COMPARISON ON
IN VITRO
DIGESTION EFFECT OF ANTIOXIDANT
AND ANTIHYPERGLYCEMIC ACTIVITY FROM ANDALIMAN
(
Zanthoxylum acanthopodium
DC.) AND JAPANESE PEPPER
(
Zanthoxylum piperitum
DC.) CRUDE EXTRACT
VANESSA KARNADY
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
STATEMENT OF THESIS AND
SOURCES OF INFORMATION AND DEVOLUTION OF
COPYRIGHT*
I hereby declare that my thesis entitled Comparison on In Vitro Digestion Effect of Antioxidant and Antihyperglycemic Activity from Andaliman (Zanthoxylum acanthopodium DC.) and Japanese Pepper (Zanthoxylum piperitum DC.) Crude Extract, is an original piece of work, written and completed on my own, based on sources as listed on the work cited section and not a duplication of other writing that has been published in other Universities.
I hereby assign copyright of my paper to the Bogor Agricultural University.
Bogor, August 2015
SUMMARY
VANESSA KARNADY. Comparison on In Vitro Digestion Effect Of Antioxidant and Antihyperglycemic Activity From Andaliman (Zanthoxylum acanthopodium DC.) and Japanese Pepper (Zanthoxylum piperitum DC.) Crude Extract. Supervised under HANNY WIJAYA and ENDANG PRANGDIMURTI.
Hyperglycemia is one of important issue lately due to it will lead into other complication such as micro and macro vascular disease. Hyperglycemia is caused by the excess of glucose in the human body. This uptake of excess glucose causes imbalance between oxidants and antioxidants in the human body.
Andaliman(Zanthoxylumacanthopodium DC.) is a traditional exotic spice that grows in North Sumatra, Indonesia. This spice is one genus with Japanese pepper (ZanthoxylumpiperitumDC.) that mainly grows in Japan. Both of spices have been used as folk medicines since their phytochemical substances possessing strong antioxidant activity and allegedly to possess α-glucosidase inhibitor activity. Their activity, however, might be altered under gastrointestinal digestion due to the structure alteration of the responsible compounds. Therefore, this study was aim to
study and compare the changes of α-glucosidase inhibitor and antioxidant activities
of andaliman and Japanese pepper crude extracts under in vitro gastrointestinal digestion
The in vitro gastrointestinal digestion is mimicking the digestion condition in the gastric and small intestine. While, the determination of activity were done in vitro by using α-glucosidase enzyme inhibition assay and DPPH radical scavenging activity methods. Acarbose, a commercial inhibitor was used as positive control for α-glucosidase inhibition assay and ascorbic acid as positive control for DPPH radical scavenging activity.
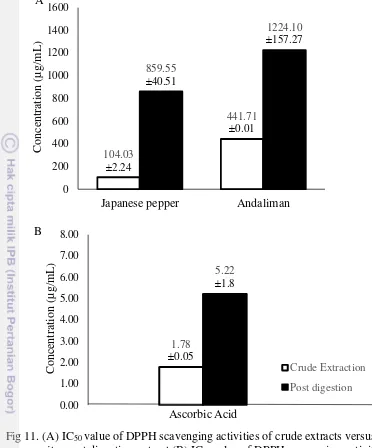
Before digestion simulation, the crude extract of Japanese pepper showed better inhibition activity towards α-glucosidase enzyme (IC50 = 3930.21µg/mL)
compare to andaliman (IC50 = 20346.94µg/mL). The crude extract of Japanese
pepper also showed better radical scavenging activity towards DPPH (IC50
=104.03µg/mL) comparing to andaliman (IC50 = 20346.94µg/mL).
In vitro gastrointestinal digestion decreased the antioxidant activity and α -glucosidase inhibition of both spices. Japanese pepper’s α-glucosidase inhibition activity were lost 1.42 times while andaliman lost 1.77 times. However, in DPPH radical scavenging activity, andaliman were lost only 2.77 times while Japanese pepper lost 8.26 times. Antioxidant activity of andaliman was more stable than Japanese pepper during the digestion simulation.
Extract of Japanese pepper had stronger antioxidant (IC50 =5580.66µg/mL)
and α-glucosidase inhibition activity (IC50 = 859.55µg/mL) compare to andaliman’s
antioxidant (IC50= 1224.10µg/mL and α-glucosidase inhibition (36089.58µg/mL)
after digestion simulation. Comparing to andaliman, Japanese pepper still showed better antioxidant activitiy as well as inhibition activity towards α-glucosidase enzyme and antioxidant.
Keywords: α-glucosidase inhibitor, antioxidant, in vitro
RINGKASAN
VANESSA KARNADY. Perbandingan Pengaruh Pencernaan Secara in vitro Terhadap Aktivitas Antioksidan dan Antihyperglikemik dari Ekstrak Kasar Andaliman (Zanthoxylum acanthopodium DC.) dan Lada Jepang (Zanthoxylum piperitum DC.). Dibimbing oleh HANNY WIJAYA dan ENDANG PRANGDIMURTI.
Kondisi hiperglikemia merupakan salah satu masalah yang penting akhir-akhir ini mengingat hiperglikemia dapat menyebabkan komplikasi lain seperti penyakit makro dan mikro vaskular. Hiperglikemia disebabkan oleh kelebihan glukosa dalam tubuh seseorang. Kelebihan glukosa tersebut akan menyebabkan ketidakseimbangan antara oksidan dan antioksidan dalam tubuh manusia.
Andaliman (Zanthoxylum acanthopodium DC.) adalah rempah-rempah tradisional dan bahan aditif alami yang tumbuh di alam liar di Sumatera Utara, Indonesia. Tanaman ini masih dalam satu genus dengan “shansho”, Lada Jepang (Zanthoxylum piperitum DC.) yang tumbuh terutama di Jepang. Kedua tanaman ini sudah banyak digunakan sebagai obat tradisional karena banyak mengandung zat fitokimia yang memiliki aktivitas antioksidan yang kuat dan juga diduga memiliki kemampuan menghambat α-glukosidase. Namun, aktivitasnya mungkin akan berubah di bawah kondisi pencernaan disebabkan oleh berubahnya struktur kimia senyawa-senyawa aktifnya. Oleh karena itu, penelitian ini bertujuan untuk mempelajari aktivitas dan sekaligus perubahan aktivitas tersebut yang dimiliki oleh ekstrak kasar lada Jepang dan andaliman saat pencernaan secara in vitro.
Simulasi pencernaan secara in vitro dilakukan dengan meniru kondisi pencernaan dalam lambung dan usus kecil. Sementara, penentuan aktivitas dilakukan secara in vitro dengan menggunakan pengujian penghambatan terhadap enzim α-glucosidase dan penghambatan aktivitas radikal DPPH. Acarbose, yang merupakan obat komersial digunakan sebagai kontrol positif dalam uji penghambatan α-glucosidase dan asam askorbat sebagai kontrol positif untuk penghambatan aktivitas radikal DPPH.
Sebelum simulasi pencernaan, ekstrak kasar lada Jepang memiliki aktivitas penghambatan yang lebih baik terhadap enzim α-glucosidase (IC50=3930.21μg/mL)
dibandingkan dengan andaliman(IC50=20346.94μg/mL). Ekstrak kasar lada Jepang
juga menunjukkan aktivitas antiradikal yang lebih baik terhadap DPPH (IC50=104.03μg/mL) dibandingkan dengan andaliman (IC50=20346.94μg/mL).
dibandingkan dengan kemampuan antioksidan (IC50 = 1224.10μg / mL dan inhibisi α-glucosidase (36089.58μg / mL) dari andaliman setelah simulasi pencernaan. Lada Jepang masih memiliki aktivitas penghambatan terhadap enzim α -glucosidase dan aktivitas antioksidan yang lebih baik dibandingkan dengan andaliman.
