KHIDIR TAJELSEIR OTHMAN MUSTAFA
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
I declare that this thesis titled “Antibacterial Activity of Aqueous Acetone
Extracts from
Acacia nilotica
Seeds” is my own work. Information and quotes that
were sources from journals and books have been acknowledged and mentioned where
in the thesis they appear. All complete references are given at the end of the paper.
acetone extracts from
Acacia nilotica
seeds. Supervised by SUMINAR SETIATI
ACHMADI and NISA RACHMANIA MUBARIK.
The present study aimed at evaluating the
in vitro
antimicrobial activity of
aqueous acetone extracts of medicinal plant
Acacia nilotica
subsp.
nilotica
(Family:
Fabaceae
) seeds against Gram-negative bacteria
Escherichia coli
, Gram-positive
organisms
Staphylococcus aureus,
and Gram-negative marine bacteria
Vibrio
harveyi.
The crude extract of
A. nilotica
presented the highest anti-
S. aureus
activity
and was effective against all bacterial strains tested. The antibacterial activity of
aqueous acetone extract and isolated constituents of seeds and pods of
A. nilotica
were evaluated by the cup diffusion method against the three species of bacteria
.
The
crude extract was subsequently fractioned and monitored by bioassay leading to the
isolation of active fractions by further chemical analysis. These active fractions
showed highest antibacterial activity
in vitro
at 1000 ppm concentrations which
capable of inhibiting the growth of bacteria. The inhibition diameters were varied
from 0.1-0.6 cm. Sephadex LH-20, a liquid chromatography media, was used to
purify and to separate the active compounds, the result of the separations was 3
fractions, two were ethanol fractions, and one was acetone fraction. Two–dimension
thin layer chromatography showed these two fractions were not pure. Acute toxicity
test towards
Artemia salina
Lethal Dose 50 (LD
50) result showed that the crude
extract of the seed has sufficient toxicity in low concentration, indicating that the
crude extract should be effective towards microorganism as well. Elucidation by
Fourier transform infrared and ultraviolet spectroscopy for these two fractions
presented good result which indicated they were part of tannins.
KHIDIR TAJELSEIR OTHMAN MUSTAFA. Antibacterial activity of aqueous
acetone extracts from
Acacia nilotica
seeds. Supervised by SUMINAR SETIATI
ACHMADI and NISA RACHMANIA MUBARIK.
Many efforts have been made to discover new antimicrobial compounds from
various kinds of plants. One of such resources is folk medicines. The increasing
prevalence of multidrug resistant strains of bacteria and the recent appearance of
strains with reduced susceptibility to antibiotics raises the specter of untreatable
bacterial infections and adds urgency to the search for new infection-fighting
strategies.
Acacia nilotica
is a tree 5-20 m high with a dense spherical crown, stems and
branches usually dark to black colored, fissured bark, grey-pinkish slash, exuding a
reddish low quality gum. Pods are strongly constricted, hairy, white-grey, thick and
softly tomentose. Its seeds number approximately 8000/kg.
A. nilotica
and other
acacia species are used in folk medicine by peoples in rural areas for illnesses caused
by bacteria, but this has not been confirmed by experiments.
Condensed tannins are natural preservatives as anti-fungal agents. This is the
reason for their presence in the outer tissues of plants – to help protect against attack
by a variety of pathogens including fungi. Condensed tannins are larger tannins and
toxic to many microbes. When condensed tannins are broken down their subunits
they are believed to be able to bind proteins but low molecular weight tannins are
toxic to fewer microbes because once the molecule is broken down the subunits loses
their protein binding abilities.
From 400 g of the
A. nilotica
seeds which were extracted with aqueous
acetone
70%yielded 6% crude extract of the weight and its moisture content was
12.58%. The addition of the FeCl
310% to the filtrate of the sample showed blackish
blue color, indicated the presence of tannins in the sample. Tannin content was
determined using butanol-HCl assay method (as % catechin) using U-2010
Spectrophotometer, wavelength 530 nm, and found the catechin content was 46.61%.
The crude extract of
A. nilotica
presented the highest anti-
S. aureus
activity
and was effective against all bacterial strains tested. The antibacterial activity of
aqueous acetone extract and isolated constituents of seeds and pods of
A. nilotica
were evaluated by the cup diffusion method against the three species of bacteria
.
The
crude extract was subsequently fractioned and monitored by bioassay leading to the
isolation of active fractions by further chemical analysis. These active fractions
showed highest antibacterial activity
in vitro
at 1000 ppm concentrations which
capable of inhibiting the growth of bacteria. The inhibition diameters were varied
from 0.1-0.6cm. Sephadex LH-20, a liquid chromatography media, was used to purify
and to separate the active compounds, the result of the separations was 3 fractions,
two were ethanol fractions, and one was acetone fraction. Two–dimension thin layer
chromatography showed these two fractions were not pure. Acute toxicity test
towards
Artemia salina
Lethal Dose 50 (LD
50) result showed that the crude extract of
the seed has sufficient toxicity in low concentration, indicating that the crude extract
should be effective towards microorganism as well. Elucidation by Fourier transform
infrared and ultraviolet spectroscopy for these two fractions presented good result
which indicated they were part of tannins.
© Copyrights property of Khidir Taj-Elseir Othman Mustafa, the year 2009
Copyrights is protected
Prohibited multiplies without written permission from Bogor Agricultural University,
partly or entirely in any form or by any means, electronic, photocopying,
ANTIBACTERIAL ACTIVITY OF AQUEOUS ACETONE
EXTRACTS FROM Acacia nilotica SEEDS
KHIDIR TAJELSEIR OTHMAN MUSTAFA
Thesis
As one of requirements to obtain degree of
Master of Science at
The Department of Chemistry
GRADUATE SCHOOL
Name : Khidir TajElseir Othman Mustafa
Registration Number : G451078131
Major : Chemistry
Approved by
Advisory Committee
………. ……...……….
Prof. Dr. Ir. Suminar S. Achmadi
Dr. Nisa Rachmania Mubarik, MSi
(Chairman) (Member)
Agreed
Coordinator of Major Dean of Graduate School
Programmer
………. ……..….……….
Prof. Dr. Ir. Latifah K. Darusman Prof. Dr. Ir. Khairil A. Notodiputro, M.S.
KHIDIR TAJELSEIR OTHMAN MUSTAFA
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
I declare that this thesis titled “Antibacterial Activity of Aqueous Acetone
Extracts from
Acacia nilotica
Seeds” is my own work. Information and quotes that
were sources from journals and books have been acknowledged and mentioned where
in the thesis they appear. All complete references are given at the end of the paper.
acetone extracts from
Acacia nilotica
seeds. Supervised by SUMINAR SETIATI
ACHMADI and NISA RACHMANIA MUBARIK.
The present study aimed at evaluating the
in vitro
antimicrobial activity of
aqueous acetone extracts of medicinal plant
Acacia nilotica
subsp.
nilotica
(Family:
Fabaceae
) seeds against Gram-negative bacteria
Escherichia coli
, Gram-positive
organisms
Staphylococcus aureus,
and Gram-negative marine bacteria
Vibrio
harveyi.
The crude extract of
A. nilotica
presented the highest anti-
S. aureus
activity
and was effective against all bacterial strains tested. The antibacterial activity of
aqueous acetone extract and isolated constituents of seeds and pods of
A. nilotica
were evaluated by the cup diffusion method against the three species of bacteria
.
The
crude extract was subsequently fractioned and monitored by bioassay leading to the
isolation of active fractions by further chemical analysis. These active fractions
showed highest antibacterial activity
in vitro
at 1000 ppm concentrations which
capable of inhibiting the growth of bacteria. The inhibition diameters were varied
from 0.1-0.6 cm. Sephadex LH-20, a liquid chromatography media, was used to
purify and to separate the active compounds, the result of the separations was 3
fractions, two were ethanol fractions, and one was acetone fraction. Two–dimension
thin layer chromatography showed these two fractions were not pure. Acute toxicity
test towards
Artemia salina
Lethal Dose 50 (LD
50) result showed that the crude
extract of the seed has sufficient toxicity in low concentration, indicating that the
crude extract should be effective towards microorganism as well. Elucidation by
Fourier transform infrared and ultraviolet spectroscopy for these two fractions
presented good result which indicated they were part of tannins.
KHIDIR TAJELSEIR OTHMAN MUSTAFA. Antibacterial activity of aqueous
acetone extracts from
Acacia nilotica
seeds. Supervised by SUMINAR SETIATI
ACHMADI and NISA RACHMANIA MUBARIK.
