MOLECULAR ANALYSIS OF OIL PALM (Elaeis guineensis Jacq.)
FLOWERING ASSOCIATED GENES AND THEIR POTENTIAL
APPLICATION IN BREEDING PROGRAMMES
WALTER AJAMBANG NCHU
GRADUATE SCHOOL
BOGOR AGRICUTURAL UNIVERSITY BOGOR
This is to declare that the dissertation titled “Molecular Analysis of Oil Palm (Elaeis guineensis Jacq.) Flowering Associated Genes and their Potential Application in Breeding Programmes” is the result of my personal research under the direction of the supervising committee and has never been presented in any form wherever. Any other sources of information that have been mentioned in this dissertation from published or unpublished works of other authors have been acknowledged in the text and included in the reference chapter.
Based on this assertion, I therefore transfer the copyrights of this dissertation to the Bogor Agricultural University.
Bogor, February 2015
WALTER AJAMBANG NCHU. Molecular Analysis of Oil Palm (Elaeis guineensis
Jacq.) Flowering Associated Genes and their Potential Application in Breeding Programmes. Under the supervision of SUDARSONO as head, SINTHO WAHYUNING ARDIE and HUGO VOLKAERT as members of the committee.
The oil palm (Elaeis guineensis Jacq.) is an important economic crop that is used in the food, chemical and bio-diesel industries. Breeding to increase yields in oil palm has been focused on improving the sex ratio of more female to male inflorescences. Environmental and genetic factors largely influence oil palm growth and yields. Soil water availability, especially in Sub Saharan Africa and source-sink balances have been the main factors affecting sex ratio and inflorescence production in oil palm. Male inflorescences have been induced in the highly feminine Pisifera race of oil palm by carbohydrate depletion through complete defoliation. However, the efficiency of this process was still a major problem especially in this era of climatic instability. Also, the molecular genetic mechanisms underlying the process of male inflorescence induction by complete defoliation are yet to be uncovered. The objective of this study was to investigate the phenotypic responses and identify the molecular genetic mechanisms responding to complete defoliation of oil palm in order to efficiently increase oil palm seed production through improved pollen production and expand existing breeding programmes. In this dissertation, we present the quantity of environmental factors and the periods of treatment for which male inflorescence induction or sex determination is effective and determine the exact moment at which sex differentiation genes are initiated in response to complete defoliation treatment. Knowledge of this moment enabled us to accurately isolate the genes and study the molecular mechanisms underlying male inflorescence induction in oil palm caused by complete defoliation using RNA-seq.
Complete defoliation significantly induced male inflorescence of up to 104%. An acute soil water deficit of 16.8 mm between the 30th and 60th days after complete defoliation (DAD) had a positive effect on male inflorescence induction. Generally, complete defoliation treatments carried out during the dry seasons produced more male inflorescences than during the wet season. Unlike inflorescence induction, inflorescence emergence was highest during the wet season irrespective of the date of its induction. Regression analysis on 18 time-specific, climate related variables indicated that oil palm initiates response mechanisms between day 30 and day 60 after complete defoliation stress. Coincidentally, total soluble sugar measured on completely defoliated trees and control trees at 45 DAD, showed a sugar depletion of 55% in leaf tissues and 21% in inflorescence tissues. Preferential sex induction should be a way of acclimation in oil palm under carbohydrate depletion stress caused by complete defoliation.
genes (DEG) responding to complete defoliation stress were located in the chloroplast and mitochondrion. The chloroplasts and mitochondria are the two powerhouses of the cell, one regulating carbohydrate production through photosynthesis and the other regulating cell energy availability in the form of ATP. Similarly, the biological processes that were functionally enriched from the DEG data set were photosynthesis and carbohydrate related processes. Gene network analysis showed that abiotic stress response DEGs were co-expressed with vegetative to reproductive phase transition of the meristem, gibberellin, auxin, jasmonic acid and photoperiodism. This co-expression confirmed that abiotic stress caused by complete defoliation was involved in male inflorescence induction in oil palm, regulated by sugar status and plant growth hormones. Genes linked to carbohydrate metabolism, flower development, stress response, circadian rhythm, PGR and vegetative to reproductive phase transition of the meristem may be responsible for the regulation of inflorescence emergence in oil palm. Sequence data generated from this study should be used to compare the expression from other races of the oil palm especially the commercial race, Tenera and Dura. These sequences should be used to quantify gene expression on breeding populations in the process of selecting parents for seed production
!
WALTER AJAMBANG NCHU. Analisis Molekuler Gen-Gen yang Berkaitan dengan Pembungaan dan Aplikasi dalam Pemuliaan Tanaman Kelapa Sawit. Dibimbing oleh SUDARSONO sebagai ketua, SINTHO WAHYUNING ARDIE dan HUGO VOLKAERT sebagai anggota komisi pembimbing.
Kelapa sawit (Elaeis guineensis Jacq.) adalah tanaman tropis yang digunakan dalam industri makanan, kosmetik dan bio-diesel. Tujuan utama dalam pemuliaan kelapa sawit adalah menaikan sex ratio bunga betina terhadap bunga jantan. Faktor lingkungan dan genetik mempunyai peran besar dalam produktivitas kelapa sawit. Ketersediaan air, terutuma di Afrika, dan ketersediaan kandungan kabohidrat mempengaruhi sex ratio kelapa sawit. Pemangkasan daun sawit adalah salah satu cara untuk menginduksi bunga jantan di kelapa sawit namun masih ada beberapa hal terkait efisiensi teknik tersebut, apalagi gen-gen yang berperan dalam proses ini belum diketahui. Penelitian ini bertujuan untuk menyelidiki perubahan fenotipe dan menentukan gen-gen yang terekspresi akibat pemangkasan daun sawit. Dalam disertasi ini, diberikan informasi tentang berapa jumlah faktor lingkungan yang dibutuhkan untuk menginduksi bunga jantan pada kelapa sawit secara efisien. Selain itu, diberitahukan juga berapa waktu yang dibutuhkan oleh kelapa sawit sebelum merespon terhadap stres pemangkasan daun. Informasi ini digunakan untuk pelajari ekspresi gen terkait pembungaan dalam kelapa sawit yang diakibatkan oleh stres pemangkasan daun.
Data menunjukan bahwa teknik pemangkasan daun bisa menaikan produksi bunga jantan kelapa sawit sampai 104%. Teknik ini paling efisien di musim kering dan optimum saat cekaman air sama dengan 16.8 mm dalam waktu 60 hari pertama setelah pemangkasan daun. Analisis regresi menunjukan bahwa tanaman kelapa sawit akan memberikan respon terhadap cekaman kabohidrat yang diakibatkan oleh pemangkasan daun merupakan ekspresi gen dalam waktu 30 sampai 60 hari setelah pemangkasan. Kadar gula yang diambil dari jaringan bunga dan daun kelapa sawit 45 hari setelah pemangkasan menunjukan bahwa kadar gula menurun 21% di jaringan bunga dan 55% di jaringan daun dibanding kontrol.
