the data derived from batch (bath) equilibrium measurements for R1-PI and HSA with DEAE-Spherodex. In addition, competi-tive binding was demonstrated when solutions containing mixtures of HSA andR1-PI having a protein concentra-tion>1 mg mL-1were loaded onto packed beds of DEAE-Spherodex. Under these competitive conditions, a sig-nificant reduction in the amount of bound HSA andR1 -PI was observed. The results thus raise a caution with regard to the choice of the adsorption model which is employed to simulate the binding behavior of multicom-ponent protein mixtures of proteins derived from human plasma as well as other complex biological sources, since both the noncompetitive and competitive Langmiurean adsorption models significantly overestimated the com-petitive binding capacities for HSA andR1-PI with the ion exchange binding sites.
Acknowledgment
This investigation was supported by the Australian Research Council. During the course of this work, G. M. S. Finette was a recipient of a Centre for Bioprocess Technology Graduate Scholarship and M. T. W. Hearn was a recipient of an Alexander von Humboldt Fors-chungspreis. The very useful discussions with former graduate students, Drs. Anna Johnston and Jeff Davies, are also acknowledged.
Literature Cited
Anspach, F. B.; Wirth, H.-J.; Unger, K. K.; Stanton, P.; Davies, J. R.; Hearn, M. T. W. High performance liquid affinity chromatography with phenylboronic acid, benzamidine tri-L-alanine and concanavalin A immobilized on 3-isothio-cyanatopropyltriethoxysilane-activated non porous monodis-perse silicas.Anal. Biochem.1989,179, 171-181.
Beelaram, A.; Sadana, A. High resolution fractionation proc-esses: chromatographic techniques.Isol. Purif.1994,1, 45
-62.
Beissinger, R. L.; Leonard, E. F. Sorption kinetics of binary protein solutions: general approach to multi-component systems.J. Colloid Interface Sci.1982,85, 521-533.
Bellot, J. C.; Condoret, J. S. Modelling of liquid chromatographic equilibria.Process. Biochem.1983,28, 365-376.
Bjorklund, M.; Hearn, M. T. W. Characterisation of silica-based heparin affinity sorbents through column chromatography of plasma fractions containing thrombin.J. Chromatogr.1996, in press.
Chase, H. A. Purification of proteins by adsorption chromatog-raphy in expanded beds.TEBTECH1994,12, 296-303. Draeger, N. M.; Chase, H. A. Liquid fluidised bed for protein
purification.Trans. Inst. Chem. Eng.1991,69,45-52. Dowd, V.; Yon, R. J. Heterogeneous binding of aldolase to
phosphocellulose: interpretation in terms of a concerted cluster model of multivalent affinity.J. Chromatogr.1992,
627, 145-151.
Fayed, A. E.; Zidan, Z. H.; Abou, Z.; Arab, A. A. K.; Magdoub, M. N. I. Ultrafiltration membrane permeability of some milk contaminants.Int. Dairy J.1995,5, 569-576.
Finette, G. M. S.; Mao, Q. M.; Hearn, M. T. W. Studies on the expansion characteristics of fluidised beds with silica-based adsorbents used in the purification of proteins. J. Chro-matogr.1996a,743, 57-73.
Finette, G. M. S.; Mao, Q. M.; Hearn, M. T. W. Comparative studies on the isothermal characteristics of proteins adsorbed under batch equilibrium binding conditions to coulombic, immobilized metal ion and dye affinity matrices with different ionic strength and temperature conditions.J. Chromatogr.
1996b, in press.
Graham, D. The characterization of physical adsorption systems. 1. The equilibrium function and standard free energy of adsorption.J. Phys. Chem.1953,57, 665-669.
Harris, E. L. V.; Angal, S.Protein purification methods; IRL Press: Oxford, 1989; pp 1-317.
Hearn, M. T. W.; Anspach, B. Chemical, Physical and biological concepts in isolation and purification of proteins. In Separa-tion Processes in Biotechnology; Asenjo, J. A., Ed.; Marcel Dekker, Inc.: New York, 1990; pp 17-65.