Many efforts have been made to discover new antimicrobial compounds from
various kinds of plants. One of such resources is folk medicines. The increasing
prevalence of multidrug resistant strains of bacteria and the recent appearance of
strains with reduced susceptibility to antibiotics raises the specter of untreatable
bacterial infections and adds urgency to the search for new infection-fighting
strategies.
Acacia nilotica
is a tree 5-20 m high with a dense spherical crown, stems and
branches usually dark to black colored, fissured bark, grey-pinkish slash, exuding a
reddish low quality gum. Pods are strongly constricted, hairy, white-grey, thick and
softly tomentose. Its seeds number approximately 8000/kg.
A. nilotica
and other
acacia species are used in folk medicine by peoples in rural areas for illnesses caused
by bacteria, but this has not been confirmed by experiments.
Condensed tannins are natural preservatives as anti-fungal agents. This is the
reason for their presence in the outer tissues of plants – to help protect against attack
by a variety of pathogens including fungi. Condensed tannins are larger tannins and
toxic to many microbes. When condensed tannins are broken down their subunits
they are believed to be able to bind proteins but low molecular weight tannins are
toxic to fewer microbes because once the molecule is broken down the subunits loses
their protein binding abilities.
From 400 g of the
A. nilotica
seeds which were extracted with aqueous
acetone
70%yielded 6% crude extract of the weight and its moisture content was
12.58%. The addition of the FeCl
310% to the filtrate of the sample showed blackish
blue color, indicated the presence of tannins in the sample. Tannin content was
determined using butanol-HCl assay method (as % catechin) using U-2010
Spectrophotometer, wavelength 530 nm, and found the catechin content was 46.61%.
The crude extract of
A. nilotica
presented the highest anti-
S. aureus
activity
and was effective against all bacterial strains tested. The antibacterial activity of
aqueous acetone extract and isolated constituents of seeds and pods of
A. nilotica
were evaluated by the cup diffusion method against the three species of bacteria
.
The
crude extract was subsequently fractioned and monitored by bioassay leading to the
isolation of active fractions by further chemical analysis. These active fractions
showed highest antibacterial activity
in vitro
at 1000 ppm concentrations which
capable of inhibiting the growth of bacteria. The inhibition diameters were varied
from 0.1-0.6cm. Sephadex LH-20, a liquid chromatography media, was used to purify
and to separate the active compounds, the result of the separations was 3 fractions,
two were ethanol fractions, and one was acetone fraction. Two–dimension thin layer
chromatography showed these two fractions were not pure. Acute toxicity test
towards
Artemia salina
Lethal Dose 50 (LD
50) result showed that the crude extract of
the seed has sufficient toxicity in low concentration, indicating that the crude extract
should be effective towards microorganism as well. Elucidation by Fourier transform
infrared and ultraviolet spectroscopy for these two fractions presented good result
which indicated they were part of tannins.
© Copyrights property of Khidir Taj-Elseir Othman Mustafa, the year 2009
Copyrights is protected
Prohibited multiplies without written permission from Bogor Agricultural University,
partly or entirely in any form or by any means, electronic, photocopying,
ANTIBACTERIAL ACTIVITY OF AQUEOUS ACETONE
EXTRACTS FROM Acacia nilotica SEEDS
KHIDIR TAJELSEIR OTHMAN MUSTAFA
Thesis
As one of requirements to obtain degree of
Master of Science at
The Department of Chemistry
GRADUATE SCHOOL
Name : Khidir TajElseir Othman Mustafa
Registration Number : G451078131
Major : Chemistry
Approved by
Advisory Committee
………. ……...……….
Prof. Dr. Ir. Suminar S. Achmadi
Dr. Nisa Rachmania Mubarik, MSi
(Chairman) (Member)
Agreed
Coordinator of Major Dean of Graduate School
Programmer
………. ……..….……….
Prof. Dr. Ir. Latifah K. Darusman Prof. Dr. Ir. Khairil A. Notodiputro, M.S.
blessings that have seen this research work completed, in titled “Antibacterial
Activity of Aqueous Acetone Extracts from
Acacia nilotica
Seeds”. I would like to
express my gratitude to all those who gave me the possibility to complete this thesis. I
want to thank the Government of Indonesia for giving me scholarship and the
Government of Sudan for giving me permission to study in Indonesia and helping me
with support in Indonesia. Also I thank Bogor Agricultural University, IPB; I gave
my special thanks to the Department of Chemistry and Department of Biology to
their unlimited helping which made this work succeed. I have furthermore to thank
the Dean of the Graduate school, Mr. Khairil Anwar, and Miss Anissa Anwar, and
Mr. Drajat Martianto for their continuous helping and looking after us.
I am deeply
indebted to my supervisors Prof. Dr. Ir. Suminar Setiati Achmadi, and Dr. Nisa
Rachmania Mubarik, M.Si whose guidance, stimulating suggestions, and
encouragement helped me in all the time of research for and writing of this thesis. I
will not forget thanks Dr. Irma Suparto as the examiner and Dr. Iman Rusmana for
usage of
Vibrio harveyi
. My former colleagues from the Department of Chemistry
supported me in my research work. I wont to thank them for all their help, support,
interest and valuable hints. Especially I am obliged to Miss Wulan Tri Wahyuni, Mrs.
Hariyanti, Miss Hasu Marty Linda, and Mr. Paulyasi Tokasaya Fiji. I also want to
thank Mr. Sabur, Mr. Iman, and Mrs. Heny for all their assistance on the laboratories
work. My Father was of great help in difficult times. My Daughter Naziha, as well as
my brother Mutaz for their stimulating support to do this thesis. Especially, I would
like to give my special thanks to my wife, Sewasin, who patient love enabled me to
complete this work. God bless you all.
CONTENT
Page
CONTENT ………...…. xi
TABLES ………..…. xii
FIGURES ………..………. xiv
APPENDICES ……….…..………... xv
INTRODUCTION
Background ……….………….….. 1
Objectives, Purpose, and
Scope of Research ……….……….… 2
Place and Time of Study ...……….……….…. 2
LITERATURE REVIEW
Acacia nilotica
subsp.
nilotica
…………...……….………... 3
Tannins
……….……….… 5
Staphylococcus aureus ………...………..
7
Escherichia coli
………...
8
Vibrio harveyi
………... 9
Artemia salina
………..……….. 10
Lethal Dose 50 (LD
50) ……….. 10
MATERIAL AND METHODS
Apparatus and Materials ...…... 12
Chemical Analysis
……… 12
Tannin Fractionation ……….. 13
Toxicity Test (LD
50) ………..…... 14
Antimicrobial tests …..………... 14
RESULTS
DISCUSSION
Phytochemical analysis ……… 20
Tannin content ……….. 21
Separation ………... 21
Acute toxicity test toward
A. salina
(LD
50)
……..………...
22
Antibacterial Test ………. 23
Elucidation ………... 26
Comparing with Previous Reference about Antimicrobial from
TABLES
Page
FIGURES
Page
1
Acacia nilotica
(a) Pods and (b) Seeds …………..……… 3
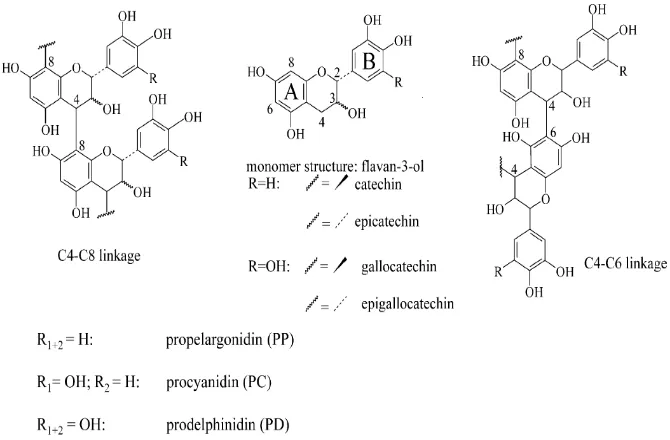
2 Chemical structure of flavan-3-ol; monomer units and condensed tannins ... 6
3 The presence of tannin using butanol-HCl assay ……….………... 15
4 TLC (a) one and (b) two dimensions of ethanol fraction, acetone fraction,
APPENDICES
Page
Background
Many efforts have been made to discover new antimicrobial compounds from various kinds of plants. One of such resources is folk medicines. Systematic screening of them may result in the discovery of novel effective compounds. The increasing prevalence of multidrug-resistant strains of bacteria and the recent appearance of strains with reduced susceptibility to antibiotics raises the specter of untreatable bacterial infections and adds urgency to the search for new infection-fighting strategies.