Ekspresi gen di jaringan bunga kelapa sawit mengunakan Next Generation Sequencing (NGS) menujukan bahwa mayoritas gen gen yang berperan dalam regulasi cekaman kabohidrat di kelapa sawit terletak di kloroplas dan mitokondria. Analisis fungsi menggunakan Gene Ontology, menujukan bahwa gen gen tersebut berperan dalam metabolisme kabohidrat dan fotosintesis. Analisis ko-ekspresi menujukan keterkaitan antara cekaman kabohidrat dan induksi bunga serta peran zat pertumbuhan tanaman seperti auxin, gibberellin dan jasmonic acid. Ini membuktikan bahwa gen gen dalam lintasan cekaman kabihidrat yang diakibatkan oleh pemangkasan daun berperan dalam induksi bunga di jantan tanaman kelapa sawit.
© Copyright IPB, 2015
Copyright protected under the law
.No part or all of this work may be reproduced without citing the source. Copying may be done only on the basis of education, research, scientific writing, reports or review; and which should not cause any prejudice to IPB.
!
MOLECULAR ANALYSIS OF OIL PALM (
Elaeis guineensis
Jacq.)
FLOWERING ASSOCIATED GENES AND THEIR POTENTIAL
APPLICATION IN BREEDING PROGRAMMES
WALTER AJAMBANG NCHU
Dissertation
Submitted in partial fulfilment of the requirements for the degree Doctor of Philosophy
in
Plant Breeding and Biotechnology
GRADUATE SCHOOL
BOGOR AGRICULTURAL UNIVERSITY BOGOR
!
Examiners during Closed Exam: Prof. Dr. Ir. Iskandar Z. Siregar M.Sc
Dr. Desta Wirnas, SP, M.Si
Examiners during Public Defence: Dr. Ir. Sudrajat, MS
!
Dissertation Title : Molecular Analysis of Oil Palm (Elaeis guineensis Jacq.) Flowering Associated Genes and their Potential Application in Breeding Programmes
Name : Walter Ajambang Nchu
NIM : A263118081
Approved by
Supervisory committee
Prof. Dr.Ir.Sudarsono, M.Sc. Head
Dr.Sintho W Ardie, SP, M.Si. Member
Dr. Hugo Volkaert Member
Endorsed by
Head of Mayor
Plant Breeding and Biotechnology
Dean Graduate School
Dr. Ir. Yudiwanti Wahyu, E.K, MS. Dr. Ir. Dahrul Syah, M.Sc. Agr.
!
FOREWORD
Glory be to God the Father Almighty for his ever increasing guidance and protection especially during the difficult moments the author went through while undertaking studies in Bogor, without which all human efforts wouldn’t have borne any fruits.
The author wishes to convey his sincere and special thanks to the head of the supervisory committee Prof. Dr. Ir. Sudarsono, MSc. for his advice and remarks during the preparation, execution and presentation of this dissertation. The author pays gratitude to members of the supervising committee, Dr. Sintho W. Ardie, SP, M.Si and Dr. Hugo Volkaert, for their resourceful instructions and comments on this work. The author is grateful to Prof. Dr. Ir. Iskandar Z. Siregar M.Sc, Dr. Desta Wirnas, SP, M.Si, Dr. Ir. Sudrajat, MS and Dr. Adi Pancoro who gave their precious time to respectively serve as closed examiners and open examiners of this dissertation.
This project would never have existed without the vision and wisdom of Dr. Zok Simon and Bapak Widya Wiryawan, former General Manager of IRAD Cameroon and present CEO of ASTRA Agro Lestari Indonesia respectively. Special thanks go to Bapak Bambang Palgoenadi, Dr. Ngeve Mbua Jacob and Dr. Woin Noe for bravely executing the training aspect of this project. The author is indebted to Bapak Satyoso Hartodedjo of ASTRA Agro, Dr. Koona and Dr. Ngando of CEREPAH for the smooth management of this project.
The Late Joseph Asende Nchu has a special place in the author’s mind for initiating and accompanying him in this long and exciting journey. The author is grateful to Mama Comfort Namondo for her constant support during his stay abroad.
The author presents special gratitude to his family members especially the wife Lylie Michele Ajambang and children Jeff, Megane and Naomi for their endurance during his long absence from home. High appreciation is extended to the colleagues of PMB and ASTRA labs for their technical and scientific assistance during the execution of this project.
The author hopes to receive public criticisms that may improve the quality of this dissertation. The author wishes that this dissertation makes an impact in science and development especially in the field of oil palm breeding and genomics.
Bogor, February 2015
!
TABLE OF CONTENTS
TABLE OF TABLES xiii
TABLE OF FIGURES xiii
TABLE OF APPENDICES xv
1 INTRODUCTION 1
Background 1
Problem statement 1
Objectives of research 3
Importance of research 3
Novelty 3
Scope, framework and limitations 4
2 LITERATURE REVIEW 7
Origin and botany of oil palm 7
Major changes caused by carbohydrate depletion 7
Hormones associated with flowering 9
Genes involved in floral induction and initiation in oil palm 10 The role of environmental factors and assimilates 10 The oil palm trunk as a carbohydrate reserve 13 Previous work on gene expression in oil palm with molecular methods 14
3 MASSIVE CARBOHYDRATE RESERVES BUFFERS AND DELAYS RESPONSE TO STRESS CAUSED BY COMPLETE DEFOLIATION IN OIL PALM (Elaeis guineensis Jacq.)
15
Abstract 15
Introduction 16
Materials and Methods 18
Results and discussions 21
Conclusion 29
4 INFLORESCENCE EMISSION TIME IN OIL PALM (Elaeis guineensis Jacq) IS SEASONAL, IRRESPECTIVE OF INDUCTION DATE
30
Abstract 30
Introduction 31
Materials and Methods 32
Results and discussions 34
Conclusion 46
5
COMPARATIVE EXPRESSION PROFILING OF THREE EARLY INFLORESCENCE STAGES OF OIL PALM RESPONDING TO COMPLETE DEFOLIATION SHOWS THAT VEGETATIVE TO
REPRODUCTIVE PHASE TRANSITION OF MERISTEM IS CAUSED BY SUGAR DEPLETION
!
Abstract 47
Introduction 48
Materials and Methods 50
Results and discussions 52
Conclusion 68
6 GENERAL DISCUSSIONS 69
7 GENERAL CONCLUSIONS AND RECOMMENDATIONS 71
REFERENCES 72
APENDICES 83
!