Hortacsu, A.; McCoy, B. J. Chromatographic separation of dynamically interchanging protein isomers.Isol. Purif.1996,
2, 133-148.
Hossain, Md. M.; Do, D. D. Displacement chromatography of a binary mixture of proteinsseffect of protein aggregation.Isol. Purif.1996a,2, 149-164.
Hossain, Md. M.; Do, D. D. Displacement chromatography of mixtures containing a protein and an impurity: effect of protein denaturation.Isol. Purif.1996b,2, 201-216. Huang, J. X.; Horvath, Cs. Adsorption isotherms on
high-performance liquid chromatographic sorbents. I. Peptides and nucleic acid constituents on octadecylsilica.J. Chromatogr.
1987a,406, 275-284.
Huang, J. X.; Horvath, Cs. Adsorption isotherms on high-performance liquid chromatographic sorbents. II. Proteins on cation exchangers with silica support.J. Chromatogr.1987b,
406,285-294.
Jacobson, S.; Golshan-Shirazi, S.; Guiochon, G. Chromato-graphic band profiles and band separation of enantiomers at high concentration.J. Am. Chem. Soc.1990,112, 6492-6498.
Jain, J. S.; Snoeyink, V. L. Adsorption from bisolute systems on active carbon.J. Water Res.1973,45, 2463-2479. Jensen, W. A.; Armstrong, J. McD.; De Giorgio, J.; Hearn, M.
T. W. Stability studies on maize leaf phosphoenolpyruvate carboxylase: Effect of salts.Biochemistry1995,34, 472-480. Johnston, A.; Hearn, M. T. W. Protein-protein displacement effects in preparative ion exchange chromatography of mul-ticomponent protein mixtures: Studies with plasma proteins derived from Cohn Fraction II+ III Supernatant.Int. J. Biochromatogr.1996, submitted.
Johnson, R. D.; Arnold, F. H. The Temkin model describes heterogeneous protein adsorption. Biochim. Biophys. Acta
1995,1247, 293-297.
Katti, A. M.; Guiochon, G. Prediction of band profiles in displacement chromatography by numerical integration of a semi-ideal model.J. Chromatogr.1988,449, 25-40.
Katti, A. M.; Guiochon, G. Quantitative comparison between experimental band profiles of binary mixtures in overloaded elution chromatography and then profile predicted by the semi-ideal model.J. Chromatogr.1990,499, 21-35. Katti, A. M.; Huang, J.-X.; Guichon, G. Prediction of the profiles
of the elution bands of proteins in preparative liquid chro-matography.Biotechnol. Bioeng.1990,36, 288-292. Koj, A.; Hatton, M.; Wong, K. L.; Regoeczi, E. Isolation and
partial characterization of rabbit plasma R-1-antitrypsin.
Biochemistry1978,169, 589-596.
Lee, R. G.; Adamson, C.; Kim, S. W. Competitive adsorption of plasma proteins onto polymer surfaces.Thrombosis Res.1974,
4, 485-490.
Lee, T.-H.; Thompson, P. E.; Aguilar, M. I.; Hearn, M. T. W. Conformational stability of aâ-turn motif in human growth hormone [6-13] peptide analogues at hydrophobic surfaces.
Int. J. Pept. Protein Res.1996, in press.
Leterme, S.; Boutry, M. Purification and preliminary charac-terization of mitochondrial complex I (NADH: Ubiquinone reductase from broad bean (Vicia faba L). Plant Physiol.
1993,102, 435-443.
Levison, P. R.; Badger, S. E.; Toome, D. W.; Streater, M.; Cox, J. A. Process scale evaluation of a fast flowing anion-exchange cellulose.J. Chromatogr.1994,658, 419-428.
Mast, A. E.; Enghild, J. J.; Salvesen, G. Conformation of the reactive site loop of R1-PI probed by limited proteolysis.
Biochemistry1992,31, 2720-2728.
Mao, Q. M.; Hearn, M. T. W. Optimisation of affinity and ion-exchange chromatographic processes for the purification of proteins.Biotechnol. Bioeng.1996,52, 202-222.