© All Rights Reserved IPB, 2015
Copyright Reserved
Quoting some or all of this paper without including or mentioning the source is prohibited. Quoting is only for educational purposes, research, scientific thesis, report writing, criticism, or review an issue; and citations are not detrimental to the interests of IPB
Thesis
partial fulfillment of the academic requirements to obtain Magister Sains
on
Food Science Study Program
COMPARISON ON
IN VITRO
DIGESTION EFFECT OF ANTIOXIDANT
AND ANTIHYPERGLYCEMIC ACTIVITY FROM ANDALIMAN
(
Zanthoxylum acanthopodium
DC.) AND JAPANESE PEPPER
(
Zanthoxylum piperitum
DC.) CRUDE EXTRACT
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
2015
ACKNOWLEDGEMENTS
Praise Lord for His endless blessings and continuous leading for the writer throughout the research and completion of this thesis report titled “Comparison on In Vitro Digestion Effect of Antioxidant and Antihyperglycemic Activity from Andaliman (Zanthoxylum acanthopodium DC.) and Japanese Pepper (Zanthoxylum piperitumDC.) Crude Extract”. The writer clearly realizes that the research and this report would not possible to be completed without the support from many people. Writer would like to express gratitude to:
1. Prof.Dr.Ir. C. Hanny Wijaya, M.Agr and Dr.Endang Prangdimurti as thesis supervisors for for the time and guidance during the research and completion of the thesis report.
2. My beloved family, mama, papa and Manda for always be my rock
3. My 2012 IPN friends, Eron, Diana, Ka Tiwi, Rosana, Eren, Laras, Kamil, Novan, Mas Syafii, Tuti, Mia, Trina, Ara, Faris, Wulan, Rina, Anis and other friends, thank you for the precious friendship and the knowledge you shared with me.
4. Irena a friend, my andaliman supplier, thank you for sharing me your knowledge and sharing everything about andaliman, especially the pictures and journal writing.
5. The Laboratory members, Bu Irdha and Mba Sherly. Thank you for your guidance, knowledge that you shared with me and for always there when I am in trouble.
6. All Laboratory Staff, Pa Taufik, Pa Yahya, Mba Ari, Pa Rojak, Mba Irin, Teh Yayam and Pa Sob for your help and instruction when using the instruments,
7. Biofarmaka Laboratory Staff, Mba Ella, Mba Wiwik, Bu Ninik and Mba Ina, thank you for the help, guidance and knowledge sharing when I did the experiment.
8. Hashidoko sensei, for your guidance in doing lab experiments, data analyzing and presentation practice which is very precious.
9. Echochem Laboratory member, Reika, Yokota, Nishiyama, Sharon, Nie, Bocky, Nizhisuka, Haba, Aki, Yoshida, Zetry, Wang Lei, Hiroyo Hanai, thank you for the friendship, concern, guidance, help and knowledge shared when I was alone in Hokudai Lab.
10.Dhina and Namfon thank you for being my friends so I can finish this thesis master, and thank you for teaching me about chemistry and lab technique.
The author is aware that this report is far from perfect and it may contain many mistakes. The author would like to deliver apology and welcome any critics and/or suggestions given to this report with great pleasure. Finally, the author hopes that this report would be useful for the readers.
Vanessa Karnady
TABLE OF CONTENT
LIST OF FIGURES xi
LIST OF APPENDICES xi
1 INTRODUCTION 1
Background 1
Research Problem 2
Research Objectives 2
Research Benefit 2
2 Literature Review 2
Zanthoxylum Genus 2
Zanthoxylum acanthopodium DC. (Andaliman) 3
Zanthoxylum piperitum DC. (Japanese pepper) 6
Hyperglycemia 7
Oxidative Stress in Hyperglycemia 8
Alpha glucosidase enzyme and its inhibitor 8
3 Methods 9 Materials 9 Research Methods 9 Crude Extract Preparation 10
In vitro Gastrointestinal Digestion 11
Antihyperglycemic Activity Assay 11
Antioxidant Activity Assay 12
4 Results and Discussion 13
Crude Extract Preparation 13
Antihyperglycemic Activity 14
Antioxidant Activity 18
CONCLUSION AND SUGGESTION 22
Conclusion 22
Suggestion 23
BIBLIOGRAPHY 23
APPENDICES 28
LIST OF FIGURES
1 Andaliman field in Goting Raya, Simalungun North Sumatra 4
2 Zanthoxylum acantophodium DC. 5
3 (A) Mature (red) andaliman 5
(B) Young (green) andaliman 5
4 (A) Young (green andaliman) 5
(B) Mature (red andaliman) 5
(C) Senescent (black andaliman, seeds exposed) 5
5 Zanthoxylum piperitum DC. 7
6 Research Flow Chart 10
7 (A) Andaliman 14
(B) Japanese pepper 14
8 (A) α-glucosidase inhibition activities of crude extracts 16 (B) α-glucosidase inhibition activities of in vitro post digestion
extracts 16
9 (A)IC50 value of α-glucosidase inhibition activity of crude extracts
versus in vitro post digestion extracts 17
(B) IC50 value of α-glucosidase inhibition activity of crude extracts
versus in vitro post digestion extracts 17
10 (A) DPPH scavenging activities of crude extracts 21 (B) DPPH scavenging activities of in vitro post digestion extracts 21 11 (A) IC50 value of DPPH radical scavenging activity of crude extracts
versus in vitro post digestion extracts 22
(B) IC50 value of DPPH radical scavenging activity of crude extracts
versus in vitro post digestion extracst 22
LIST OF APPENDICES
1 Moisture content and yield 28
2 Antioxidant activity 29
1 INTRODUCTION
Background
The type 2 diabetes represents by the asymptomatic hyperglycemia, which is an elevation in blood glucose levels, result from the inadequate insulin secretion or action. Moreover, chronic hyperglycemia will produce reactive oxygen species (ROS) which is a main role in microvascular and macrovascular complication (Singh & Poonam 2009). Thus, controlling hyperglycemia is believed to be important on treating diabetes mellitus. According to Shibano et al. (2008) combination of the antihyperglycemic inhibitor and antioxidant will be more effective for the treatment and prophylaxis of hyperglycemia.
Alpha glucosidase is an enzyme located in the small intestine brush border, which will catalyzes the final step of carbohydrate digestion to glucose, the absorbable monosaccharide. Synthetic acarbose is used as well-known antihyperglycemic inhibitor which will limit the availability of the glucose by hampering the rate of final step hydrolysis of complex carbohydrate into glucose in the intestine (Jaiswal et al. 2012). However the use of synthetic acarbose is not suitable for all because of the contraindication in patient with inflammatory bowel disease and renal impairment (Kim et al. 2005), beside that synthetic acarbose does not possess natural antioxidant as in natural compound. Therefore, in the field of food science much interest has been focused on the development of food functional, including screening of natural bioactive compound which have less side effect and considering the phytochemical from natural compound.
Zanthoxylum acanthopodium DC. (andaliman) is an endogenous plant from North Sumatra, Indonesia. It has lemony aroma and unique taste which give tingling sensation on the tongue. Batak tribes widely used it as spices and folk medicine (treating stomachache). According to Wijaya (2000), the tingling sensation came from the trigeminal compound that has similar structure with a compound that was isolated from Zanthoxylum piperitum DC. (Japanese pepper) which is known as sanshool. The similar uniqueness made them more interesting to be further explored. Some studies already showed the bioactivity of Japanese pepper, it had strong antioxidant activity (Yamazaki et al. 2007), antimicrobial activity (Lee et al. 2012), hepatoprotective effect (Lee & Kye 2008) and gave relaxation effect on circular muscle of gastric (Hashimoto et al. 2001). Likewise, andaliman has also shown some bioactive capability such as antioxidant (Tensiska et al. 2003), antimicrobial (Parhusip et al. 2005), xanthin oxidase inhibitor (Kristanti et al. 2012) and anti-inflammatory (Yanti et al. 2011) but the bioactivity of andaliman is still less popular than Japanese pepper, therefore it will need a study which can compare their bioactivity.
influence the antioxidant and antihyperglycemic activity (Bermudez-Soto et al. 2007). However, antioxidant and antihyperglycemic activity of the extract obtained from in vitro digestion has not been investigated yet.
Research Problem
Hyperglycemia has become common disease in both developed and developing countries in connection with life style changes a dietary habits. The α -glucosidase inhibitor generally used to medically treat hyperglycemia. Despite powerful synthetic α-glucosidase inhibitor, such as acarbose, they usually induce side effect such as hepatoxicity and disorder gastrointestinal symptoms (flatulence, diarrhea and abdominal boating). Therefore, a natural α-glucosidase inhibitor from food sources like andaliman or Japanese Pepper become an attractive therapeutic approach on treating hyperglycemia. Moreover, the treatment of hyperglycemia will be more effective if they have combination roles as antioxidant and α -glucosidase inhibitors. Unfortunately, the alteration of activity under gastrointestinal condition has not yet been studied before. Therefore a further study about the changes of antioxidant and antihyperglycemic activities of both andaliman and Japanese pepper during gastrointestinal simulation will give better understanding of both spices’ efficacy as antihyperglycemic ingredients.