TABLE OF TABLES
2.1 Cell and physiological responses of some major phyto-hormones involved in flower sex determination and development, including related references
9
3.1 Correlation coefficients for selected variables that had a significant relationship with male inflorescence number
26
4.1 Number of inflorescences produced before and after defoliation 34 4.2 T-test for number of inflorescence emission between the wet and dry
season
36
4.3 Functional networks involving down regulated and up regulated DEGs
42
4.4 Ten top DEGs based to FC and p-value 43
5.1 Summary data from the different libraries 53
TABLE OF FIGURES
1.1 Flow chart of research activities
6 2.1 Un-emerged female inflorescence extracted from leaf axil number 14 and
un-emerged male inflorescences extracted from leaf number 13
8
2.2 Possible interactions resulting from the severe pruning of oil palm as proposed by Adam et al. (2011). The circles represent the biological
processes while the squares represent the products
11
2.3 Apical meristem of oil palm tree showing undetermined flower bud and future leaves
12
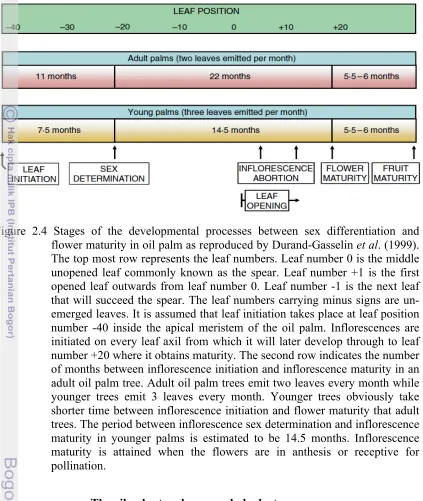
2.4 Stages of the developmental processes between sex differentiation and flower maturity in oil palm as reproduced by Durand-Gasselin et al. (1999).
The top most row represents the leaf numbers. Leaf number 0 is the middle unopened leaf commonly known as the spear. Leaf number +1 is the first opened leaf outwards from leaf number 0. Leaf number -1 is the next leaf that will succeed the spear. The leaf numbers carrying minus signs are un-emerged leaves. It is assumed that leaf initiation takes place at leaf position number -40 inside the apical meristem of the oil palm
13
3.1 Complete defoliation on mature pisifera trees. (a) Mature non defoliated pisifera palm (b) completely defoliated pisifera palm. (c) A recovering pisifera palm 45 DAD. The arrow shows the number of leaves that have been produced after complete defoliation. (d) A mature male inflorescence at anthesis. Anthesis is when pollen on the flowers has stained maturity and is capable of fertilising female flowers. The white bars on (a) and (b) are drawn to 1 m scale while that of d is 10 cm scale. Arrow points to newly
!
developed oil palm leaves after complete defoliation.
3.2 Leaf counting during inflorescence extraction 20 3.3 Monthly soil water deficit (curve) and monthly precipitation (bars)
recorded between 2007 and 2013 at La Dibamba Cameroon
22
3.4 Male inflorescence production and mean annual precipitation between 2001 and 2012 at La Dibamba before and after treatment. The broken line
between year 2006 and 2007 represents the start of defoliation treatment. The number of male inflorescences produced is represented by bars corresponding to the primary vertical axis while precipitation curve is represented by the secondary vertical axis
23
3.5 The relationship between precipitation, rainy days and male inflorescence induction on plants subjected to complete defoliation stress
24
3.6 Stages leading to tissue extraction for total soluble sugar analysis. (a) a mature pisifera being felled 45 DAD, (b) transportation of the reduced crown to the laboratory for tissue extraction, (c) leaf and inflorescence extraction (d) inflorescence located at leaf axil + 5 (on red background) placed on the leaf petiole
26
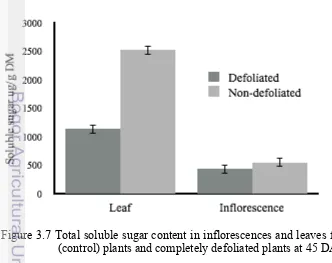
3.7 Total soluble sugar content in inflorescences and leaves from non defoliated (control) plants and previously defoliated plants 45 days after defoliation
27
3.8 The effect of increasing soil water deficit on male inflorescence production 28 4.1 Distribution of inflorescence emission in months after complete defoliation 35 4.2 Average monthly emission of male inflorescences based on the season of
defoliation treatment
37
4.3 Distribution of gene expression level in non-defoliated (blue) and defoliated tissues (red)
39
4.4 Venn diagram of DEGs on stress and control tissues 39 4.5 Volcano plot for DEGs between non-defoliated and defoliated samples.
The y-axis represents the level of significance of the expression change between samples measured on –log10 p-value (pval = 0.05), while the x-axis represents the fold change of DEGs. DEGs located above the red line are significant based on the p-value while those below are not significant. The DEGs located between the two blue lines are non DEGs based |FC| ≥ 2
40
4.6 Functional categoristaion of DEGs based on biological processes 41 4.7 Average monthly male inflorescence production and mean monthly
precipitation for the period between 2007 and 2012 recorded at La Dibamba Oil Palm Research Centre
44
4.8 Average monthly FFB production and mean monthly precipitation between 2007 and 2012 at La Dibamba Oil Palm Research Centre
45
5.1 Extracted inflorescences from axils of palm leaves 50 5.2 A dissected oil palm showing a series of inflorescence buds enclosed in the
axils of future leaves
52
5.3 Distribution of expression levels based on FC between the non defoliated (blue) and defoliated (red) tissues
54
5.4 Volcano plots for DEGs for the three different comparisons (a) axil -20/axil -27 (b) axil +5/axil -27 (c) axil +5/axil -20
55
5.5 Differential gene expression at FC ≥ 2 and p ≤ 0.05 between inflorescence stages under stress
!
5.6 Gene bar chart for (a) up regulated DEGs (b) down regulated DEGs from three inflorescence stages based on cellular component
57
5.7 Gene bar chart for (a) up regulated DEGs and (b) down regulated DEGs from three inflorescence stages based on molecular functions
58
5.8 Gene bar chart for (a) up regulated DEGS and (b) down regulated DEGs from three inflorescence stages based on biological process
60
5.9 Gene set enrichment score from the comparison between inflorescence at leaf axil +5 against inflorescence of leaf axil -27
61
5.10 Gene set enrichment score from the comparison between inflorescence located at leaf axil -20 against inflorescence of leaf axil -27
62
5.11 Gene set enrichment score from the comparison between inflorescence at leaf axil +5 against inflorescence located at leaf axil -20
63
5.12 Network of related genes from the abiotic stress process analysed on Gene MANIA
66
5.13 Network of related genes from the reproductive process analysed on Gene MANIA
67
TABLE OF APPENDICES
1 Male flower and pollen production before and after complete defoliation 83 2 List of RNA samples extracted on both leaves and flowers for RNA-seq 84
3 Box plot for normalised transcripts 85
4 Tree diagram for samples based on inter sample correlation 86 5 Differential expression of genes between treatments 87 6. Differential expression of genes between samples under stress 88
7 Functional annotation chart from DAVID 89
CHAPTER I
INTRODUCTION
Background
Oil palm (Elaeis guineensis Jacq.) is the world’s leading oil crop with 35% of total world consumption of vegetable oils (Soy stats 2014). Oil palm is cultivated on approximately 15 million hectares across the world and consumption is expected to double by the year 2020 (FAO 2009). Apart from its traditional use as a source of food, oil palm is used in the manufacture of margarine, pharmaceuticals, soap and cosmetics, animal feed and organic manure, building material, furniture and biofuel. Production of biodiesel from oil palm has been increasing in recent years, particularly in Africa and Latin America (FAO 2010; Mitchell 2011).