Sudan throughout its long history has accumulated a rich body of empirical knowledge of the use of medicinal plants for the treatment of various diseases. Chemical studies of Sudanese medicinal plants provide a valuable material base for the discovery and development of new drugs of natural origin (Cowan 1999). Acacia nilotica and other acacia species are used in folk medicine by people in rural areas as a remedy for tuberculosis, leprosy, small pox, dysentery, cough, ophthalmic, toothache, skin ulcers, cancer, as astringents, antispasmodics, and aphrodisiac. A. nilotica species have been reported to have antihyper-glycaemic, antimicrobial, antiplasmodial, anti-inflammatory, analgesic, and antipyretic properties.
The bark and seeds are used as a source of tannins. The species is also used for medicinal purposes. The gum of A. nilotica is sometimes used as a substitute for gum Arabic (obtained from A. senegal) although the quality is inferior. The species is suitable for the production of paper and has similar pulping properties to a range of other tropical timbers (Nasroun 1979).
anti-fungal agents. This is the reason for their presence in the outer tissues of plants – to help protect against attack by a variety of pathogens including fungi (Peter 2004). Aqueous acetone is more effective extractive than that of alcoholic solvents and acetone extract inhibits tannin-protein interaction (Reed et al. 1985). The methanol extract of the pods of A. nilotica shows antihypertensive activities.
Objectives, Purpose, and Scope of Research
The objectives of this research were (1) to determine the antimicrobial activity of A. nilotica subsp. nilotica seeds extracts; and (2) to define the effective fraction of the extracts towards three species of bacteria(Staphylococcus aureus, Escherichia coli, and Vibrio harveyi).
This research would like to confirm the usefulness of this plant as a
medicinal plant. By carrying out this research, safety of people from illnesses
caused by bacteria may be enhanced and also medicinal effects of A. nilotica
seeds can be established. Trials were undertaken to examine the anti-bacterial activity of the seeds aqueous acetone extract against both Gram–negative and Gram–positive bacteria (three species, i.e. E. coli, S. aureus, and V. harveyi).
Furthermore, the effective fractions against these three bacteriawere determined.
Place and Time of Study
LITERATURE REVIEW
Acacia nilotica subsp. Nilotica
Acacia nilotica subsp. nilotica (Family: Fabaceae) is a tree 5-20 m high with a dense spherical crown, stems and branches usually dark to black colored, fissured bark, grey-pinkish slash, exuding a reddish low quality gum. The tree has thin, straight, light, grey spines in axillaries pairs, usually in 3 to 12 pairs, 5 to 7.5 cm long in young trees, mature trees commonly without thorns. The leaves are bipinnate, with 3-6 pairs of pinnulae and 10-30 pairs of leaflets each, tomentose, rachis with a gland at the bottom of the last pair of pinnulae. Flowers in globulous heads 1.2-1.5 cm in diameter of a bright golden-yellow color set up either axillaries or whorl on peduncles 2-3 cm long located at the end of the branches. Pods are strongly constricted, hairy, white-grey, thick and softly tomentose. Its seeds number approximately 8000/kg (Fagg 2001).
A. nilotica (thorn mimosa) is a species of Acacia (wattle) native to Africa
and the Indian subcontinent. It is also currently an invasive species of significant
concern in Australia (Fagg 2001). A. nilotica which was introduced to Indonesia is subsp. indica (Djufri 2004).
[image:30.612.199.464.453.539.2](a) (b)
Figure 1 Acacia nilotica (a) pods and (b) seeds (Attenborough 1995).
recognized, the most important are subsp. adstringens; subsp. kraussiana; subsp. nilotica; and subsp. tomentosa (Kongevej 2008).
Uses of A. nilotica. A. nilotica is one of the most important multipurpose tree species of dry land Africa and South Asia. The wood is hard and used for general construction purposes and implements. It is fairly termite resistant. The wood yields an excellent fuel wood. Flowers are attracting bees and make a good base for apiculture. Pods are nutritious and make a high quality fodder for livestock especially during the dry season. The plant has alleged medical properties as the bark is used for treatment of cough, cancers, tumours of the ear, intestine pains and the roots to treat impotence (Kongevej 2008).
The pods and leaves are very popular with cattle. Pods are also used as a
supplement to poultry rations in India (Fagg 2001). A. nilotica makes a good protective hedge because of its thorns. In Italian Africa, the wood is used to treat
small pox. In Ethiopia certain parts of the tree are used as alactogogue. The wood is "very durable if water-seasoned" and its uses include tool handles and lumber for boats (Fagg 2001).
Also, A. nilotica be used as medicine. African Zulu take the (bark) for
cough. Furthermore, it acts as an astringent and it is used to treat diarrhea,
dysentery, and leprosy. In West Africa, the bark or gum is used to treat cancers
and/or tumors (of ear, eye, or testicles) and indurations of liver and spleen, and
condylomas. Sap or bark, leaves, and young pods are strongly astringent due to
tannin, and are chewed in Senegal as an antiscorbutic. The bruised leaves are poulticed and used to treat ulcers. In Lebanon, the resin is mixed with orange-flower infusion for typhoid convalescence. The Chipi use the root for tuberculosis.
In Tonga, the root is used to treat tuberculosis. Egyptian Nubians believe that
diabetics may eat unlimited carbohydrates as long as they also consume powdered
pods (Fagg 2001).
when there is no grass. The digestible crude protein and total digestible nutrient values of acacia leaves are 10.21% and 66.46%, respectively (Barman & Rai 2003).
The total tannin content of acacia pods is 18.71% and that of the seeds. The mature seeds contain 234 g/kg crude protein, 126 g/kg crude fiber, 66.6 g/kg crude fat, and 234 g/kg carbohydrates. Potassium, phosphorus, magnesium, iron, and manganese occurred in high concentrations (Siddhuraju et al. 1995).
Storage and Viability. The dry seeds can be stored for several years even at ambient temperature. Bruchid infested seed can be damaged during storage but the insects rarely re-infest new seed in storage (Kongevej 2008).
Tannins
Tannins are water-soluble polyphenols that are commonly foundin higher herbaceous and woody plants. They can be classified into two categories: hydrolysable and non-hydrolysable (condensed). Tannic acid is an important gallotannin belonging to the hydrolysableclass, while catechinbelongs to the non-hydrolysable class (Fig 2).Hydrolysabletannins are esters of phenolic acids and a polyol,usually glucose. The phenolic acids are either gallic acidin gallotannins, or hexahydroxydiphenic acid in ellagitannins. The hexahydroxydiphenic acid of ellagitannins undergoes lactonization to produce ellagic acid (Hagerman et al. 2006).
Figure 2 Chemical structure of flavan-3-ol; monomer units and condensed tannins (Hagerman et al. 2006).
and the effect ofincubating S. aureus for 24 h in the combination of conventional chemotherapy and tannic acid below the MIC.
Staphylococcus aureus
Staphylococcus aureus is a facultatively anaerobic, Gram-positive coccus, which appears as grape-like clusters when viewed through a microscope and has large, round, golden-yellow colonies, often with hemolysis, when grown on blood agar plates. The golden appearance is the etymological root of the bacteria's name: aureus means "golden" in Latin.
Staphylococcus aureus is catalase positive (meaning that it can produce the enzyme "catalase") and able to convert hydrogen peroxide (H2O2) to water and
oxygen, which makes the catalase test useful to distinguish staphylococci from enterococci and streptococci. A large percentage of S. aureus can be differentiated from most other staphylococci by the coagulase test: S. aureus is primarily coagulase-positive (meaning that it can produce "coagulase", a protein product, not an enzyme) that causes clot formation while most other Staphylococcus species are coagulase-negative. However, while the majority of S. aureus are coagulase-positive, some may be atypical in that they do not produce coagulase. Incorrect identification of an isolate can impact implementation of effective treatment and/or control measures.
Staphylococcus aureus may occur as a commensal on human skin; it also
occurs in the nose frequently (in about a third of the population) and throat less commonly. The occurrence of S. aureus under these circumstances does not always indicate infection and therefore does not always require treatment (indeed, treatment may be ineffective and re-colonization may occur). It can survive on domesticated animals such as dogs, cats and horses, and can cause bumble foot in chickens. S. aureus can infect other tissues when normal barriers have been breached (e.g., skin or mucosal lining). This leads to furuncles (boils) and carbuncles (a collection of furuncles). In infants S. aureus infection can cause a severe disease Staphylococcal scalded skin syndrome (SSSS).
producing hyaluronidase that destroy tissues, and contact with objects such as towels, sheets, clothing, or athletic equipment used by an infected person.