Objectives
The purpose of this study was to compare the activity of andaliman and Japanese Pepper crude extracts as α-glucosidase inhibitor and antioxidant, particularly the changes of the antihyperglycemic and antioxidant activity during an in vitro gastrointestinal digestion simulation.
Research Benefit
The research provided information regarding the antihyperglycemic and antioxidant efficacy from andaliman and Japanese pepper which could be implemented for further development as a natural antidiabetic ingredient from plant. The result also indicated the other possibility of andaliman utilization besides it being use as regular spices.
2 LITERATURE REVIEW
Zanthoxylum Genus
species of Zanthoxylum distributed mainly in tropical area, most of the trees are deciduous and shrubs. It also could be identified by its fruit which usually are follicles or esquizocarp, contains from one to five carpels, commonly aromatic, and they are ordinarily bivalve with a single red or black, shiny seeds. This genus are popular because its phytochemistry activity (Patino et al. 2012).
In the food industry essential oil from Zanthoxylum species such as Z. xanthoxyloides, Z. gillettii, Z. simulans are obtained from their leaves or fruits.
The bark from Z. Tessmannii are usually being utilized as emulsifying agent and encapsulants (Adesina 2005). Many species has been traditionally used as a remedy throughout world, such as in Taiwan they used it as remedy for snakebite and aromatic tonic for fever. The bark of Z .liebmannianum, is used in Mexico for the treatment of stomach pains, amebiasis and intestinal parasites and as a local anesthetic agent (Ross et al. 2004). Z. monophyllum in Venezuela, it used as a remedy for runny nose or nasal mucosal inflammation. In Batak tribes, the fruits of Z. acanthopodium DC. has been used to heal stomachache and toothache (Suryanto et al. 2004). In East Asia, all part of the plant from Z.piperitum commonly used to heal vomiting, diarrhea and abdominal pain (Yamazaki et al. 2007).
Zanthoxylum acantophodium DC. (Andaliman)
Zanthoxylum acanthopodium DC. (Andaliman) is a traditional exotic spice and natural food additive that grows in the wild in North Sumatra, Indonesia, especially in the area of Lake Toba. It is widely available in the Dairi, North Tapanuli, Tobasa (Malau et al. 2002), Humbang, Silindung, and Toba Holbung areas (Napitupulu et al. 2004). Its habitat is sandy loam soil and grows at elevation of 0.9 kilometers above sea level where there can be up to 2500 mm of annual rainfall with rain on 170-180 days/year (Napitupulu et al. 2004). Nowadays,
andaliman is cultivated, for example, in Gotting Raya, Simalungun North Sumatera (Fig 1).
Andaliman which belongs to Family Rutaceae, is often mistaken as a member of Family Piperaceae and identified as Piper ribesiodes. According to Hsuang (1978), andaliman is classified as follows:
Division : Spermatophyta Sub division : Angiospermae Class : Dicotyledoneae Order : Geraniales Family : Rutaceae Genus : Zanthoxylum
Species : Zanthoxylum acanthopodium DC.
surfaces and reddish green bottoms. Flowers are in the auxiliary, small with flat or conical shaped bases, 5-7 free petals, 1-2 cm long, pale yellow color, androgynous, having 5-6 stamens in flowers sitting on the base, reddish anthers, 3-4 pistils, boarded apocarp ovaries. The fruit is box or capsule shaped, or rounded, 2-3 mm diameter, green for the young fruit and red in mature fruit (Fig 3). It has one seed per fruit, hard skin, and a shiny black color. Andaliman is commonly used in the green form. However, on some occasions the red is utilized. Senescent andaliman turns black and black seeds come out (Fig 4). In this condition, the typical flavor of andaliman no longer exists.
Andaliman has traditionally been used in Batak cuisine served at a variety of cultural events. Recently, it has been used in daily food. Almost every dish at a Batak birth and marriage ceremonies is cooked with andaliman. This spice is believed to have medicinal properties that improve the appetite of sick persons (Napitupulu 2004). Traditional foods are prepared using andaliman such as arsik (yellow cooked gold fish), naniura (uncooked or fermented gold fish), tombur (a condiment for roasted fish or meat), and is also used as sauce (with crushed green chili).
Fig 2. Zanthoxylum acantophodium DC.
Fig 3. (A) Mature (red) andaliman and (B) Young (green) andaliman
Fig 4. (A) Young (green andaliman), (B) mature (red andaliman), and (C) senescent (black andaliman, seeds exposed).
Alongside its anti-inflammatory ability, andaliman also traditionally used for preserving cooked meat for few days against rancidity and spoilage, the scientific studied bear out that andaliman has antiradical activity on its ethanolic extract, this extract at 1000 ppm has higher radical scavenging than 1000 ppm of α -tocopherol (Suryanto et al. 2004). Food preservatives potency is also supported by
A B
study which done by Parhusip et al.(2006), that etyl acetate extract of andaliman has a high inhibition towards either Staphylococus aureus or Bacillus cereus cell and cell protoplast. Both of S.aureus and B.cereus, they are pathogen bacteria which often contaminating food.
Tensiska et al. (2003), the antioxidant extract from andaliman fruit has its activity on aqueous system. However, on emulsion system antioxidant activity is less intense than Butylated Hydroxy Toluene (BHT) which was used as the positive control. As well as in oil system, the antioxidant activity also less intense than BHT which was observe by its longer induction time than BHT has. As antioxidant the fruit extract from andaliman is relatively heat stable on aqueous system on 175ºC in 2 hour. The pH stability on emulsion system showed protection of antioxidant increase consequently to increase from pH 3 to 7.
Antiradical activity which is isolated from n-butanol andaliman extract compound were type of hydroxy ester sterol and cholesterol-3-o-β-glucosidase and they have xanthine oxidase inhibition were IC50 0,34μg/mL and 0,06μg/mL
respectively, and their antioxidant activity were IC50 68,35μg/mL and 60,52μg/mL
respectively, which is higher than BHT or quercetin. It was shown that compound hydroxy ester sterol has better activity in DPPH reduction, and cholesterol-3-o-β -glucosidase was better as xanthine oxidase inhibitor (Kristanti et al. 2012).
Zanthoxylum piperitum DC. (Japanese Pepper)
Japanese pepper (Zanthoxylum piperitum DC) is a decidous and shruberry tree distributed in Japan, China and Korea (Fig 5.). The plant can grow into 3 up to 6 meters high. Various parts of this plant such as leaves, flowers and fruits are used in of Japanese cuisine for its unique flavor, such as for sprinkling on the broiled eel dish. In Asia around 1980’s the pericarp of the fruits from Japanese pepper has been used as an antihelmintic and also treatments of digestive organs. Whereas, the fruits and leaves was founded to contain terpenoid, aliphatic acid amides, alkaloid, flavonoids and other phenolics (Hur et al. 2003).
The methanol extract from the leaves was proven to prevent lipid peroxidation which was induced by bromobenzene. The extract was capable of reducing the activity of analine hydrolase, epoxide producing enzyme and by enhancing activity of epoxide hydrolase which is an epoxide removing enzyme. However, it did not give effect to aminopyrine, N-dimethylase and gluthahione S-transferase (Hur et al. 2003). Research which done by Jeong et al. (2011) stated that Z.piperitum leaf also have a radical-scavenger and reducing agent. It was presented by protective effects against H2O2 induced neurotoxicity in a
dose-dependent manner. This protective effect was lead to the fact that the extract has strong antioxidative and neuronal protective effects that are correlated with its high level of phenolics, particularly quercetin, afzelin, and hyperoside.
Japanese pepper fruit was found to be equal to that α- tocopherol and stable under heat treatment, and the compound were identified to be hyperoside and quercetin. They were found to be a good scavenger for DPPH.
Figure 5. Zanthoxylum piperitum DC. Source: Suehiro, 2014
Glycoprotein from Japanese pepper also has positive effect as hepatoprotective agent as a natural antioxidant. The result showed that Japanese pepper’s glycoprotein has an inhibitory effect on hypoxanthine/xanthine oxidase- or glucose/glucose oxidase-induced cytotoxicity in a dose-dependent manner. In addition, administration of Japanese pepper glycoprotein (20 mg/kg) lowers the levels of lactate dehydrogenase, alanine transaminase, and thiobarbituric acid reactive substances, whereas increases that of nitric oxide, accompanying the normalizing effects on the activity of hepatic anti-oxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) in mouse model of carbon tetrachloride-stimulated acute liver injury (Lee et al. 2008).