Socio-economic benefits of a sustainable oil palm plantation could include poverty alleviation and long-term employment opportunities (Badrun 2010; Kurniawan 2010; Norrochmat and Hadianto 2010; Pahan 2011). Profit sharing may provide a further incentive, attracting more workers to the palm oil sector, along with better living and working conditions (Albán and Cárdenas 2007). Indonesia’s palm oil export was the major force behind the stabilisation of its economy during the 2008-2009 global financial crisis. Oil palm production has favoured the development of upstream companies such as the seed industry, fertiliser, agrochemicals, agro mechanicals and financial services; side stream companies and downstream companies in Cameroon.
Problem statement
The commercial cultivation of oil palm is faced with many problems ranging from land availability, environmental issues, social issues, agronomy, diseases, climate change and the availability of quality seeds. Seed availability is the foundation for any agricultural project. There are three different races of the cultivated oil palm species, categorised basically by the thickness of the shell, which is controlled by a co-dominant monogenic inheritance (Beinaert and Vanderweyen 1941). Moretzsohn et al. (2000) identified the allele pairs of the gene as sh+/sh+ for dura race, sh+/sh- for tenera race and sh-/sh- for the pisifera race. The commercial oil palm cultivar is a hybrid called Tenera denoted as T or DP derived from a cross of two homozygous parents based on shell thickness known as the Dura or female parent represented by DD and the Pisifera or male parent represented by PP. In the process of seed production in oil palm, a trained pollinator artificially carries out pollination. Pollen is harvested from the Pisifera parent and then manually pollinated on to the Dura inflorescences. The quantity and quality of pollen used in this pollination process is highly related to the quantity and quality of total seed produced.
programmes are the number of bunches per tree (BNO) and the total bunch weight (BW), which are both heritable (Hardon et al. 1985; Rafii et al. 2002). Under normal environmental conditions, seed producers may have to import pollen from countries with sub optimal environmental conditions such as the arid zones of Africa. In addition to high expenditure, pollen importation has to undergo the tight regulations concerning the transfer of biological organisms. In Cameroon, the seed production unit spends 50% of its production cost on pollen importation. Thus it was imperative for each seed production centre to be able to produce its own pollen locally in order to reduce seed production costs and also to save time and energy in handling complex international regulations regarding international transfer of biological material.
Hardon and Corley (1976) reported that oil palm produces male inflorescence after it has undergone a high production of fruit bunches. When the plant produces Fresh Fruit Bunches (FFB), it losses energy as bunches are continuously harvested from the plant. In contrast, the Pisifera race aborts its fruits immediately after fertilisation and hence does not produce bunches. Therefore most of its energy is stored in the trunk and a lesser part of it is used for plant maintenance. This means that the Pisifera plant shall never loss enough energy to let it undergo the required depletion of stored energy that will enable it to produce male inflorescences. Corley (1976) and Durand Gasselin et al. (1999) reported that severe defoliation of Pisifera leaves induces male inflorescence in oil palm, and that the treatment was most effective during the dry season in Ivory Coast. This is an indication that oil palm sex differentiation is strongly affected by climatic factors, with male inflorescence being influenced by carbon depletion and water deficit. Although phenological studies have been carried out which suggests points of interaction between genetic and the environment factors in the induction of male inflorescences on oil palm (Corley 1976; Uhl 1988; Van Heel et al. 1987; Tomlinson 1990; Durand-Gasselin et al. 1999; Adam et al. 2011), nothing is yet known about the nominal values of climatic factors, the exact amount of time necessary for the oil palm to respond and initiate the mechanism of male inflorescence induction, genetic basis of sex determination, nor the molecular mechanisms by which these processes are regulated in oil palm. A number of questions concerning the mode of interaction between molecular-genetic processes and sex differentiation still remain unanswered (Adam et al. 2011; Walter et al. 2012).
The development of molecular biology, supported by sophisticated bioinformatics, can be used to understand the genetic basis and molecular mechanisms underlying the process of sex differentiation in oil palm. Molecular biology methods such as Next Generation Sequencing (NGS) have been used on genomic DNA and RNA transcripts by several researchers in order to assign putative functions to genes (Jouannic et al. 2005; Adam et al. 2005; Ho et al. 2007; Adam et al. 2007; Low et al. 2008; Adam et al. 2011; Jen et al. 2013).
et al. (2013) reported that among the three commonly RNA-seq used technologies, Illumina showed better gene coverage than Roche 454 and Sanger on oil palm tissues because of its higher sequencing depth.
Objective of research
Investigate the phenotypic responses and identify the molecular genetic mechanisms responding to complete defoliation of oil palm in order to efficiently increase oil palm seed production and also expand existing breeding programs.
Specific objectives
1. Explore the effects of complete defoliation of Pisifera on male inflorescence induction.
2. Estimate the critical moment in which genes are expressed in response to complete defoliation in order to be able to isolate and clone these genes for further molecular and downstream research.
3. Determine the effects of voluntary inflorescence induction by complete defoliation on the seasonal trend of inflorescence emergence.
4. Identify the molecular mechanisms associated to complete defoliation of oil palm.
Importance of research
1. This research shall permit us to know the exact time at which genes are switched on in response to complete defoliation treatment.
2. Understand the precise period during the year for which complete defoliation is effective in producing male flowers.
3. The specific period of gene expression will permit a successful isolation of target genes expressed as a result of complete defoliation.
4. The molecular processes regulating male inflorescence induction and emergence in oil palm shall be identified.
Novelty
Durand-Gasselin et al. (1999) initiated work on oil palm defoliation in the Ivory Coast as a method of male inflorescence induction in oil palm. They based their study on the phenotypic response to severe pruning of oil palm leaves. Some work has been done on oil palm gene expression by researchers such as Jouannic et al. (2005), Adam et al. (2007), Ho et al. (2007), Tranbarger et al. (2011) and Morcillo et al. (2013) focusing on different oil palm tissues using techniques such as EST, micro array and other hybridisation. An incomplete oil palm genome is available from the Malaysian Oil
Palm Board (Singh et al. 2013) and is stored in the domain
Previous research has not indicated the moment at which genes are expressed in response to complete defoliation. ESTs collected from oil palm flower tissues were not based on any previous flower induction treatment. The collection of ESTs were not time specific nor tissue specific. This present research is unique in the sense that;
i. Samples were collected from tissues that have been stressed and compared with
tissues in normal conditions.
ii. Samples were collected with respect to the spatial-temporal expression of genes responsible for sex differentiation.
iii. Flowering was induced by the application of a treatment whereas the other researchers did not induce flowering.
iv. NGS through RNA-seq was used to identify genes associated with male floral induction. Specific primers from MADS box genes were not used as was done by other authors.
v. No prior hybridisation such as Suppression Subtractive Hybridisation (SSH) or
Fluorescence in situ Hybridisation (FISH) techniques in order to identify only previously identified genes but carried out sequencing, assembly and annotation with bioinformatics enabling us to identify new transcripts.
vi. An attempt was made to identify genes associated with floral induction and sex differentiation rather than studying genes associated with floral morphology.