Deeply situated S. aureus infections can be very severe. Prosthetic joints put a person at particular risk for septic arthritis, and Staphylococcal endocarditic (infection of the heart valves) and pneumonia, which may be rapidly spread (Kluytmans et al. 1997).
Escherichia coli
Escherichia coli is Gram-negative, facultative anaerobic and non-sporulating. The cells are about 2 m long and 0.5 m in diameter, with a cell volume of 0.6-0.7 m3. It can live on a wide variety of substrates. E. coli uses mixed-acid fermentation in anaerobic conditions, producing lactate, succinate, ethanol, acetate and carbon dioxide. Since many pathways in mixed-acid fermentation produce hydrogen gas, these pathways require the levels of hydrogen to be low, as is the case when E. coli lives together with hydrogen-consuming organisms such as methanogens or sulfate-reducing bacteria.
Optimal growth of E. coli occurs at 37 °C, but some laboratory strains can multiply at temperatures of up to 49 °C. Growth can be driven by aerobic or anaerobic respiration, using a large variety of redox pairs, including the oxidation of pyruvic acid, formic acid, hydrogen and amino acids, and the reduction of substrates such as oxygen, nitrate, and dimethyl sulfoxide.
do not acquire genetic elements encoding for virulence factors, they remain benign commensalism.
Virulent strains of E. coli can cause gastroenteritis, urinary tract infections, and neonatal meningitis. In rare cases, virulent strains are also responsible for hemolytic-uremic syndrome (HUS), peritonitis, mastitis, septicemia and Gram-negative pneumonia. Recently it is thought that E. coli and certain other food borne illnesses can sometimes trigger serious health problems months or years after patients survived that initial bout (Vogt &Dippold 2005).
Vibrio harveyi
It’s a species of Gram negative, bioluminescent, marine bacteria in the genus Vibrio. V. harveyi are rod-shaped, motile (via polar flagella), facultatively anaerobic, halophilic, and competent for both fermentative and respiratory metabolism. They do not grow at 4°C or above 35°C. V. harveyi can be found free-swimming in tropical marine waters, commensally in the gut micro flora of marine animals, and as both a primary and opportunistic pathogen of marine animals, including Gorgonian corals, oysters, prawns, lobsters, the common snook, barramundi, turbot, milkfish, and seahorses. V. harveyi is responsible for luminous vibriosis, a disease that affects commercially-farmed penaeid prawns. Additionally, based on samples taken by ocean-going ships, V. harveyi is thought to be the cause of the milky seas effect, in which, during the night, a uniform blue glow is emitted from the seawater. Some glows can cover nearly 6,000 square miles (Austin & Zhang 2006).
Artemia Salina
Artemia salina is a species of brine shrimp, primitive, aquatic crustacean that are more closely related to Triops and cladocerans than to true shrimps. They can tolerate fluctuations in the salinity of water — they can live in water having much more or much less salt content than normal seawater. The resilience of these creatures makes them ideal test samples in experiments. The females can produce fertilized eggs in parthenogenesis. These eggs can remain in a dormant state, in cysts, for a number of years until they are placed in water. Some animals are capable of living in very odd and extreme environments. Excellent examples of extreme environments are found in highly saline lakes and pools. These vary considerably in their total osmotic concentration, and in the nature of the solutes present.
The ability of A. salina to live in such brines has long been known. Artemia can survive in several most unlikely media. For example, it has been known that Artemia is capable of living for days in solutions of potassium permanganate, potassium bichromate, and silver nitrate. Such statements as these might be taken as evidence that Artemia is highly impermeable. A study of the survival of Artemia in various media may thus give information concerning the permeability of the animal and possibly also about the regulatory mechanisms (Croghan 1957). Brine shrimp eggs were supplied by Ocean Star International Inc. Larvae were used within 1 d of hatching (Lee et al. 1999)
Lethal Dose 50 (LD50)
LD50 test involves the administration of a substance to a group of animals
LD50 provides no information on what system failure led to the death of the
animals. Some deaths may be due to the quantity of the test substance causing gastric rupture or other morbidity unrelated to the toxicity of the test substance.
The designers of the LD50 test in 1927 acknowledged its serious
inadequacies, intending it only for certain narrow medical purposes. Nevertheless, use of the LD50 test has become widespread as a general measurement of chemical
toxicity. The LD50 has been challenged for decades as both unreliable and
MATERIALS AND METHODS
Apparatus and Materials
The apparatuses used were extraction apparatus, rotary evaporator, freeze dryer, TLC silica gel (thin layer chromatography), Sephadex LH 20, instruments for bacterial test, Fourier Transform Infrared Shimadzu 2010A Spectro-photometer, UV U- 2800 Spectrophotometer.
Acacia nilotica subsp. nilotica seeds collected from Gadarif area in east of
Sudan were the plant parts examined for the antimicrobial test. These seeds were saved in dark plastic storage box set at room temperature. Three species of bacteria (S. aureus, E. coli, V. harveyi) were collected in Microbiology Laboratory, Department of Biology, Bogor Agricultural University.
Chemical Analysis
Moisture Content. About 1 g of the finely ground material was transferred into a tarred wide-mouth weighing bottle and weighed accurately. The sample was dried at 105 °C in an oven for 4 hours, cooled in a desiccator for about 20 minutes and weigh again accurately. The procedure was repeated until three weightings at an interval of one hour until a constant weight. The moisture content (%) was calculated from the sample weight before and after drying. The equation used was the following:
MC % = {(W2 −W3)/ (W3 – W1)} × 100 ……… (1)
where W1 = weight of weighing bottle (g), W2 = weight of moist sample +
weighing bottle (g), W3 = weight of dried sample + weighing bottle (g), MC % =
moisture content (%).
Tannin Test. About 1 g of the extract was dissolved in 20 ml of hot distilled water and filtered. Two to three drops of FeCl310% was added to 2 ml of
The Butanol-HCl Assay (Hagerman et al. 2006). A colorimetric assay involves the oxidative cleavage of proanthocyanidins with ferrous sulfate. About 1g of the aqueous acetone extract was dissolved in 100 ml of the aqueous acetone. To (0.5 and 0.1) ml of aqueous acetone plant extract solution and to(0.0, 0.1, 0.2 and 0.5) ml of standard (catechin which was solved in aqueous acetone 70%), was added a 5-mL portion of an acidic solution of ferrous sulfate (77 mg of FeSO4⋅7H2O dissolved in 500 mL of 2:3 HCl/n-butanol). The tubes was loosely
covered and placed in a water bath at 95 °C for 15 min. The absorbance was read at 530 nm. Tannin content in aqueous acetone extracts was determined (as % catechin) using U-2010 Spectrophotometer, wavelength 530 nm (used for the standard curve). The molecular extinction coefficient ε mol that can be used to convert the absorbance values to a concentration is equal to 34700 L mol-1cm-1.
% Tannin (mg/100 g) = (As/Ast) × Fd × 100% ……… (2)
where As = absorbance of the sample; Ast = absorbance of the standard; Fd = dilution factor.
Tannin Fractionation
About 400 g of seeds of A. nilotica were cleaned and dried in open air and powdered, submerged in 70% aqueous acetone, and 1% ascorbic acid, then the aqueous phase was cleaned up by several washing with n-hexane (25 ml, 6 times). The water layer was washed with ethyl acetate (10 ml, 10 times). Then the solvents were removed by rotary evaporation and freeze dried (Lorenz 2002). The crude extract was applied to the liquid column chromatography using Sephadex LH 20 (Van Soest et al. 1999) as stationary phase and washed with 95% ethanol to elute the tannin with low molecular weight. The substance which was adsorbed at Sephadex LH 20 was treated with aqueous acetone of 50%.
fractions that had microbial activity were elucidated using FT-IR and UV spectrophotometer (Appendix 1).
Toxicity Test (LD50)
Crude extract was dissolved in sea-water to obtain concentration series of 10, 15, 20, 25, 50 ppm; Tween-80 was added to the homogenized solvents. To each mixture ten A. salina larvae were introduced. After 24 hours, observation was done on the living and the dead of the larvae (Sukardiman et al. 2004). The equation used is the following:
log LD50 = Xk – d/2 ∑ (Pi + Pi+1) ………. (3)
where i = group number, 1, 2, 3, 4, 5, and 6; Xk = the max of logarithmic dosage; d
= the difference of two border upon logarithmic dosage, d = lgDi+1 - lgDi; and Pi=
the death rate of each group (Jun et al. 2006).