From above information showed that Japanese pepper is one of important aromatic and medicinal plant which used widely in East Asia. Hwang and Kim (2012), assessed the potential health risk of Japanese pepper derived essential oil, based on bone marrow micronucleus, bacterial reverse mutation, and chromosome aberration tests. The Z.piperitum derived essential oil contains myrcene, octanal, d-limonene and linalool which used as antimicrobial agents against foodborne pathogens, from the study it showed no indication of bone marrow micronucleus abnormalities, mutagenicity, or chromosomal aberration.
Hyperglycemia
glucose uptake. After insulin circulate with glucose in the blood stream it will exit the tissue to reach body cell. Body cell is a place where insulin receptor will bind to circulation of insulin. The receptor will act as a lock whereas the insulin will act as the key, when the receptor act normal it will lead glucose enter the body cell in normal level. Therefore, the body cell can produced energy (Giugliano et al. 2008). Otherwise in people who ail with diabetes type 2, the problem will be either the βcell in pancreas does not produce enough insulin or the cell resist the effect of insulin, or the problem could be both of them. When the cell resist the effect of insulin, insulin cannot unlock the cell to let glucose enter the body cell because of the abnormality of the receptor. Which means the glucose will be locked up outside the cell and the glucose level in the blood will be high, this is known as hyperglycemia. To compensate the hyperglycemia, the βcell excrete more and more insulin, this will lead to the overworked of βcell which will lose its ability to produce enough insulin (McDonnell et al. 2012).
Oxidative Stress in Hyperglycemia
Oxidative stress is generated by an imbalance between the production of reactive oxygen species (ROS) and the antioxidant defense system. The overworked of β cell caused by continuous excretion of insulin will be toxic for β cell itself, which called β cell dysfunction. According to Wu et al. (2004) the dysfunctional of β cell accompanied by increasing activity of glycolysis with lower production of ATP, increasing the accumulation of intracellular ROS, oxidative damage to mitochondria and also increasing the apoptotic cell death. The increasing of glycolysis rate is because when the blood glucose level is too high, glucose will be deposit in the liver where the glycolysis took place.
Actualy in glucose metabolism which is one of the oxidative metabolism, ROS is a normal product from glucose metabolism that formed from the reduction of molecular oxygen or by oxidation of water to yield products such as superoxide anion and hydrogen peroxide. When the metabolism rat, in this case glycolysis, increase it means that will be an excessive ROS in pancreatic β-cell, this will make cellular damage on pancreatic β cell. The damage of pancreatic β-cell will increase the development and worsening hyperglycemia. Therefore, it will need a scavenger for the ROS which will be expected to prevent the toxic effect of increased glycolysis (Hartati et al. 2012, McDonnell et al. 2012, Wu et al. 2004).
Alpha glucosidase enzyme and its inhibitor
Alpha glucosidase enzyme (EC 3.2.1.20) is enzyme which catalyse the degradation of α-1,6 glycoside. The function of this enzyme is to hydrolyze α-limit dextrin into glucose. Alpha glucosidase in mammal digestion located on the surface of the brush border membrane of the small intestine which catalyse the final process of carbohydrate digestion in the digestion. (Jaiswal 2012, Berdanier et al. 2006).
that the bioactive component in Alstonia scholaris that could inhibited the activity of α-glucosidase enzyme is quercetin 3-O-β-D-xylopyranosyl (1”-2”)-β-D— galactopiranosid and (-)-lioniresinol 3-O-β-D-glucopiranosid (Jong-Anurakkun et al. 2007). Another study showed that the whole flavonoid mixture from Crapesium abrotanoides plant extract showed non-competitive inhibition against α -glucosidase enzyme activity which originated from yeast (Mayur et al. 2010). Chaenomeles sinensis was shown to be a potent α-glucosidase enzyme which related to the high phenolic content that have high antioxidant activity (Sancheti et al. 2009). According to Arsiningtyas et al. (2014) mentioned the caffeoylquinic acid derivatives shown to be important for the inhibitory activity of α-glucosidase enzyme.
Acarbose known as fermented product from some species of Actinoplanes. Acarbose is effective in inhibiting some carbohydrate degradable enzim such as : α-glucosidase, cyclomaltodextrin glucaniltransferase (CGTase), α-amylase and dextran sucrase. Acarbosa is pseudo oligosaccharide which has pseudo oligosaccharides ring [[4,5,6-trihydroksi-3-(hidroksimetil)-2-cyclohexene-1-yl]amino]-alpha-D-glucopyranosil-1(1-4)-O-(alpha)-D-glucopyranosil-(1-4)-D- Glucose. The acarbose inhibition mechanism against those enzymes above because of cyclohexene ring and the nitrogen linkage that mimics the transition state for the enzymatic cleavage of glycosidic linkages (Yoon & Robyt 2002).
3 METHODS
Materials
Fresh andaliman fruit was obtained from Simalungun farm, in North Sumatra, Indonesia. Japanese pepper fruit was obtained from traditional market in Kyoto, Japan. α- glucosidase enzyme (G5003), pancreatin (P1750), bile extract (B8631), pepsin (P7000), substrate p-nitrophenyl-α-glucopyranoside (N-1377) and 1-1-diphenyl-2-picrylhydrazyl (D9132) were supplied by Sigma Aldrich (USA). L-ascorbic acid (F951727) was supplied from Merck. Glucobay (100 miligram acarbose) was supplied from Bayer (PT. Bayer Indonesia, Jakarta), ethanol and ethyl acetate were supplied by JT Baker (USA), methanol and ethanol was supplied from Merck (Darmstadt, Germany). All chemicals used were analytical grade.
Research Methods
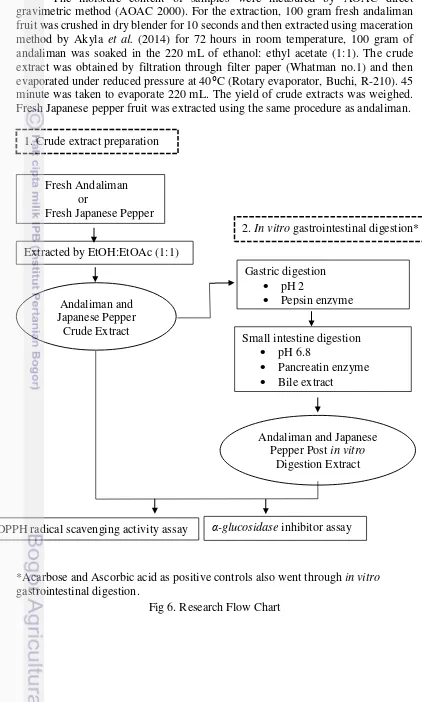
Crude Extract Preparation
The moisture content of samples were measured by AOAC direct gravimetric method (AOAC 2000). For the extraction, 100 gram fresh andaliman fruit was crushed in dry blender for 10 seconds and then extracted using maceration method by Akyla et al. (2014) for 72 hours in room temperature, 100 gram of andaliman was soaked in the 220 mL of ethanol: ethyl acetate (1:1). The crude extract was obtained by filtration through filter paper (Whatman no.1) and then evaporated under reduced pressure at 40⁰C (Rotary evaporator, Buchi, R-210). 45 minute was taken to evaporate 220 mL. The yield of crude extracts was weighed. Fresh Japanese pepper fruit was extracted using the same procedure as andaliman.
*Acarbose and Ascorbic acid as positive controls also went through in vitro gastrointestinal digestion.
Fig 6. Research Flow Chart Fresh Andaliman
or
Fresh Japanese Pepper
Extracted by EtOH:EtOAc (1:1)
Andaliman and Japanese Pepper
Crude Extract 1. Crude extract preparation
2. In vitro gastrointestinal digestion*
Gastric digestion
pH 2
Pepsin enzyme
Small intestine digestion
pH 6.8
Pancreatin enzyme
Bile extract
Andaliman and Japanese Pepper Post in vitro
Digestion Extract
In vitro Gastrointestinal Digestion
The crude extract was diluted in distilled water, then it was passed through upper digestive tract (gastric and small intestine) simulation based on the following
in vitro method by Cilla et al. (2011). The crude extract pH value was adjusted (pH Meter, Eutech Instruments pH 700) into pH 2 using HCl (6M) and allowed to stand
for 30 minutes, to make sure the adjusted pH was done. To start the gastric
simulation, freshly prepared pepsin sufficient to yield 0.02 g pepsin/g extract was added to the crude extract, then it was incubated in the shaking water-bath (Incubator Shaker, GFL 1083) at 37ºC for 2 hour. The enzyme was terminated in the cold condition inside ice bath for 10 minutes. Then, the extract was adjusted to pH 6.8 by NaHCO3 (1M) in order mimicking the condition in the small intestine
and an amount of pancreatin and bile extract solution sufficient to provide 0.005 g pancreatin and 0.03 g bile extract/g extract was added, and incubation was continued for another 2 h at 37ºC. The enzyme was terminated in the cold condition inside the ice bath for 10 minutes. The extract pH was adjusted to pH 7.2 by dropwise of 0.5 M NaOH. All post digestion extract was transferred to centrifuges tubes and was centrifuged at 3500 g (Heraeus, Labofuge 400 R) for 1 hour, followed by collection of the supernatants which continued to antihyperglycemic and antioxidant assay.