Scope, framework and limitations of the research
This research is based on four inter-linked objectives aiming at contributing to the development of oil palm, specifically oil palm genomics, breeding and seed production. These activities were carried out in different locations because of the perennial nature of the plant and also because of the availability of laboratory facilities. The four activities were:
1. The complete defoliation of Pisifera parent plants.
2. RNA-seq on flower related tissues extracted on Pisifera parents after complete defoliation.
3. Analysis of gene expression levels associated to male flower induction. 4. The quantification of gene expression levels associated to male inflorescence
induction
Experiment two shall be developed based on the first results obtained in experiment one. By using mathematical models, we shall be able to predict the time and amount of environmental conditions necessary for switching gene expression in response to complete defoliation. Samples of tissue shall be extracted from the completely defoliated tree and unstressed tree to identify the group of Differentially Expressed Genes (DEG) associated with this stress and male inflorescence induction in Pisifera. Next Generation Sequencing will be used to sequence transcripts expressed in response to the complete defoliation of the Pisifera. A simulated defoliation activity, tissue extraction and RNA isolation shall be carried out on Pisifera at the PT ASTRA Research Centre in Kumai Central Kalimantan. NGS shall be carried out in the Macrogen laboratories in South Korea.
Experiment three and four shall be based on the results of experiment two. The level of expression of different transcripts found in the different tissues shall be compared with the normal level of expression contained in the oil palm reference genome.
Figure 1.1 Flow chart of research activities
Exp 1. Complete defoliation of Pisifera
Phenotypic and physiological responses Analysis of climatic and time factors
Exp 2. Extraction of flower related tissues for mRNA isolation
mRNA isolation
Quality control
NGS
Sequence processing
Quality control
Transcriptome mapping to reference genome
Transcriptome characterisation/ gene
annotation
Differential gene expression analysis
Gene ontology
CHAPTER II
LITERATURE REVIEW
Origin and botany of oil palm
The oil palm presently exists in the cultivated and wild states in the equatorial and tropical regions of Africa, South East Asia and South and Central America. Many authors have put forward various arguments on the origin of the oil palm (Zeven 1964; Hartley 1988; Soh et al. 2003). There is no doubt that the cultivated African oil palm Elaeis guineensis Jacq. is of African origin because there is supporting pre historic evidence that it originated from the area around Nigeria, Cameroon (Gulf of Guinea) and the Congo (Zeven 1964; Hartley 1988). Early European traders collected oil palm seeds from Africa and planted them in The Amsterdam Botanic Gardens, Netherlands and later transferred it to the Bogor Botanic Gardens Indonesia, then a Dutch colony in the year 1848. The four palms planted in Bogor then became the foundation stock for the development of the world oil palm industry (Hunger 1917).
The oil palm is a diploid (2n = 2x = 32) classified under the family Arecaceae (Dransfield et al. 2005). The crown of a mature palm consists of between 30 to 50 leaves. The number of leaves produced annually by a plantation palm decreases from 30 to 40 after 2 years of age to an average of 20 to 25 per annum after 8 years of age. The roots of the oil palm can reach a horizontal distance of 16 m and a vertical distance of 8 m in well drained soils. Secondary roots develop on primary roots and tertiary roots develop on secondary roots forming a network of dense root system (Corley and Tinker 2003). The oil palm is monoecious, with male and female inflorescences occurring separately on the same tree (Dransfield et al. 2008). The female and male inflorescences are shown in Figure 2.1.
The oil palm is commonly divided into three races based on their shell thickness (sh). The sh gene had been discovered by Beinaert and Vanderweyen (1941). The Dura has a shell thickness between 2 to 8 mm; the Tenera 0.2 to 2 mm and the Pisifera has no shell. The fleshy mesocarp of Dura yields between 15 to 17%, oil that of Tenera yields between 21 to 23% oil and Pisifera more than 23% oil. The Pisifera mostly aborts its fruits and thereby produces virtually empty bunches, thus it is not cultivated on large scale for commercial purpose.
Major changes caused by carbohydrate depletion in plants
Figure 2.1 Un-emerged female inflorescence extracted from leaf axil number 14 (left) and un-emerged male inflorescences extracted from leaf number 13 (right). The inflorescence from leaf axil number 14 is two weeks older than the inflorescence from leaf axil number 13. Female inflorescences are always longer and larger than their corresponding male inflorescence of similar age and from the same plant.
Hormones associated with flowering
Concerning hormones associated with oil palm sex differentiation, Corley (1976) observed that the growth regulator auxin NAA (naphthalene acetic acid), favoured female flower production in palms grown in polythene bags. The masculinising effect of GA3 has also been reported in cucumber (Fuchs et al. 1977; Pimenta Lange et al. 2012). GA, ABA, IAA and Cytokinin content were found to be higher in the developmental stages of male flower than in female flowers in poplar (Song et al. 2013). In the much-studied Arabidopsis, individual pathways that include photoperiod (Srikanth and Schmid 2011), vernalisation (Kim et al. 2009), autonomous (Mouradov et al. 2002), ageing (Fornara and Coupland 2009) and GA (Mutasa-Gottgens Hedden 2009), regulate flowering time. The GA4 which is most likely produced in the leaves and transported to the meristem up regulates one or both of the
LFY genes, a floral meristem identity gene and the SUPRESSOR OF
OVEREXPRESSION OF CONSTANS 1 (SOC1), a floral integrator leading to flowering (Bernier and Perilleux 2005).
Table 2.1 Cell and physiological responses of some major phyto-hormones involved in flower sex determination and development, including related references
Hormones Cell and physiological responses Species Reference
Abscisic acid Flowering initiation, flower, leaf
and fruit senescence and
abscission, stomata closing,
concentration increases during drought
Populus tomentosa
Song et al. (2013)
Auxin Increases cell elongation and
division, initiation of floral primordia, specifies the number and identity of floral organs
Oil palm,
Populus tomentosa
Corley (1976); Song et al. (2013) Brassinosteroid Slows abscission, stimulates cell
division
Maize Hartwig et
al. (2011)
Cytokinin Regulates cell division and delays
senescence, affect meristem size, required for pollen development
Soybean, Nicotiana plumbaginifolia
Wong et al. (2013); Vincentz et al. (1993)
Ethylene Floral transition, feminising role
in flower development, induces fruit ripening, synthesis increases as a result of stress
Arabidopsis Achard et
al. (2007)
Gibberellic acid Masculinising effect, induce
phase transitions and stimulates fruit development
Lolium temulentum
King and
Evans 1991
Jasmonic acid Developing inflorescences,
defensive responses to stress
Arabidopsis Reinbothe et
al. 2009
responsible for stamen abortion in maize inflorescences (DeLong et al. 1993; Dellaporta and Calderon-Urrea 1993) although it has the opposite effect on cucumber. The role of phytohormones in flower development and sex determination is summarised in Table 2.1.