Antimicrobial Tests
RESULTS
Moisture Content of The Seeds
From 400 g of the A. nilotica seeds which were extracted with aqueous acetone yielded 21g and its moisture content was 12.58% (Appendix 2).
The result of moisture content led to the dry weight of the sample was 349.68 g and the crude extract was 21 g then the percentage of the crude extract was 6% of the weight.
Tannin Content
The addition of the FeCl310% to the filtrate of the sample showed blackish
blue color (Figure 3) indicated the presence of tannins in the samples. In aqueous acetone extracts as determined using butanol-HCl assay method using U-2010 Spectrophotometer at wavelength 530 nm, the tannins was 40.75% (Appendix 3) as % catechin. The data in Appendix 4 and the calculation is shown in Appendix 5.
[image:42.612.231.380.424.554.2]
Figure 3 The presence of tannin using butanol-HCl assay.
Ethanol and Acetone Extracts
ethanol fraction F1 and acetone fraction F3 were more pure than F2 fraction (Figure 4).
[image:43.612.154.486.457.583.2](a) (b)
Figure 4 TLC (a) one and (b) two dimensions of ethanol fraction, acetone fraction, and crude extract.
Acute Toxicity
The result of acute toxicity as tested toward LD50 of A. salina showed that
the crude extract of A. nilotica has enough toxicity in low concentration, indicating that the crude extract may be effective towards microorganism as well (Table 1).
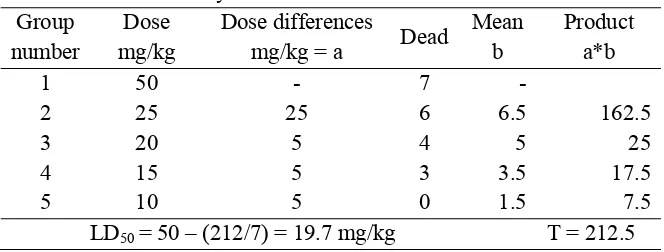
Table 1 Acute toxicity of Karber’s method Group
number
Dose mg/kg
Dose differences
mg/kg = a Dead
Mean b
Product a*b
1 50 - 7 -
2 25 25 6 6.5 162.5
3 20 5 4 5 25
4 15 5 3 3.5 17.5
5 10 5 0 1.5 7.5
LD50 = 50 – (212/7) = 19.7 mg/kg T = 212.5
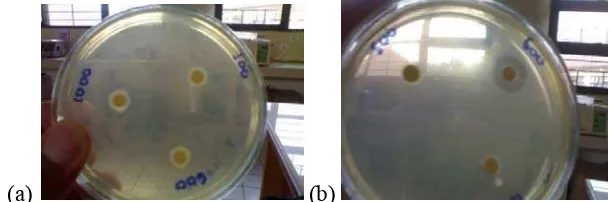
Antibacterial Properties
inhibition zone (Figure 5). The antibacterial activity of aqueous extract were evaluated by the cup diffusion method against three species of bacteria: S. aureus, (Gram-positive organisms), E. coli (Gram-negative), and V. harveyi (Gram negative). One marine bacteria in the genus Vibrio and two human pathogenic bacteria.
The raw data of antibacterial test were presented in Appendix 6.
[image:44.612.146.451.217.318.2](a) (b)
Figure 5 Inhibition zones of (a) V. harveyi and (b) S. aureus.
[image:45.612.132.508.281.464.2]
Figure 6 Inhibition zone of V. harveyi.
Figure 7 Inhibitionzone of S. aureus.
[image:45.612.131.506.507.667.2]Chemical Stucture
Ethanol fraction and acetone fraction were characterized by FT-IR and UV spectrophotometer. UV spectrum of ethanol fraction (Figure 9-a) with solvent ethanol read in max = 248nm, 305 nm and 418 nm, and FT-IR absorbance of
ethanol fraction (Figure 10-a), and FT-IR spectra of ethanol fraction (Figure 10-a) resemble FT-IR spectra of catechin (Appendix 8), but the UV showed only one
max = 320 nm (Figure 9-b) and FT-IR spectrum of acetone fraction (Figure 10-b).
(a)
(b)
Figure 9 The UV spectrum of (a) ethanol fraction (b) acetone fraction.
(a)
(b)
DISCUSSION
Phytochemical Analysis
Addition of 1% FeCl3 to the solution of crude extract in hot water gave blue
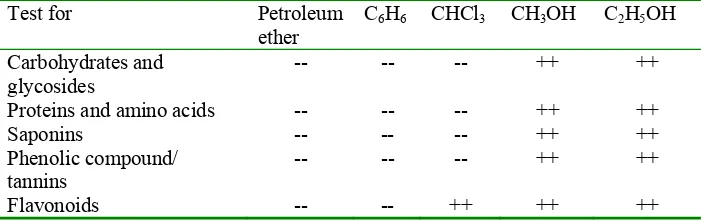
color; meaning that A. nilotica seeds contained tannins. According to Table 5, Satish et al. (2006) phenolic compounds and tannins were found in both ethanol and methanol extracts, indicating that these compounds are favorably extracted in polar solvent. In addition to that, Reed et al. (1985) mentioned that aqueous acetone is a more effective extracting agent than alcoholic solvents. Aqueous acetone was used because acetone inhibits tannin-protein interaction (Hagerman et al. 1986). Phytochemical analysis of the aerial parts of the A. nilotica demonstrated the presence of tannin as shown by the blue color in the FeCl3 test
evidence flavonoids and polyphenolic compounds in the flowers, tannin glycosides, volatile oil organic acids, coumarins, and carbohydrates in the seeds (Haj-Ali & Yagoub 2007). Phytochemical analysis of all the solvent extracts revealed the presence of carbohydrates and glycosides, phytosterols, phenolic compounds, saponins, flavonoids, proteins and amino acids, gums and mucilages in both methanol and ethanol extract in the leaves (Table 2).
[image:47.612.129.481.479.590.2]
Table 2 Phytochemical analysis of Acacia nilotica leaves (Satish et al. 2006)
Test for Petroleum
ether
C6H6 CHCl3 CH3OH C2H5OH
Carbohydrates and glycosides
-- -- -- ++ ++
Proteins and amino acids -- -- -- ++ ++
Saponins -- -- -- ++ ++
Phenolic compound/ tannins
-- -- -- ++ ++
Flavonoids -- -- ++ ++ ++
2006). The stem ethanolic bark extract of the plant possessed the active compounds e.g. tannins, terpenoids, saponins, and glycosides (Banso 2009).
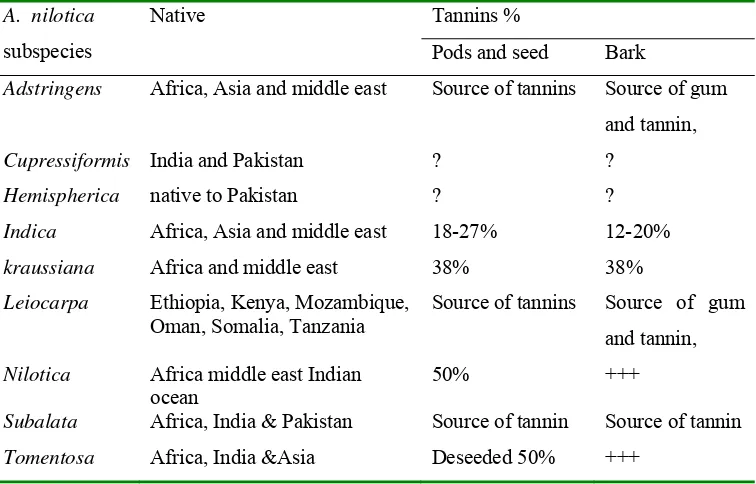
Table 3 Tannin content of various Acacia nilotica subspecies (Barnes 2006) A. nilotica
subspecies
Native Tannins %
Pods and seed Bark
Adstringens Africa, Asia and middle east Source of tannins Source of gum and tannin,
Cupressiformis India and Pakistan ? ? Hemispherica native to Pakistan ? ? Indica Africa, Asia and middle east 18-27% 12-20% kraussiana Africa and middle east 38% 38% Leiocarpa Ethiopia, Kenya, Mozambique,
Oman, Somalia, Tanzania
Source of tannins Source of gum
and tannin,
Nilotica Africa middle east Indian ocean
50% +++
Subalata Africa, India & Pakistan Source of tannin Source of tannin Tomentosa Africa, India &Asia Deseeded 50% +++
Tannin content
According to the result of moisture content which was 12.58%, tannin content was determined using butanol-HCl assay method (as % catechin) using U-2010 Spectrophotometer, wavelength 530 nm, and found the catechin content was 46.61% (Appendix 5). A. nilotica subsp. nilotica conformed to Table (3) Barnes (2006) content tannins in pod seeds around 50% compared with 46.6% in this research. In many plants, there is a large fraction (some times >50%) of the tannins that can not be extracted (insoluble tannins) (Reed et al. 1985).