Antihyperglycemic Activity Assay
Antihyperglycemic activity was determined using the α-glucosidase inhibition method described by Sancheti et al. (2009), α-glucosidase stock (1 units/mL) was diluted with phosphate buffer (pH7, 0.1M) to a final concentration of 0.04 units/mL. The inhibitory activity against the α-glucosidase was measured using the following procedures. The working samples at difference concentration (10µL) were added to the sample and control B, while distilled water (10µL) was added to the control and blank, and substrate 4-nitrophenyl α-D-glucopyranoside 10 mM (25µL) was added to all. Then, the α-glucosidase solution (25µL) was added to the sample and control A, then the phosphate buffer was added to the blank and control B. Control A defines as the total glucose after hydrolysis of substrate and enzyme, control B as the initial glucose in the sample and substrate, sample as the total of hydrolysis between sample and substrate, the blank as the initial glucose in the substrate. The reaction was carried out at 37⁰C for 30 minutes, and was terminated by adding 100 µL Na2CO3 0.2M, the optical density of the wells was
digestion were 0.5, 0.3, 0.1, 0.05 and 0.01µg/mL. The inhibitory activity was calculated from the following equation.
%� ℎ����� = � �− � %
A1 = Abs Control A – Abs Blank A2 = Abs Sample – Abs Control B
The result was presented as half the maximal inhibitory concentration value (IC50 value). IC50 value defined as the concentration of the substance required to
inhibit 50% of α-glucosidase activity under the assay conditions. It was determined by constructing a dose response curve between the logarithm of the concentration of substances on the X-axis and the inhibitory activities on the Y-axis.
Antioxidant Activity Assay
Antioxidant activity was determined using DPPH radical scavenging assay according to Awah et al. (2010). The procedure was started by adding 150 µL of the working samples at different concentration and mixed with 75 µL of 0.2 mM DPPH in ethanol solution inside the micro well. The mixture was allowed to stand at room temperature in dark room for 25 minutes. Blank solution was consisted of the sample solution (150 µL) and ethanol (75 µL), for the control was consisted of DPPH solution (75 µL) and ethanol (150 µL). The experiments were done triplicate and used ascorbic acid as the positive control. The absorbance was measured at 518 nm (Microplate reader, Epoch, Biotek). The concentration of crude extract of andaliman that were used were 2090.34, 1045.17, 522.58, 261.29, and 130.65 µg/mL of dry weight of andaliman, for post digestion extract of andaliman were 5225.84, 2090.34, 1045.17, 522.58, and 261.29 µg/mL of dry weight of andaliman. The concentration of crude extract of Japanese pepper that were used were 476.50, 238.25, 119.12, 59.56, and 29.78 µg/mL of dry weight Japanese pepper and for post digestion extract were 2382.49, 953.00, 476.50, 238.25, and 119.12 µg/mL of dry weight Japanese pepper. Ascorbic acid concentration before digestion were 20, 10, 5, 2.5 and 1.25 µg/mL, while post digestion concentration were 40, 20, 10, 5 and 2.5 µg/mL. The inhibition activity was calculated using the following equation:
DPPH Inhibition activity (%)= − [{A s s p e−A s
� � } × ]
The result was presented as half the maximal inhibitory concentration value (IC50 value). The IC50 value defined as the concentration of the sample required to
4 RESULT AND DISCUSSION
Crude Extract Preparation
Extraction was purposed to separate compounds from a homogen mixture using a solvent as the separator agent, in order to get the bioactive compound. In this study the extraction from both andaliman and Japanese Pepper fruit were done using organic solvent, which were ethanol and ethyl acetate (1:1) and the extraction method was maceration for 72 hours in the room temperature (Akyla et al. 2014). The extraction was using only the fruits of andaliman and Japanese pepper. Fruit means the pericarp and seed of andaliman and Japanese Pepper (Fig 7A&B).
In this study the extraction was done based on the polarity since the active compound which has the physiological active on the fruits have not been fully discovered yet. Therefore the solvents were expected to extract most of the compounds in andaliman and Japanese Pepper fruit including the flavour compound in andaliman. The solvent were represent the polar (ethanol) and less polar (ethyl acetate) solvent. The utilization of ethanol was expected to extract the phenolic compounds which have antioxidant activity, this was proven according to Suryanto et al. (2004) showed that ethanolic extract contained the highest phenolic compounds which gave the highest radical scavenging activity. According to Akyla et al. 2014 the crude extract from the mixture of ethyl acetate and ethanol was also given a similar aroma to the fresh andaliman. However, in this study the mixture was expected to yield an extract contain both polar and less polar compound since it is still an early study.
The result from maceration showed that Japanese Pepper were shown to have green colour and andaliman was shown to have brownish green extract. Both of the samples were went through the same procedure and condition until became a crude extract, the final step of crude extract preparation. Then, the step that was not in control was the process after harvesting, especially for the Japanese pepper, while the andaliman was available directly from the Simalungun farm. The colour that formed were different since the polyphenol oxidase enzyme in the andaliman fresh fruit while in the Japanese pepper the enzyme was might be already inactivated by the blanching process after harvesting.
Fig 7. (A) Andaliman (B) Japanese pepper
The differences also seen in the andaliman crude extract yield was 9.57% (db), while Japanese Pepper crude extract yield was 20.98% (db). It was most likely influenced by the compound that extracted with the solvent, Japanese Pepper showed higher yield which represent the more compounds were extracted from Japanese pepper than andaliman. This also indicated that the solvent ratio between ethyl acetate and ethanol was suitable to give a high yield of extract, while the ratio might be less appropriate to obtain higher yield. The other factor which might be influenced was the differences of the moisture content of both of fresh fruit, Japanese Pepper (75.13%) was shown to have higher moisture content than andaliman (80.13%) this moisture content influenced the calculation of the yield. The moisture content might be influenced by the higher hydration after the blanching in the Japanese pepper fruit (Gowen et al. 2007).
Antihyperglycemic Activity
Hyperglycemia is a high blood glucose due to inadequate insulin in the body. One of the way to treat hyperglycemia is by hampering the key enzyme in the small intestine which catalysed carbohydrate breakdown into glucose. Alpha glucosidase is the key enzyme which will catalyse the broken down of α-1,6 glycosidic bond in alpha limit dextrin to glucose. The inhibition of the reaction could hamper the main metabolic pathway by preventing the production of metabolite, which is glucose. The compound which has the inhibition activity might be possible act as α-glucosidase inhibitor.
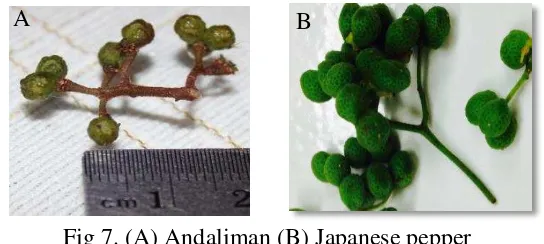
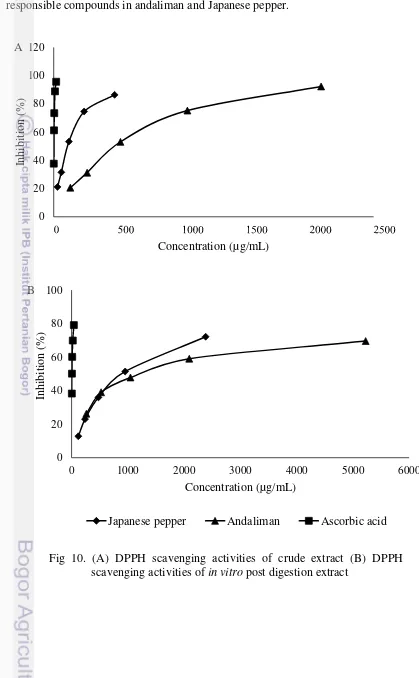
In this study, the crude extract of andaliman and Japanese pepper were shown to have inhibition activity towards the α-glucosidase enzyme. The whole series of concentration inhibition activity of andaliman and Japanese pepper were shown in Fig 8 (A). The range of andaliman concentration were from 13064.60µg/mL of andaliman dry weight to 209033.61µg/mL of andaliman dry weight, while Japanese pepper were from 2382.49µg/mL of Japanese pepper dry weight to 47649.88µg/mL of Japanese pepper dry weight. The series of concentration were determined based on the inhibition activity below and above 50% inhibition activity that were needed in the IC50 activity.