Genes involved in floral induction and initiation in oil palm
Flower initiation includes all of the development processes necessary for the irreversible commitment by the meristem to produce a flower or inflorescence. Vegetative to reproductive phase transition of meristem results from consequential activities of floral meristem identity genes that specify floral meristem fate and of floral organ identity genes that determine the pattern of whorl establishment. Floral meristem identity genes stimulate floral organ identity genes to promote floral development. The floral organ identity genes are designated as ABC classes of homeotic genes. The ABC model includes the APETALA 1,2,3 (AP); PISTILLATA 1 (P1) and AGAMOUS (AG). The class A genes (AP1 and AP2) are responsible for sepals identity, the class B (AP3 and P1) plus the class A genes are responsible for petal identity, the class C (AG) plus the class B genes are responsible for stamens identity and the class C genes alone specifies the carpel identity. The SUPERMAN genes maintain whorl boundaries. Superman mutants in Arabidopsis develop extra stamens at the expense of carpels, rendering carpels defective or under developed (Schultz et al. 1991; Nibau et al. 2011).
It is believed that in unisexual flowers, sex is determined by the selective repression of growth or abortion of either the male or female reproductive organs. It is known that the identity of reproductive organs is controlled by homeotic genes belonging to the MADS box gene family. Kater et al. (2001) reported that the arrest of either male or female organ development is dependent on their positions in the plant. Although sex determination in animals is well studied, little is known about the molecular processes involved in plant sex determination. Only few genes that influence sex have been cloned. The TASSELSEED2 gene in maize which is responsible for the abortion of pistil primordia in the tassel (DeLong et al. 1993) and the ANTHER EAR1 gene (Bensen et al. 1995) which encodes an enzyme in the gibberellin biosynthesis pathway responsible for stamen abortion in maize ear. The class B and C members of the MADS box family encode putative transcription factors with a highly conserved DNA binding domain and may influence the sex of plants. They suggest a role of the class C genes in controlling the arrest of the reproductive organs in cucumber.
The role of environmental factors and assimilates
flowering phenotypes and promote dark phase flowering (Roldan et al. 1999) while in some cases, it was shown to significantly reduce flowering (Zhou et al. 2008). Sugar concentration changes are associated to plant tissue senescence and abortion (Rolland et al. 2002; Yoshida et al. 2002; Kakumanu et al. 2012). The expression of hexokinases was linked to senescence in transgenic plants (Xiao et al. 2000). Carbohydrate related genes have been linked to embryo abortion in maize (Zhuang et al. 2007). Adam et al. (2011) proposed a mechanism of sex differentiation in oil palm that is presented in Figure 2.2.
Figure 2.2 Possible interactions resulting from the severe defoliation of oil palm as proposed by Adam et al. (2011). The circles represent the biological processes while the squares represent the products.
Adam et al. (2005) noticed a small group of cells at the base of oil palm leaves and suspected them to be precursor cells for inflorescence meristem and suggested that these cells should be identified to detect mRNA transcripts of putative genes. Figure 2.3
Water
Indeterminate inflorescences
Female inflorescence
Male inflorescence Hormone status
Glucose Starch
shows inflorescence buds at the base of leaves in a dissected meristem of a mature pisifera palm.
Figure 1.3 Apical meristem of oil palm tree showing undetermined flower bud and future leaves. The meristem was obtained from a dissected cross section of a mature pisifera plant in the PT ASTRA Agro Lestari oil palm plantation in Kalimantan.
!
The results of Adam et al. (2005; 2011) arise from the conclusions of previous researchers such as Corley (1976) and Durand-Gasselin et al. (1999) who indicated that the period between flower sex differentiation and flower maturity takes about 22 months. A diagrammatic representation of the activities between flower initiation and flower maturity is shown in Figure 2.4.
Figure 2.4 Stages of the developmental processes between sex differentiation and flower maturity in oil palm as reproduced by Durand-Gasselin et al. (1999). The top most row represents the leaf numbers. Leaf number 0 is the middle unopened leaf commonly known as the spear. Leaf number +1 is the first opened leaf outwards from leaf number 0. Leaf number -1 is the next leaf that will succeed the spear. The leaf numbers carrying minus signs are un-emerged leaves. It is assumed that leaf initiation takes place at leaf position number -40 inside the apical meristem of the oil palm. Inflorescences are initiated on every leaf axil from which it will later develop through to leaf number +20 where it obtains maturity. The second row indicates the number of months between inflorescence initiation and inflorescence maturity in an adult oil palm tree. Adult oil palm trees emit two leaves every month while younger trees emit 3 leaves every month. Younger trees obviously take shorter time between inflorescence initiation and flower maturity that adult trees. The period between inflorescence sex determination and inflorescence maturity in younger palms is estimated to be 14.5 months. Inflorescence maturity is attained when the flowers are in anthesis or receptive for pollination.
The oil palm trunk as a carbohydrate reserve
[image:30.612.99.522.84.585.2]Severe defoliation as well as high density planting and fruit pruning are aspects which reduce total carbon reserves, and are thus compatible with the notion that higher sex ratios are associated with higher levels of carbon assimilates (Sparnaaij 1960).
CHAPTER III
MASSIVE CARBOHYDRATE ASSIMILATES DELAY RESPONSE
TO STRESS IN OIL PALM (
Elaeis guineensis
Jacq.) CAUSED BY
COMPLETE DEFOLIATION
1Abstract
Understanding how and when crops cope with and respond to stress during reproductive development offers the possibility for researchers to forecast production in situations of abrupt climate change. A research was conducted to study the effect of complete defoliation under time-specific climate-related conditions on inflorescence sex differentiation in oil palm. A total of 162 pisifera oil palm trees were completely defoliated at the rate of three trees per month between July 2007 and December 2011 at La Dibamba Cameroon. Complete defoliation significantly increased male inflorescence induction by 104% than those of control without defoliation. Acute soil water deficit (SWD) of 16.8 mm between the 30th and 60th day after complete defoliation (DAD) had an additional positive effect on male inflorescence production. A regression analysis on 18 time-specific, climate-related research and two inflorescence-related variables resulted in high regression coefficients for the time period 30th to 60th DAD. This is an indication that oil palm responds to complete defoliation stress after a 30-day delay period. Total soluble sugars measured at 45 DAD showed a depletion of 55% in the leaves and 21% in inflorescence of defoliated trees compared to those of control trees without defoliation. Preferential sex differentiation in oil palm towards maleness is an acclimation response to the depletion of total soluble sugar inflected by mechanical and soil water deficit stresses. Information obtained from this research shall permit the simulation of male inflorescence induction and yield forecasting in other geographical locations. Molecular scientists who wish to study genes responding to complete defoliation and soil water deficit stress in oil palm can also use this knowledge to isolate related genes.