McKay, G.; Duri, B. A. Prediction of multicomponent adsorption equilibrium data using empirical correlations.Chem. Eng. J.
1989,41, 9-23.
Ming, F.; Howell, J. A. Operational properties of an inverted matrix cellulose CM ion exchanger.Bioseparation1994, 4, 63-70.
Murthy, R.; Hercz, A. Purification of human a1antitrypsin by affinity chromatography on sepharose bound to Concanavalin A.FEBS Letts.1973,32, 243-246.
Musiani, P.; Tomasi, T. Isolation, chemical, and physical proper-ties ofR-1-antitrypsin.Biochemistry1976,15, 798-804. Myerowitz, R.; Handzel, Z.; Robbins, J. Human Serum a1
-antitrypsin. Isolation and demonstration of electrophoretic and immunologic heterogeneity.Clin. Chim. Acta1972,39, 307-317.
Nicoud, R. M.; Seidel-Morgenstem, A. Adsorption isotherms: Experimental determination and application to preparative chromatography.Isol. Purif.1996,2, 165-200.
Oddie, G.; Mao, Q. M.; Hearn, M. T. W. Unpublished results, 1996.
Peterson, E. A.; Sober, H. A. InThe plasma proteins; Putman, F. W., Ed.; Academic Press: New York, NY, 1975; pp 105
-127.
Phillips, M. W.; Subramanian, G.; Cramer, S. M. Displacement chromatography of biomolecules.J. Chromatogr.1988,439, 341-351.
Ragoport, E. M.; Zhigis, L. S.; Vlasova, E. V.; Piskanev, V. E.; Bovin, N. V.; Zubou, V. P. Purification of monoclonal antibod-ies to Le-y and Le-d carbohydrate antigens by ion exchange and thiophilic adsorption chromatography. Bioseparation
1995,5, 141-146.
Ruthven, D. M. In Principles of Adsorption and Adsorption Processes; Wiley Interscience: New York, 1984; pp 1-247.
Ruzgas, T. A.; Razumas, V. J.; Kulys, J. J. Sequential adsorption of gamma interferon and bovine serum albumin on hydro-phobic silicon surfaces.J. Colloid Interface Sci.1990,151, 136-143.
Saha, K.; Case, R.; Wong, D. K. Y. A simple method of concentrating monoclonal antibodies from culture super-natant by ultrafiltration.J. Immunol. Methods 1992, 151, 307-308.
Scopes, R. K. InProtein Purification; Springer Verlag: New York, 1994; pp 22-43.
Spangna, G.; Pifferi, P. G. Purification and Separation of Oenocyanin anthocyanins on sulphoxyethylcellulose. Food Chem.1992,44, 185-188.
Sponen, M.; Nick, M. P.; Schnebli, H. P. Different susceptibility of elastase inhibitors to inactivation by proteinases from Staphylococcus aureus and Pseudomonas aeraginosa.Biol. Chem. Hoppe-Seyler1991,372, 963-970.
Srivastava, S. K.; Tyagi, R. Competitive adsorption of substi-tuted phenol by activated carbon developed from the fertiliser waste slurry.J. Water Res.1995,29, 483-488.
Subramanian, G.; Cramer, S. M. Displacement chromatography of proteins under elevated flow rate and crossing isotherm conditions.Biotechnol. Prog.1989,5, 92-105.
Tanford, C. Protein denaturation.Adv. Protein Chem.1968,23, 121-175.
Thevenod, F.; Hvaase, W.; Hopfer, U. Large scale purification of calf pancreatic zymogen granule membranes.Anal. Bio-chem.1992,202, 54-60.
Wankat, P. C. InLarge scale adsorption and chromatography; CRC Press: Boca Raton, FL, 1986; pp 1-356.
Wei, J.; Hearn, M. T. W. Protein interaction with immobilized metal ion affinity ligands with high ionic strength buffers.
Anal. Biochem.1996, in press.
Yamamoto, S.; Nakanishi, K.; Matsuno, R. In Ion-exchange chromatography of proteins; Marcel Dekker, Inc.: New York, 1988; pp 1-401.
Accepted January 27, 1997.X
BP9700059