Andaliman’s crude extract were giving 92.28% inhibition in 209033.61µg/mL, while Japanese pepper were giving 93.505% inhibition in 47649.88µg/mL. The 40% inhibition were given by 13064.60µg/mL of andaliman crude extract and 2382.49µg/mL of Japanese pepper crude extract. The concentration indicated that the crude extract of andaliman showed lower activity
A B
than Japanese Pepper’s crude extract, because andaliman need higher concentration to inhibit α-glucosidase enzyme. This might be caused by different condition of the bioactive compound between fresh andaliman and Japanese pepper, according to some studies, the phenolic phytochemical has the natural α-glucosidase inhibitor (Kim et al. 2005; McDougall et al. 2005, Wang et al. 2012). Therefore, the different condition of the fresh samples affected most of the phenolic in andaliman which might have oxidized by the polyphenol oxidase, while most of the phenolic compounds in Japanese pepper still have not oxidized yet, because of the blanching process which make the inhibition activity of Japanese pepper were stronger. The other reason which made Japanese pepper were stronger Japanese Pepper fruit contains quercetin which also found to be antioxidant, this might be one of the responsible compound that have the α-glucosidase inhibition activity (Yamazaki et al. 2007). However, andaliman could contain quercetin just like Japanese pepper fruit, however its fresh fruit condition was differed because of the blanching after the harvest. The uniformity of the fresh fruit condition possibly gave a great effect on the inhibition activity as well.
Quercetin is a type of flavonoid, that have been known to inhibit α -glcosidase enzyme such as quercetin 3-O-β-D-xylopyranosyl (1”-2”)-β -D-galactopyranoside and (-)-lyoniresinol 3-O-β-D-glucopyranoside (Jong-Anurakkun et al. (2007). According to Tadera et al. (2006), the glycosylation at the C-3 positions on the C rings of flavones enhances the inhibitory activity, C-3-OH are favourable to the inhibitory activity. Glycosylation is the process by which a sugar is covalently attached to a target protein which is the α-glucosidase. This glycosylation at C-3 in the C-ring of quercetin plays an important part in α -glucosidase inhibitory activities of flavonols (Jo et al. 2010).
Fig 8A shown the inhibition activity of acarbose before digestion gave the most effective inhibition activity compare to Japanese pepper and andaliman’s crude extract. It shown to give 42% inhibition in 0.01µg/mL, while Japanese pepper need 2382.49µg/mL and andaliman needed 13064.60µg/mL. Acarbose intended to be very potent, which sometimes could have lead consumer in flatulence since there are to much undigestable carbohydrate in the digestion system. The crude extract might give more effective concentration to inhibit the enzyme, but it still contain non active components along with the active ones, while the acarbose was already the active compound which already proven to have a strong inhibition towards α -glucosidase. Therefore isolation from andaliman and Japanese pepper should be done since it will help to be a potent natural inhibitor (Mayur et al. 2010).
0 undetected structural forms with different chemical properties which consequently differ its biological activity.
Post digestion of acarbose which shown in Fig 8B, showed that the acarbose also influenced by the digestion. The 70% inhibition needed 0.5 µg/mL post digestion, while the crude extract needed 0.3 µg/mL, as well post digestion acarbose needed twice higher concentration, from 0.005 µg/mL to 0.01 µg/mL to have 27% inhibition activity. Moreover to inhibit 39% post digestion acarbose needed five times higher concentration than acarbose before digestion.
Fig8. (A) α-glucosidase inhibition activities of crude extract (B) α-glucosidase inhibition activities of in vitro post digestion extract
Fig 9. (A) IC50 value of α-glucosidase inhibition activity of crude extracts versus
in vitro post digestion extract (B) IC50 value of α-glucosidase inhibition
activity of acarbose before and post digestion
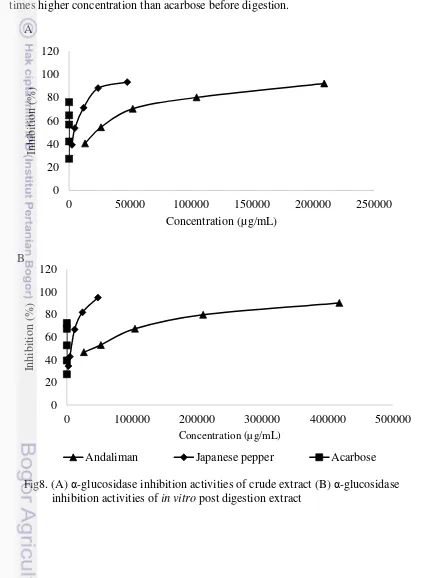
After, the whole series concentration was shown the IC50 was determined.
IC50 showed the half maximal inhibitory concentration of the effectiveness of
andaliman and Japanese pepper in inhibiting α-glucosidase inhibitor, which means the less IC50 value the more effective the inhibition. In Fig 9A it was also shown by
an increase in IC50 value for all samples post digestion extracts, including acarbose.
Acarbose shown the best activity inhibiting the enzyme, IC50 = 0.03 µg/mL,
however its post digestion extract shown 2.6 times increase to IC50 = 0.08 µg/mL
(Fig 9A). Whereas, andaliman and Japanese Pepper inhibition activity only decrease 1.77 and 1.42 times, respectively (Fig 9B), Japanese pepper were more effective and stable inhibitor after digestion than andaliman. While acarbose become the least stable after the in vitro digestion and it is the most effective inhibitor among tested samples.
(Bermudez-Soto et al. 2007). This might explain the decreasing activity in both andaliman and Japanese pepper. Bermudez- Soto et al.2007 showed that the digestion especially the pancreatic digestion decreased 43% of the anthocyanin concentration, 26% of flavanols, 19% flavan-3ol, 28% neochlorogenic acid and 24% chlorogenic acid. However, still there will need further study to explain the active compound which responsible for the antihypeglycemic activity in Japanese pepper and andaliman to show which compound decreased because of the digestion. Since there might be other compound has responsibility cause the both andaliman and Japanese Pepper fruit also contain other compounds such as essential oils (terpenes and sequisterpenes) (Suryanto et al. 2004) and amides (Hatano et al. 2004, Wijaya2000).
Antioxidant activity
Hyperglycemia induced over production of superoxide which is the casual link between high glucose and the pathways responsible for hyperglycemic damage. In fact, diabetes is typically accompanied by increased production of free radicals and/or impaired antioxidant defence capabilities. Free radicals are a factors that augment the pathogenesis a diseases via oxidative modification of DNA, protein and vital molecules. Thus consuming antioxidant is believed to protect from oxidative damage which can prevent the progression of disease, including chronic hyperglycemia.
In present study, the DPPH free radical-scavenging activity was used for the antioxidants activity of Japanese pepper and andaliman. DPPH as stable organic free radical has deep violet color, when DPPH receive proton from any hydrogen donor and converts it into α-α-diphenyl-β-picryl hydrazine (DPPH-H*) the color turn colorless. The amount of DPPH is reduced by hydrogen donor was quantified by measuring a decrease in absorbance. The less color is produced the stronger the antioxidant activity of the compounds, and more hydrogen donor for DPPH radical. Phenol is a hydroxyl group bonded directly to an aromatic hydrocarbon group which mostly produced by plants, including andaliman and Japanese pepper. The phenol group were allegedly responsible for the antioxidant activity in both andaliman and Japanese pepper. In accordance with the principle of DPPH radical scavenging activity, the hydroxyl group from phenols will be the hydrogen donor for the DPPH radical. Therefore the DPPH radical scavenging activity method is suitable for this study.