Key words: Fruit bunches, Male inflorescence induction, Mechanical stress, Sugar depletion, Sex differentiation, Stress responses
!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
1
INTRODUCTION
Abiotic stresses such as drought, flood, extreme temperatures, chemical toxicity and oxidative stress are serious threats to plants. All forms of stress usually have a negative impact on plant assimilates, which are primarily made up of carbohydrates and are used for plant growth and development. Abundance or depletion of carbohydrates may regulate plant gene expression (Ohto et al. 2001; Lee et al. 2010). It is known that carbohydrate regulated genes play important roles in responding to changing environments (Koch 1996; Sheen et al. 1999; Smeekens 2000; Liu et al. 2013). Carbohydrate depletion enhances expression of genes associated with photosynthesis, reserve mobilisation and export (Koch and Zeng 2002; Rolland et al. 2002). Carbohydrates have also been associated with plant cell and organ differentiations (Gibson 2000; Eastmond and Graham 2001; Lopez-Molina et al. 2001; Pien et al. 2001). Sugar pulses to apical meristem can lead to meristem differentiation through cell division. High sugar levels reduce photoperiod effects on floral evocation (Corbesier et al. 1998; Roldan et al. 1999) or even completely replace it. The later has been observed in Lolium temulentum L. The changes response was associated with up-regulation of invertase genes at the apical meristem (King and Evans 1991). Evidence has also been provided that a decrease in assimilates supply due to defoliation or shading, increases early fruit abscission in evergreen trees (Gomez-Cadenas et al. 2000).
Fresh Fruit Bunches (FFBs) in oil palm are fertilised female inflorescences that have developed into mature fruits. The FFB development requires much more energy than the male inflorescences. Production of FFB mobilises much primary photosynthate and or carbohydrate reserves. Under certain conditions, such reserve mobilisation may alter the inflorescence sex ratio and FFB production in the next season (Nouy et al. 1999). Hardon and Corley (1976) reported that oil palm would produce predominantly male inflorescences after suffering serious loss of carbohydrate through previous year’s harvests.
Development of male inflorescences does not require much energy compared to the female ones because they only produce pollen, which falls off two weeks after anthesis. Hence, the male inflorescence development does not require carbohydrate reserve mobilisation and more photosynthate is conserved when oil palm produces principally male inflorescences. Subsequently, increase in carbohydrate reserve results in the production of predominantly female inflorescences from the oil palm trees in the following season.
Oil palm producing the pisifera fruit type is used as male parent in breeding and seed production programs. However, the pisifera is very feminine under normal plantation conditions. The female inflorescences of pisifera do not develop into mature fruits since they are aborted. Thus FFB is not produced in pisifera, resulting in accumulation of reserve carbohydrate. Subsequently, such conditions lead to continual evocation of female inflorescences and the absence of male inflorescences. This is undesirable condition for pisifera in oil palm breeding and seed production since there will be less pollen provider (Durand-Gasselin et al. 1999).
inflorescences in oil palm. This complete defoliation stress treatment may alter the balance of sugar in the treated oil palm, leading to the evocation of male inflorescences (Campbell et al. 2012). Durand-Gasselin et al. (1999) suggested that the effects of severe defoliation stress treatment on male inflorescence evocations are probably immediate. However, the exact moment for which the oil palm initiates male inflorescence formation in response to stresses is not yet known. On the other hand, a number of researchers (Gray 1969; Corley 1976; Killmann and Lim 1987; Henson et al. 1999; Legros et al. 2009; Cros et al. 2013) reported that the oil palm trunk contains large reserves of carbohydrates. These carbohydrate reserves may serve as a buffer during transitory periods of source-sink imbalances caused by a halt in photosynthetic activities. Thus, the large reserves in oil palm trunk will be used to absorb any shock arising from stress within a certain time limit. When the reserves are considerably depleted, the plant would initiate the response mechanisms through gene expression. Under abiotic stress, such as drought stress, plants tend to mobilise carbohydrate reserves. Therefore, it is expected that oil palm will mobilise some of the carbohydrate reserves stored in the trunk to response to the stress. Such response is acclimation in the form of male inflorescence induction. Moreover, abiotic stress responses have also affect sex differentiation. Association among abiotic stress responses and sex differentiation have been demonstrated in maize by Hartwig et al. (2011). Therefore, combining complete defoliation and water deficit stress may positively affect male flower induction in oil palm.
In oil palm, the inflorescences were initiated approximately at 18-26 months before ones’ visually observed them. Therefore, understanding the factors affecting and the periods of male inflorescence induction in oil palm would be interesting. Knowledge on the period of oil palm initiates response to complete defoliation stress through the expression of genes would be very interesting to molecular scientists. This knowledge is important because the expressed genes during complete defoliation stress might be the same genes involve in male inflorescence induction. Moreover, such knowledge may help us to effectively plan and produce pollen from the limited number of pisifera type of oil palm growing under current unstable climatic conditions. However, there is only limited information available about the required parameters, which will guide breeders and seed producers to efficiently induce male inflorescences from pisifera or simulate FFB production under such condition, especially in this era of climate instability.
MATERIALS AND METHODS
Experimental site
Complete defoliation and data collection were conducted from July 2007 until May 2013 at the Specialised Centre for Oil Palm Research La Dibamba Douala in Cameroon (3.948848°N 9.762726°E). The rainy season for La Dibamba lasts from March to October with limited precipitation during the 4 to 5 months dry season. Mean annual temperature between 2007 until 2013 was 28.1 °C and the average annual precipitation was 2 769 mm of rainfall for the period of this research. The soils are classified as ferralsols with clay accumulations and low base saturation. The pisifera were planted in 1993 to serve as male parents and pollen provider in breeding and seed production programs respectively.
Complete defoliation treatments
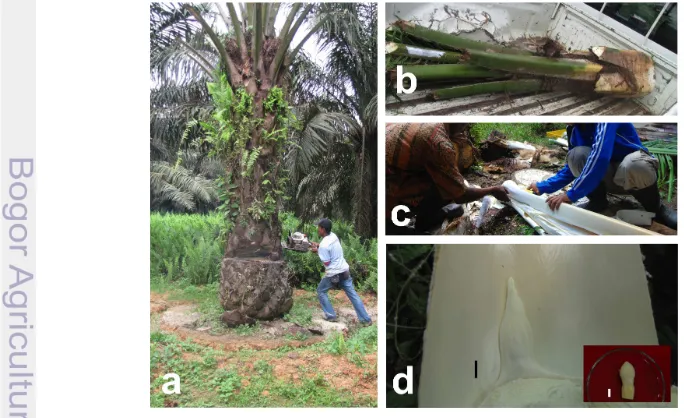
The complete defoliation treatment consisted of the removal by pruning of all the leaves and preserving only the middle un-opened one so as to avoid tree death. This activity consisted of removing all the leaves of three trees each month during the year. Pruning of the trees began in July 2007 until end of December 2011 and observations of male inflorescence formation were done until May 2013. The total number of trees pruned during the rainy seasons was equal to the total number of trees pruned during the dry seasons. The steps in complete defoliation are presented in Figure 3.1. It illustrates a mature pisifera palm in its non-defoliated form, a defoliated and a recovering pisifera 45 DAD.
Total soluble sugar
Agricultural University using 5 mL of 80 % hot ethanol (Chow and Landhausser 2014). Figure 3.2 shows the process of leaf counting during inflorescence extraction.