Separation
fraction F3 in addition to the crude extract. Ethanol fraction and acetone fraction were elucidated by FT-IR, UV spectrophotometer.
The remaining water-soluble residue of the crude acetone extract of Bruguiera gymnorrhiza bark was applied to an LH 20 Sephadex column that was eluted with methanol-water (1:1) to remove carbohydrates but retain oligomers containing a high proportion of proanthocyanidins polymers, but that were elutable with acetone-water (1:1). The acetone-water eluted polymer was reacted with phloroglucinol as a capture nucleophile in the presence of catalytic amounts of acetic acid, and the reaction product was separated on LH-20 Sephadex using ethanol as the solvent to isolate a series of oligomeric proanthocyanidin-4-phloroglucinol adducts. Epicatechin-(4β-2)-phloroglucinol and epicatechin-(4β -8)-epicatechin-(4β-2)-phloroglucinol were major products of this reaction, but other (as yet unidentified) products are also present. In the course of study of these products, a compound appearing at Rf 0.45 and 0.67 on cellulose TLC plates
developed with t-butanol-acetic acid-water (3:1:1) and 6% acetic acid, respectively, gave a red coloration after spraying with vanillin-HCI. This compound was isolated in chromatographically pure form by subsequent chromatography on LH-20 Sephadex using ethanol-water (7:3) as an eluting solvent (Achmadi et al. 1993).
Acute Toxicity Test Toward A. salina (LD50)
FromKarber’s method of calculation, LD50 values gave good result(Table
1). The result shows that the crude extract of A. nilotica has enough toxicity (LD50
19.7 mg/kg) in low concentration, indicating that the crude extract may be effective towards microorganism as well.
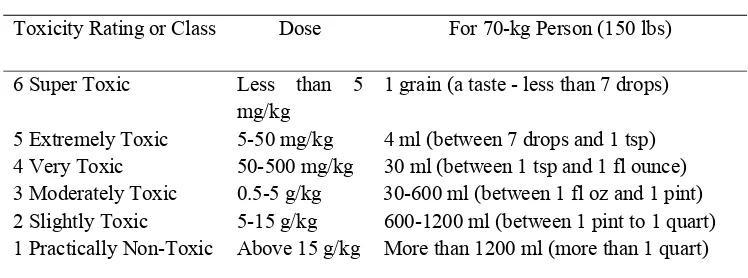
The result of LD50 =19.7 mg/kg of this research (Table 7)was denoted as
extremely toxic group according Toxicity Classes of Gosselin et al. (1984). As a measure of toxicity, LD50 is somewhat unreliable and results may vary greatly
effects that do not result in death but are nonetheless serious. There can be wide variability between species as well; what is relatively safe for rats may very well be extremely toxic for humans, and vice versa. In other words, a relatively high LD50 does not necessarily mean a substance is harmless, but a very low one is
[image:50.612.126.500.227.366.2]always a cause for concern (Hodgson 2004).
Table 4 Toxicity Classes (Gosselin et al. 1984)
Probable Oral Lethal Dose (Human)
Toxicity Rating or Class Dose For 70-kg Person (150 lbs)
6 Super Toxic Less than 5
mg/kg
1 grain (a taste - less than 7 drops)
5 Extremely Toxic 5-50 mg/kg 4 ml (between 7 drops and 1 tsp) 4 Very Toxic 50-500 mg/kg 30 ml (between 1 tsp and 1 fl ounce) 3 Moderately Toxic 0.5-5 g/kg 30-600 ml (between 1 fl oz and 1 pint) 2 Slightly Toxic 5-15 g/kg 600-1200 ml (between 1 pint to 1 quart) 1 Practically Non-Toxic Above 15 g/kg More than 1200 ml (more than 1 quart)
Antibacterial Test
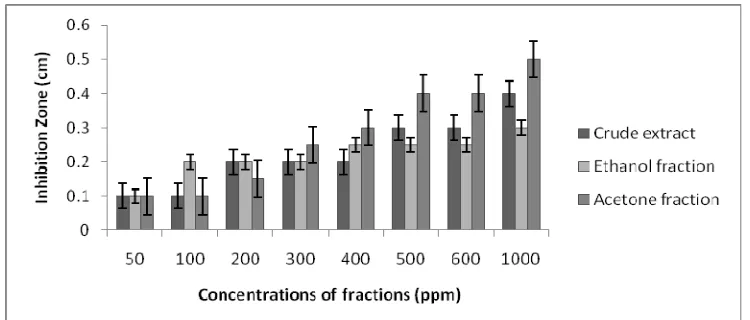
The active principles identified in this study exhibited antimicrobial activity against all the test organisms (Figures 6-8). Several plants, which are rich in alkaloids, tannins and glycosides, have been shown to possess antimicrobial activity against a number of microorganisms. For example, Adebajo et al. (1983) investigated the antimicrobial activity of leaf extract of Eugenia uniflora and reported that tannins, glycosides and alkaloids were detected and that the ethyl acetate and methanolic leaf extract of the plant were active against E. coli, P. vulgaris, K. pneumonia, and Aspergillus niger. Tannins have been reported to prevent the development of microorganisms by precipitating microbial protein and making nutritional protein unavailable for them (Banso 2009).
observed against S. aureus and the lowest degree of inhibition zone was against E. coli by the crude extract and the ethanol and acetone fractions.
The large size of the zones of inhibition indicated the potency of the active principles of the plant (Appendix 7), it was recorded that an increase in the concentration of the extract yielded higher activity as shown by the diameter of zone of inhibition. The fact that organisms may need higher concentrations of extracts to inhibit or kill them may be due to their cell wall components (Banso 2009).
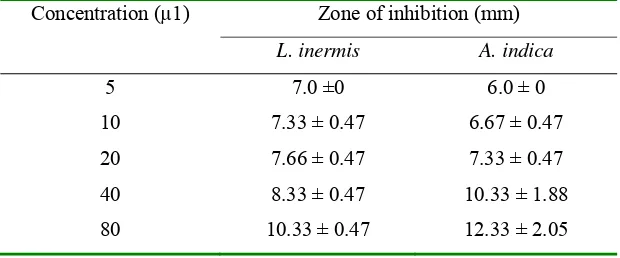
[image:51.612.165.475.412.541.2]Vibrio harveyi.Lawsonia inermis and A. indica showed high antibacterial activity against V. harveyi (Table 5). Due to its less toxic effect to shrimp and potent antibacterial activity the acetone extract of L. inermis could form one of the best options for developing novel antimicrobial compounds for eco-friendly management of disease caused by V. harveyi (Asha et al. 2008).
Table 5 Antibacterial activity of plant extracts againstV. harveyi (Asha et al. 2008)
Concentration (µ1) Zone of inhibition (mm)
L. inermis A. indica
5 7.0 ±0 6.0 ± 0
10 7.33 ± 0.47 6.67 ± 0.47
20 7.66 ± 0.47 7.33 ± 0.47
40 8.33 ± 0.47 10.33 ± 1.88
80 10.33 ± 0.47 12.33 ± 2.05
Explore application of green tea to water treatment for shrimp aquaculture, especially for rearing young penaeid shrimp, the anti-microbial activity of green tea powder (GT) and green tea polyphenols (GTPP) against pathogenic bacteria V. harveyi were examined using nutrient rich medium and sterilized sea water. The results of the experiments showed that both GT and GTPP have a high antibacterial activity against the pathogen. In the nutrient rich medium (325 agar mixed with GT and GTPP), 1000 ppm of GT or 400 ppm of GTPP exhibited antibacterial activity against Vibrio harveyi. In sterilized sea water, the antibacterial activity was demonstrated at 800 ppm of GT or 300 ppm of GTPP (Sornsanit et al. 2002).
Asha et al. (2008) and Sornsanit et al. (2002) report about A. indica antibacterial activity against V. harveyi and anti-microbial activity of green tea powder (GT) and green tea polyphenols (GTPP) against pathogenic bacteria V. harveyi. Antibacterial activity of polyphenols had being researched many a time by previous and current researchers like the polyphenols from A. nilotica in this research.