For each sample, five series of concentration (µg/mL) were tested. The concentration between Japanese pepper and andaliman were in different range since each has different capacity to acquire the IC50 value. Fig 10A, showed the inhibition
higher concentration. Mostly other concentration of andaliman crude extract needed 4.4 times higher to acquire the same inhibition activity as Japanese pepper. The data represented that between andaliman and Japanese pepper’s crude extract, Japanese pepper had shown more effective inhibition activity, while ascorbic acid as the positive control shown as the best DPPH inhibitor among all.
Fig 10B, showed the DPPH radical scavenging activity of the Japanese pepper post digestion extract (2382.49 µg/mL to 119.12 µg/mL), andaliman post digestion extract (5225.84 /mL to 261.29 µg/mL) and post digestion ascorbic acid (40µg/mL to 2.5µg/mL). It was shown that Japanese pepper and andaliman needed higher concentration to inhibit DPPH. Several concentration of Japanese pepper post digestion (953.00 µg/mL and 238.25 µg/mL) needed eight times concentration to inhibit around 51% and 22% compared to its crude extract concentration (119.12 µg/mL and 29.78 µg/mL).
In matter of IC50 value (Fig 11A&B), andaliman needed 247.74 times
higher concentration to adjust ascorbic acid while Japanese Pepper only needed 58.35 times. However, andaliman scavenging activity showed better stability after digestion since its activity only lost 2.77 times. On the other hand, IC50 of the post
digestion extract of Japanese Pepper tend to need higher concentration which activity lost up to 8.26 times. The increase of the IC50 values of the Japanese Pepper
crude extracts might be because of the decrease in phenolic radical scavenging activity after the digestion process, moreover some compound with different chemical properties could be formed (Cilla et al. 2011). There might be that the compound that responsible in andaliman is not anthocyanin since it is a compound that is very sensitive against pH changes, while andaliman tend to have stable activity after digestion. Bermudez-Soto et al. (2007) shown the total phenolic compound which might contributed the antioxidant activity was lost 22.53%, as well as study by Cilla et al. (2009,2010) determined the phenolic profile before and after in vitro gastrointestinal digestion showed a decrease in total phenolic compounds between 14.1% and 54.2%. The decrease in polyphenols is attributed to a rapid decrease in phenol stability under the mild alkaline condition in the digestion process and different chemical properties can be formed (Bermudez-Soto et al. 2007). The scavenging activity of ascorbic acid was shown lower after digestion (Fig11B). The 3 times decline might be due to the changes in pH value and also the presence of oxygen during the gastrointestinal digestion procedure. At this matter, According to Perez-Vicente et al. (2002) showed the ascorbic acid content in food product lost 16.3-56% after the digestion.
The result suggested that andaliman and Japanese Pepper have shown to possess antioxidant activity, since most spices, including andaliman and Japanese Pepper are the common sources of phenolic compound (Carlsen et al. 2010). Study reported by Lu et al. (2011), showed that mostly total phenolic compound has good correlation with the antioxidant activity and also suggested as the responsible compound for antioxidant activity in the spices and herbs. Phenolic has ideals structure for the free radical scavenging activities. Each phenolic has different antioxidant activity depend on the hydroxyl group in each phenolic structure that responsible for the hydrogen donating to DPPH (Oroian & Escriche 2015).
quercitrin-3-O-rhamnoside (IC50 =18µM) and quercitrin-3-O-galactoside (IC50=16
µM) were exhibited a strong radical scavenging activity against DPPH, which was expected to protect against peroxidative damage in living system (Yamazaki et al. 2007). Moreover, quercetin was found to be effective as α-glucosidase inhibitor since it could bind the α-glucosidase to form a new complex (Lin et al. 2009). The most widespread and diverse phenolics are the flavonoids which have the same C15 (C6-C3-C6) skeleton and possess antioxidant capacity toward a variety of easily oxidizable compounds. For example, the main flavonoid constituents are flavonol aglycones such as quercetin in onion. Generally, flavonoids containing multiple hydroxyl groups have higher antioxidant activities against peroxyl radicals. Quercetin which has hydroxyl group at position C-3 had higher peroxyl radical scavenging activity than its glycoside derivatives (Jo et al. 2010).
Japanese Pepper crude extract had higher antioxidant activity (Fig 10A), might be influenced by the sample condition. As can be seen in Fig 7, andaliman showed brownish green colour fruit while Japanese Pepper still have green colour, which might explained there were a post treatment after the Japanese Pepper fruit was harvested, which is a blanching process that would have inactivated the polyphenol oxidase enzyme (PPO). This enzyme could have alter the polyphenol in the andaliman and will affect the changes in the polyphenol (Crozier et al. 2006). According to Pujimulyani et al. 2012, blanching gave significant effect on the antioxidant activity in white saffron (Curcuma mangga Val.). The increase of the antioxidant activity might be due to hydrolysis of quercetin into aglicone quercetin which was shown to have higher antioxidant activity than the quercetin. Besides that Pujimulyani et al. 2012 also indicated that the quercetin content was not detected in the untreated white saffron, however in the blanched white saffron, it was shown to have 0.25-0.27 mg/ g of quercetin. Similar study reported by Xu and Chang (2008) showed that the heating of bilberry extract could increase the antioxidant activity, since the glycoside changed into aglicone and sugar.
On the other hand, some study showed that the oleoresin in andaliman which consisting mainly of terpenes also showed antioxidant activity, although the oleoresin did not performed high activity as the phenolic compound (Suryanto et al. 2004). According to Kristanti et al. (2012) showed that two terpenoid compound which were hydroxyl ester sterol (IC50= 68.35µg/mL) and cholesterol-3-O-β
-glucoside (IC50= 60.52 µg/mL) from andaliman showed antioxidant activity against
DPPH. The hydroxyl groups on the main chain in ester sterol and the glucose molecules of cholesterol-3-O-β-glucoside compound. The hydroxyl groups acts as proton donor to scavange the DPPH radical likewise the glucose molecule. Moreover, the conjugated double bonds, mostly the conjugated bond could have the reduction of free radicals. While has the conjugated double bond in the aromatic ring structure (ester sterol compound) and the double bond presences in the cholesterol (cholesterol-3-O-β-glucoside compound) main chain at position C-5 and C-6 (Kristanti et al.2012).
possible bioactive compound that have both of the activites is a flavonoid glycosides type. However, future identification must be done first to decide the responsible compounds in andaliman and Japanese pepper.
Fig 10. (A) DPPH scavenging activities of crude extract (B) DPPH scavenging activities of in vitro post digestion extract
B 0 20 40 60 80 100 120
Inhibi
ti
on
(%
)
Concentration (µg/mL) A
0 20 40 60 80 100
0 1000 2000 3000 4000 5000 6000
Inhibi
ti
on
(%
)
Concentration (µg/mL)
Fig 11. (A) IC50 value of DPPH scavenging activities of crude extracts versus in
vitro post digestion extract (B) IC50 value of DPPH scavenging activities
of ascorbic acid before and post digestion
5 CONCLUSION AND SUGGESTION
Conclusion
Before digestion simulation, the crude extract of Japanese pepper showed better inhibition activity towards α-glucosidase enzyme (IC50 = 3930.21µg/mL)
compare to andaliman (IC50 = 20346.94µg/mL). The crude extract of Japanese
pepper also showed better radical scavenging activity towards DPPH (IC50 =
104.03µg/mL ) comparing to andaliman (IC50 = 20346.94µg/mL).
In vitro gastrointestinal digestion decreased the antioxidant activities and α-glucosidase inhibition of both spices. The α-glucosidase inhibition activity of Japanese pepper was lost 1.42 times while andaliman’s α-glucosidase inhibition lost 1.77 times. However, in DPPH radical scavenging activity, andaliman was lost only 2.77 times while Japanese pepper lost 8.26 times. Antioxidant activity of andaliman was more stable than Japanese pepper during the digestion simulation.
Extract of Japanese pepper had stronger antioxidant (IC50 =5580.66µg/mL)
and α-glucosidase inhibition activity (IC50 = 859.55µg/mL) compare to andaliman’s
antioxidant (IC50= 1224.10µg/mL and α-glucosidase inhibition (36089.58µg/mL)
after digestion simulation. Japanese pepper still have better inhibition activity towards α-glucosidase enzyme and antioxidant activity than andaliman.
Suggestion
To improve the result of current assay condition, further analysis on responsible compounds, such as to examine the total phenol and identifying the active markers should be conducted. There is also a need to blanch the andaliman fruit before treatment and analysis in order to obtain equal condition toward Japanese Pepper sample which seemed has pre-treated as well as to minimalize the oxidation of the phenolic compounds.
BIBLIOGRAPHY
Adesina SK. 2005. The nigerian zanthoxylum: chemical and biological values. Afr J Tradit Complement Altern Med. 2:282-301.
Akyla C, Wijaya CH, Yakhin LA. 2014. Effect of spray drying encapsulation method on flavor quality of “andaliman” (Zanthoxylum acanthopodium DC.) powder [Thesis]. Karawaci (ID): Universitas Pelita Harapan.
[AOAC] the Association of Official Analytical Chemists. 2000. Official Methods of Analysis of the Association of Official Analytical Chemist. New York: Chemist Inc.
Arsiningtyas, IS, Gunawan-Puteri, MDPT, Kato E, Kawabata J. 2014. Identification of α-glucosidase inhibitors from the leaves of Pluchea indica Less., a traditional Indonesian herb: promotion of natural product use. Nat Prod Res. 28(17):1350-1353.
Awah FM, Peter NU, Julius OO, John R, Patrick L, Xiao-Jian Y, Keith RF, Michael OE. 2010. Free radical scavenging activity and immunomodulatory effect of stachytarpheta angustifolia leaf extract. J Food Chem. 119: 1409-1416.
Berdanier CD, Dwyer J, Deldman EB. 2006. Handbook of Nutrition and Food Second Edition. CRC Press.
Carlsen MH, Halvorsen BL, Holte K, Bohn SK, Dragland S, Sampson L. 2010. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr J. 9(3):1-11.
Cilla A, Perales S, Lagarda MJ, Barbera R, Farre R. 2008. Iron Bioavailability in Fortified Fruit Beverages Using Ferritin Synthesis by Caco-2 Cells. J. Agric. Food Chem., 56 (18), 8699-8703.
Cilla A, Sarrias Gonzalez-sarrias A, Tomas-Barberan FA, Espin JC, Barbera R. 2009. Availability of polyphenols in fruit beverages subjected to in vitro gastrointestinal digestion and their effects on proliferation, cell-cycle and apoptosis in human colon cancer Caco-2 cells. Food Chemistry. 114, 813-820 Cilla A, Perales S, Lagarda MJ, Barbera R, Clemente G, R Farre. 2011. Influence
of storage and in vitro gastrointestinal digestion on total antioxidant capacity of fruit beverages. J F C A. 24:87-94
Crozier A, Clifford MN, Ashihara H. 2006. Plant Secondary Metabolites;Occurance, Structure and Role in the Human Diet. Blackwell Publishing Ltd.
Giugliano D, Antonio C, Katherine E. 2008. Glucose metabolism and hyperglycemia. Am Journal Clin Nutr. 87:217S–22S.
Gowen A, Abu-Ghannam N, Frias J, Oliveira J. 2007. Influence of pre-blanching on the water absorption kinetics of soybeans. J Food Eng. 78:965-971.
Hartati S, Rizna TD, A Darmawan, Megawati. 2012. Development of Antidiabetic Active Compounds from Ethyl Acetate Extract of Acorus calamus L. Rhizomes. A T B A S Journal. Vol. 2, issue 1: 27-30.
Hashimoto K, Kazuko S, Yoshio K, Atsushi I, Masayoshi K, Hiroshi S, Shigeto N, Susumu K, Koji Y, Takashi N. 2001. Modulatory effect of aliphatic acid amides from Zanthoxylum piperitum on isolated gastrointestinal tract. Planta Med. 66:179-181.
Hatano T, Inada K, Ogawa T, Ito H, Yoshida T. 2004. Aliphatic acid amides of the fruits of Zanthoxylum piperitum. Phytochemistry. 65: 2599-2604.
Holderbaum, DF, Tomoyuki K, Tsuyoshi K, Miguel PG. 2010. Enzymatic Browning, Polyphenol Oxidase Activity, and Polyphenols in Four Apple Cultivars: Dynamics during Fruit Development. Hortscience. 45(8):1150–1154. Hsuang K. 1978. Orders and Families of Malayan Seed Plants.Singapore:
Singapore University Press.
Hur JM, Ju GP. 2003. Effect of Methanol Extract of Zanthoxylum piperitum Leaves and of its Compound, Protocatechuic Acid, on Hepatic Drug Metabolizing Enzymes and Lipid Peroxidation in Rats. Biosci. Biotechnol. Biochem. 67(5):945-950.
Hwang ES, GH Kim. 2012. Safety evaluation of Zanthoxylum piperitum Derived Essential Oil by Assessing Micronucleus Abnormalities, Mutagenicity and Chromosomal Aberration. Food Res Int. 47:267-271.
Jaiswal N, Swayam PS, Vikram B, Akansha M, Amit KS, Tadigoppula N, Arvind KS, Akhilesh KT. 2012. Inhibition of alpha-glucosidase by Acacia nilotica prevents hyperglycemia along with improvement of diabetic complications via aldose reductase inhibition. J Diabetes Metab. S 6:1-7.
Jo SH, Ka EH, Lee HS, Apostolidis E, Jang HD, Kwon YI. 2010. Comparison of Antioxidant Potential and Rat Intestinal α-Glucosidase inhibitory Activities of Querectin Rutin and Isoquerectin. J Applied Res. Nat Prod 2(4): 52-60.
Jong-Anurakkun N, Bhandari M R, Kawabata J. 2006. A-Gucosidase Inhibitors from Devil Tress (Alstonia scholaris). J Food Chem 103: 1319-1323.
Kim Y-M, Youn-Kab J, Myeong-Hyeon W, Wi YL, Hae-Ik R. 2005. Inhibitory effect of pine extract on α-glucosidase activity and postprandial hyperglycemia. Nutrition. (21): 756-761.
Kristanti RE, Mun’im A, Katrin. 2012. Isolation of antioxidant and xantine oxidase inhibitor extract of “andaliman” fruit (Zanthoxylum acanthopodium DC.). Int.J. Medical. Arom. Plants. 2:376-389.
Lee JH, Jang M, Seo J, Kim GH. 2012. Antibacterial effects of natural volatile essential oil fromZanthoxylum piperitum DC.against foodbornepathogens. J. Food Biochem. 36: 667-674.
Lee SJ, Kye TL. 2008. Glycoprotein of Zanthoxylum piperitum dc has a hepatoprotective effect via anti-oxidative character in vivo and in vitro. Toxicol in Vitro. 22:376-385.
Lin YQ, Zhou FC, Gao F, Bian JS, Shan F. 2009. Comparative Evaluation of quercetin, isoquercetin and rutin as inhibitors of α-glucosidase. J Agric Food Chem. 57:11463-11468.
Lu M, Bo Y, Maomao Z, Jie C. 2011. Antioxidant capacity and major phenolic compounds of spices commonly consumed in China. Food Res Int 44: 530-536. Malau S, Manurung A, Tobing MC. 2002. Konservasi Tanaman Khas dan Langka Kawasan Danau Toba : Plasma Nutfah dan Potensi Agrowisata. Seminar Nasional Strategi Pembangunan Berkelanjutan dan Pengelolaan Kawasan Danau Toba, Medan, Indonesia.
Mayur B, Sandesh S, Shruti S, Yum SS. 2010. Antioxidant and α-glucosidase inhibitory properties of Carpesium abrotanoides L. J. Med Plant Research. 4(15):1547-1553.
McDonnell, Marie, Gullermo EU. 2012. Insulin Therapy for the Management of Hyperglycemia in Hospitalized Patients. Endocrinol Metabolism Clinics. 175-201.
McDougall GJ, Dobson P, Smith P, Blake A, Stewart D. 2005. Assessing potential bioavailability of raspberry anthocyanins using an in vitro digestion system. J. Agri and Food Chem. 53:5896-5904.
Napitupulu B, Simatupang S, Sinaga M. 2004. Potency of Andaliman as Traditional Food Additive of Batak Ethnic North Sumatera, Prosiding Seminar Nasional Peningkatan Daya Saing Pangan Tradisional, Bogor, Indonesia.
Oh SM, Woong H, Myeong-Hyeon W. 2010. Antioxidant and α-glucosidase inhibition activity from different extracts of Zanthoxylum schnifolium fruits. Kor J Pharmacogn. 41(2):130-135.
Oroian M, Escriche I. 2015. Antioxidants: Characterization, natural sources, extraction and analysis. Food Res Int. 74:10-36.
Parhusip AJN, Jenie BSL, Rahayu WP, Yasni S. 2005. Effect of andaliman (Zanthoxylum acanhopodium DC) extract upon permeability and hidrophobicity of Bacillus cereus. J Teknol Ind Pangan. 16:24-30.