Staphylococcus aureus. According to Figure 7, the bacterial inhibition by crude extract and acetone fraction gave the best effect at the highest concentration (1000 ppm). At lower concentrations both ethanol and acetone fractions gave higher inhibition effect, whilst at higher concentrations crude extract and acetone fraction gave higher inhibition effects. Generally, ethanol extract had an average antibacterial effect around the 0.25-0.4 cm inhibition zones. Unlike V. harveyi, where acetone fraction was more effective, for S. aureus crude extract can be noted as highly effective at higher concentrations (1000 ppm), therefore its use without further fractionation can be exercised. This would be important in reducing economic burden as in more processes carried out to separate effective compounds.
difference with antibacterial effects as compared to ethanol fraction in higher concentrations.
The concentration used for antibacterial activity assay of A. nilotica leaves by Satish et al (2008) was 50µl of the aqueous extract and 106 CFU/ml of bacteria. Highly significant degree of activity was observed against S. aurous and E. coli. Antibacterial effect by 3.5 & 1.5 cm zone of inhibition was recorded in A. nilotica leaves against S. aurous and E. coli respectively (Satish et al. 2008).
According to Figures 6, 7, and 8, acetone fractions in higher concentrations from 500–1000 ppm gave the best inhibition effect for all three bacterial species which were use in concentration of 106 CFU/ml of bacteria. The average antibacterial activity against the three bacteria using all the fractions revealed that V. harveyi was the most inhibited organism. V. harveyi and E. coli they are gram negative bacteria, and the amino acid sequence of Vibrio harveyi acyl carrier protein (ACP) is 86% identical to that of Escherichia coli ACP, although five non conservative amino acid differences are concentrated in the loop region between helices I and II (Marc et al. 1997). These bacteria naturally resided from E. coli and S. aureus in humans, and animals, etc. whilst V. harveyi being in salt water.
Elucidation
Ethanol fraction showed clearer spectrum of UV and FT-IR. UV spectrum of ethanol fraction (Figure 9-a) with solvent EtOH giving a reading of max = 248nm,
305 nm and 418 nm. Maximum synthesis of catechin occurred at 190-320 nm that represented UV light, followed by blue light at 380-420 nm. MeOH max of
248nm and 305 nm (John 2003), FT-IR spectra of ethanol fraction (Figure 10-a) resemble FT-IR spectra of catechin (Appendix 8) which was indicated by the compound in ethanol fraction content structure of flavan. However, the UV spectrum of acetone fraction showed only one max = 320 nm (Figure 9-b) and
FT-IR spectrum of acetone fraction (Figure 10-b). These results could be explained by where the ethanol fraction was more pure the acetone fraction was less pure.
mode with an FTIR microscope after drying a cell wall suspension on a BaF2 microscope slide (Sené et al. 1994), or by pressing a thin potassium bromide pellet containing finely ground plant tissue and measuring the absorbance of infrared light going through the pellet with the use of an FTIR spectrometer (Vermerris et al. 2002).
Lorenz et al. (2002) reported, the extraction with 70%(v/v) aqueous acetone was standard method to purified condensed tannins, this result was confirmed with chemical analysis, spectroscopy analysis, column chromatography Sephadex LH 20 which indicated that ethanol fraction F1 was tannin, with low molecular weight from 287 to 500 unit, and the acetone fraction was condensed tannins with high molecular weight from 500 to over 9000 unit. The result of the elucidation confirmed the qualitative test which showed that these compounds were part of the polyphenols; more specifically was the condensed tannins.
Comparing with Previous Reference about Antimicrobial from A. nilotica
Previous studies revealed that Acacia species are effective antibacterial plants. Haj-Ali & Yagoub (2007) reported that A. nilotica fruits were effective against S. aureus, E. coli and V. harveyi using ethanol and chloroform extracts. Satish et al. (2006) tested A. nilotica leaves amongst 46 other plants against S.aureus and found a high zone of inhibition (0.9–3.55 cm). This zone of inhibition range is higher than this research work (highest degree of inhibition zone of 0.6 cm). These results could be from Satish et al. (2008) using A. nilotica leave extract as crude extract and in case of this research, using A. nilotica seeds as fractions and crude extract
Tannins compounds showed relevant antibacterial activity against Gram positive and Gram negative bacteria content S. aureus and E. coli they found in aqueous acetone in addition to both ethanol and methanol extracts, meaning that these compounds are favorably extracted in polar solvent.
The ethyl acetate and hexane residues of the flowers and leaves presented the best activity, which mean A. cochliacantha had a few content of tannins.
The antimicrobial activity of hot water and ethanolic extracts of six plant extracts, utilized in Pakistan for the cure of liver damage, were studied. The extracts of A. arabica, Nymphaea lotus,Sphareranthus hirtus, Emblica officinalis, Cinchorium intybus, and Cardus marianum were tested in vitro against seven bacterial species and two fungal species by well-diffusion method and microdilution methods. The patterns of inhibition varied with the plant extracts, the solvent used for extraction and the organisms tested. E. coli, Salmonella typhi, and Pseudomonas aeroginosa were the most inhibited microorganisms. The extract of Sphareranthu hirtus was the most active against multi-drug resistant P. aeruginosa and enterohemorrhagic E. coli 0157 EHEC. The ethanolic extract of S. hirtus exhibited a higher effect than the hot water extracts (Hassan et al. 2009).
Tannin toxicity tested to rumen microorganisms has been described for several bacteria species such as Streptococcus bovis, Butyvibrio fibrosolvens, Fibrobacter succinogenes, Prevotella ruminicola, and Ruminobacter amylophilis.
Enervosanone was found to have the strongest activity against four tested bacteria. Cambogin was found to be inactive against S. aureus. (-)-epicatechin was found to be inactive against all tested bacteria (Taher et al. 2005).
CONCLUSION
Acacia nilotica originated from Gadarif area in the east of Sudan, was an effective antibacterial plant as was confirmed by many other studies carried out previously. It was found that the higher the concentration, the more effective the antibacterial activity. For fraction effectiveness, out of the three fractions, acetone fraction gave the best results against all three bacteria (V. harveyi, S. aureus, E. coli) at higher concentrations (as high as 1000 ppm). However, at lower concentrations (lower than 400 ppm) ethanol fraction was more effective toward S. aureus and E. coli. The acetone fraction was elucidated as condensed tannins which were larger tannins and toxic to many microbes. When condensed tannins are broken down to their subunits they are still able to bind proteins. In the case of ethanol fraction was tannins with low molecular weight, are toxic to fewer microbes because once the molecule will break down and its subunits lose their protein binding abilities. This research and the previous research confirmed the usefulness of this plant A. nilotica as a characteristic feature of medical plant and pharmaceutical.
RECOMMENDATION
REFERENCES
Achmadi S, Syahbirin G, Choong ET, Hemingway RW. 1994. Catechin-3-0-rhamnosidechain extender units in polymericprocy anidins from mangrove bark. J Phytochem 35: 217-219.
Adebajo AO, Adewumi CO, Essein EE .1983. Antiinfective agent of higher plants Int. Syrup Med Plants 5th Ed. University of Ife, Nigeria. pp. 152-158. Adegboye MF, Akinpelu DA, Okoh, AI. 2008. The bioactive and
phytochemical properties of Garcinia kola (Heckel) seed extract on some pathogens. J Acad Afri Biotech 7: 3934-3938.
Asha S, Anitha S, Anantharjan R. 2008. Antibacterial activity of herbal plant extracts towards the fish pathogens. J Microbbiol 4: 83-89.
Attenborough D. 1995. The Private Life of Plants: A Natural History of Plant Behavior. London: BBC Books 320 p.
Austin B, Zhang X-H. 2006. Vibrio harveyi: a significant pathogen of marine vertebrates and invertebrates. J Soc Appl Microbiol 43: 119–214.
Banso A. 2009. Phytochemical and antibacterial investigation of bark extracts of Acacia nilotica. J Med Plant Res 3: 82-85.
Barman K, Rai SN. 2003. Potential of babul pods and leaves (Acacia nilotica) as an animal feed, Indi Farm 53: 26-27
Barnes R 2006. African acacias – monographs and manuals, Example of three species for the Acacia Conspectus. Oxford: Oxford Forestry Institute. CopriadyJ, Miharty, Herdini. 2002. Gallokatekin: Senyawa flavonoid lainnya dari
kulit batang rengas (Gluta renghas linn). J Nat Indo 4: 1-6.
Cowan MM.1999. Plant products as antimicrobial agents. Clin Microbiol Rev 12: 564-582.
Croghan PC. 1957. The survival of artemia salina (L.) in various media Arch.ne’e Zool 4: 213-218.
Fagg C. 2001. Acacia nilotica pioneer dry land. In Roshetko JM, Editor. Agroforestry Species and Technologies. Arizona: Winrock International. pp 23-24.
Gary L, Furtado, Antone A. Medeiros. 1980. single-disk diffusion testing (kirby-bauer) of susceptibility of proteus mirabilis to chloramphenicol: significance of the intermediate category. J Clin Microbiol 12: 550-553.
Gosselin RE, Smith RP, Hodge HC. 1984. Clinical Toxicology of Commercial Products. 5th ed. Baltimore:Williams and Wilkins.
Hagerman AE, Karonen M, Barbehenn RV, Jones CP, Salminen JP. 2006. Ellagitannins have greater oxidative activities than condensed tannins and galloyl glucoses at high pH: Potential Impact on Caterpillars. J Chem Ecol 32:2253-2267.
Hagerman AE, Klucher KM. 1986. Tannin-protein interaction. In: Cody V Middleton EJr, Harbone J, Alan R. Plant flavonoids in biology, and medicine: biochemical, pharmacological, and structure-activity relationships. New York Wiley-Interscience. pp 67-76.
Haj-Ali AM, Yagoub SO. 2007.Antimicrobial Activity of Acacia nilotica extracts against some bacteria isolated from clinical specimen. Res J Med Plants 1: 25-28.
Harbone JB. 2006. Metode Fitokimia, Penuntun Cara Modern Menganalisis Tumbuhan. Bandung: Penerbit ITB.
Hassan A, Rahman S, Deeba F, Mahmud S. 2009. Antimicrobial activity of some plant extracts having hepatoprotective effects. J Med Plant Res 3:20-023. Hodgson-A E. 2004. Textbook of Modern Toxicology. Third Ed. North Carolina: Wiley-Interscience.
John KMM, Sasikumar R, Balasubramanium M, Saravanan M, Kumar RR. 2003. Influence of light on catechin biosynthesis in tea. J Chi Tech 24: 2.
JunZ, XiaL, ChanggenF, YanglinX, HuaqiangC. 2006. Studies on synthesis and toxicity of polyoxometalate containing sulfanilamide. Acad Cen Res 62: 2509-2515.
Kluymans J, Belkum A, Verbrugh H. 1997. Nasal carriage of staphylococcus aureus underlying mechanisms and associated risks. Clin Microb Rev 10: 502-520.
Kongevej H. 2008. Acacia nilotica, Forest & Landscape Denmark, Seed Leaflet. Copenhagen. Forest & Landscape,No. 137.
Lee TH, Chen YM, Chou HN. 1999. Toxicity assay of cyanobacterial strains using Artemia salina in comparison with the mouse bioassay. Acta Zool Taiw 1:00-00.
Lorenz K, Preston CM. 2002. Characterization of high-tannin fraction from humus by carbon-13, cross-polarization and magic-angle spinning nuclear magnetic resonance. J Environ Qual 31:431–436.
Manríquez-Torres JJ, Zúñiga-Estrada A, González-Ledesma M, Torres-Valencia JM. 2007. The Antibacterial metabolites and proacacipetalin from Acacia cochliacantha. J Mex Chem Soc 51: 228-231.
Marc A, Roche DL, Shen Z, David M, Byers. 1997. Hydrodynamic properties of Vibrio harveyi acyl carrier protein and its fatty-acylated derivatives. Biochem Biophys 344: 159-164.
Nasroun TH. 1979. Pulp and paper making properties of some tropical hardwood species grown in the Sudan.Sudan Silva 4: 22-32.
Peter KV. 2004. Handbook of Herbs and Spices Vol. 2- New York: Woodhead Ltd.
Reed JD, Horvath PJ, Allen MS, VanSoest PJ. 1985. Gravimetric determination of soluble phenolics including tanninfrom leaves by precipitation with trivalent yeterbium. J Sci Food Agric 36:255-261.
Saeidnia S, Yassa N, Rezaeipoor R, Shafiee A, Gohari AR, Kamalinejad M, Goodarzy S. 2008. Immunosuppressive principles from Achillea talagonica, an endemic species of Iran. J Med Plant Res Daru 17: 37-41.
Satish S, Raghavendra MP, Raveesha KA. 2008. Evaluation of the antibacterial potential of some plants against human pathogenic bacteria. J Bio Res 2: 44-48
Sené CFB, McCann MC, Wilson RH, Grinter R. 1994. Fourier transforms raman and fourier-transform infrared spectroscopy. J Plant Physiol 106: 1623-1631.
Siddhuraju P, Vijayakumari K and Janardhanan K. October. 1995. Chemical Composition and nutritional evaluation of an underexploited legume, Acacia nilotica.Tamil NaduFood Chem 57:385-391.
Sornsanit K, Nomura N, Migo VP, Matsumura M. 2002. The use of green tea powder and green tea polyphenols for treatment of Vibrio-infected shrimp culture water. J Jap Waert Treat Biol 38: 111-115.
Speilman L. 2005. The Role of Fungal Competition and Tannin Chemistry in Chestnut and Oak Resistance to parasitica. Sweet Briar: Springer.
Sukardiman, Poerwono H, Mubarika S, Sismindari. 2004. Studi Kelarutan Astemizol (2): perhitungan rasio saturasi dan penentuan parameter termodinamika pada proses solubilisasi miselar menggunakan Tween 80 sebagai solubilizing agent. Maj Farm Airlang 4:852 1050.
Taher M, Idris MS, Ahmad FA, Dayar. 2005. A polyisoprenylated ketone from Calophyllum enervosum. J Phytochem 66:723-726.
Van SoestPJ, RobertsonJB, LascanoC, Reed JD, Pell AN, Giner-ChavezBI. 1999. A method for isolating condensed tannins from crude plant extracts with trivalent ytterbium. Food Agric 74: 359 – 368
Vermerris W, Thompson KJ, McIntyre LM. 2002. The maize Brown midrib1 locus affects cell wall composition and plant development in a dose-dependent manner. Heredity 88: 450-457.
Vermerris W, Nicholson R. 2006. Isolation and Identification of Phenolic Compounds, Phenolic Compounds Biochemistry. Gainesville: Springer. Vogt RL, Dippold L. 2005. Escherichia coli O157:H7 outbreak associated with
APPENDICES
Appendix 2 Calculation of moisture content
The moisture content test was repeated three times, the weight of the sample before oven (Wa) was 1.0245g, and the weight of the sample after oven (dry weight Wb) was 0.91g, then the moisture content (X%) calculated passed the following equation:
X% = ((Wa-Wb)/Wb) x 100%
Appendix 5Result of butanol-HCl analysis
Sample No. Name Abs (530.0) Conc (ppm) Avg Conc
BLK 0.001 0.000
Stand 0.00 0.028 0.462
Stand 0.1 0.059 0.986
Stand 0.2 0.120 2.025 Use as standard Abso
Stand 0.5 0.135 2.273
Sample 0.1 0.489 8.272
Sample 0.5 2.357 39.910
(0.489/o.120) x (0.01/0.1) x 100 = 40.75%
For the dry weight was calculated with the following the mathematic calculation
(Y1/400) x 100 = 40.75%
(Y2/400) x 100 = 46.67%
(Y3/400) x 100 = 12.58%
Where
Y1 the tannins content in gram = 163 g
Y2 the other components content = 186.68 g
Y3 the moisture content in gram = 50.32 g
Appendix 6 Raw data of antibacterial tests
Factor Experiments Inhibition Zone of bacteria The repeat experiments
Concentration of extracts (ppm)
0 50 100 200 300 400 500 600 1000
Fractions Type of bacteria
Area of inhibition zone in (centimeters)
Crude extract
Vibrio cholera
0.60 0.80 0.80 0.90 0.90 0.85 0.95 1.00 1.00
0.60 0.75 0.85 0.90 0.90 0.90 1.00 0.95 1.00
S. aureus
0.60 0.70 0.70 0.70 0.70 0.90 0.95 1.00 1.20
0.60 0.70 0.75 0.70 0.80 0.85 1.05 1.10 1.20
E. coli 0.60 0.70 0.70 0.80 0.80 0.80 0.90 0.90 1.00
0.60 0.70 0.70 0.80 0.80 0.80 0.90 0.90 1.00
Ethanol fraction
Vibrio cholera
0.60 1.00 1.00 1.00 1.00 0.90 0.95 1.00 1.00
0.60 1.00 1.00 0.90 1.00 1.00 1.00 0.95 1.00
S. aureus
0.60 0.85 0.90 0.70 0.90 0.90 0.90 0.95 1.00
0.60 0.85 0.90 0.90 0.90 1.00 0.90 0.90 1.00
E. coli 0,60 0.60 0.75