[image:36.612.100.502.136.531.2]Figure 3.2 Leaf counting during inflorescence extraction. Each leaf axil contains an inflorescence.
Observations
[image:37.612.88.510.81.395.2]A total of eighteen climate-related research variables and two inflorescence-related response variables were studied. The climate inflorescence-related variables were analysed for a period of three months following each treatment and included among others; precipitation 10 days after complete defoliation (0-10 mm) DAD, 20 (0-20 mm) DAD, 30 (0-30 mm) DAD and 60 (0-60 mm) DAD. Precipitation between day 10 and day 20 (10-20 mm) DAD; between day 20 and day 30 (20-30 mm) DAD and between day 30 and day 60 (30-60 mm) DAD were also calculated. The same measurements were also taken for number of rainy days (RD) and SWD after complete defoliation. The total number of male inflorescences produced before complete defoliation (FBD) and the total number of male inflorescences produced after complete defoliation (FAD) was recorded.
Statistical analysis
Analysis of data was carried out using the software SAS version 9.3 (SAS Institute Inc, Cary NC). The sum, averages and standard deviations were calculated for all the observations and they were used for subsequent analysis. A Pearson’s regression analysis was carried out to study how increasing or decreasing levels of experimental variables (amounts of rainfall, soil water and number of rainy days) would affect responses (total number of male inflorescences produced). A paired T- test was used to compare the effects on the same individuals before and after treatment. A two – sample T – test was used to compare response from averages of two different sets of data. All tests were measured at a confidence interval of 95%.
RESULTS AND DISCUSSIONS
Precipitation and soil water deficit at research site
0 20 40 60 80 100 120 0 100 200 300 400 500 600
Jan Feb Mar Apr May Jun Jul Aug Sep Oct Nov Dec
w at er d ef ic it ( m m ) P re ci p it at ion ( m m )
Precipitation Water deficit
!
Figure 3.3 Monthly soil water deficit (curve) and monthly precipitation (bars) as recorded at the La Dibamba Meteorological Station during the period of research. This represents average precipitation and average soil water deficit between 2007 and 2012. The wet season extends between March and October.
Effect of complete defoliation on male inflorescence induction
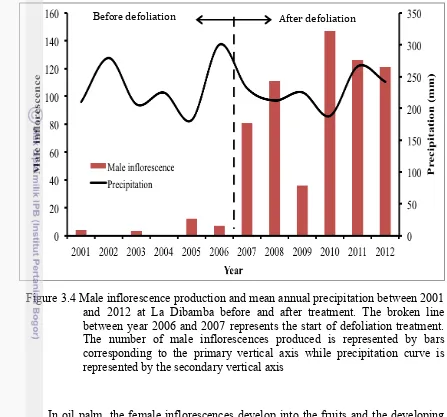
Figure 3.4 Male inflorescence production and mean annual precipitation between 2001 and 2012 at La Dibamba before and after treatment. The broken line between year 2006 and 2007 represents the start of defoliation treatment. The number of male inflorescences produced is represented by bars corresponding to the primary vertical axis while precipitation curve is represented by the secondary vertical axis
In oil palm, the female inflorescences develop into the fruits and the developing bunches are very demanding in terms of energy especially between the stages of inflorescence emergence and fruit maturity. Energy is needed in embryo, endosperm and fruit development. A single fresh bunch weighs an average of 20 kg and a single oil palm tree bears and carries between 15 and 25 bunches each year. Each female inflorescence requires between 5 to 6 months on the tree to get to fruit maturity (Corley 1976; Durand-Gasselin et al.1999; Corley and Tinker 2003). In contrast, the male inflorescence has an average weight of just 2 kg and does not remain for a long period on the palm tree, progressing to senescence in about one month after anthesis, and liberating the oil palm tree from further energy demand. Therefore the production of more male inflorescences in response to stress created by complete defoliation is a strategy developed by the oil palm to conserve limited resources for maintenance. This acclimation was thus in form of male inflorescence induction in expense of female one (Hardon and Corley 1976; Campbell et al. 2012).
After&defoliation
Relationship of climatic parameters and male inflorescence production Amount of daily precipitation and number of rainy days were analysed for a period of two months after defoliation. It should be noted the impact of complete defoliation on the oil palm trees reduce significantly after two months because the trees would have produced about five open leaves that can produce carbohydrate through photosynthesis.
!
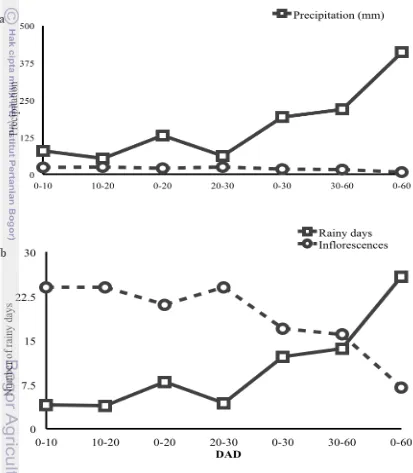
Figure 3.5 The relationship between male inflorescence production and (a) precipitation (b) number of rainy days after in the first two months after complete defoliation. Measurements were taken on each tree immediately after complete defoliation until the 60th day. These measurements were taken through out the year between 2007 and 2012.
a
[image:41.612.98.511.182.655.2]The graphs presented in Figure 3.5a show that the number of male inflorescences reduced with higher quantities of precipitation; while Figure 3.5b shows a downward trend for number of male inflorescences induced as against increasing number of rainy days. The trend is similar to that of total precipitation because of the ferralsols soil type, which retains water because of clay accumulations. Several researchers have evoked the relationship between soil water availability and inflorescence sex determination (Spalik 1991; Bikel and Freeman 1993; Campbell et al. 2012).
Period for switching on sex differentiation in response to defoliation
A test was carried out to determine the period in which the oil palm tree will initiate response mechanisms to the shock received from complete defoliation stress. The test was done on the specified time intervals resulting in 18 associated climate parameters considered as explanatory variables and two male inflorescence emergences considered as dependent variable. A regression analysis carried out on these 18 variables revealed that those climatic variables associated with the second month after complete defoliation had the highest influences on male inflorescence production. The R2 = 15.6% for precipitation recorded between the 30th and 60th day after treatment, 22.1% for the total number of rainy days recorded between the 30th and 60th day after treatment and 12.7% for precipitation recorded 60 days after treatment. These variables and their correlations are shown on Table 3.1. The other 15 variables each had an R2 close to zero and these are variables associated with the first 10, 20 and 30 days after complete defoliation respectively. It should be noted that other variables such as genetic, physiological and farm management do influence inflorescence induction and represent the other part of correlations that are not measured in this study.
There was a significant relationship observed between the number of male inflorescences produced and three related soil water deficit variables. These variables are the amount of precipitation that was registered in the second month, the number of rainy days registered in the second month and the total amount of precipitation registered during the first two months after complete defoliation. These variables, which are environmental data collected during the second 30 days after complete defoliation gives us a clue on when the oil palm senses the impact of complete defoliation and then responses to it. The correlation coefficients presented in Table 3.1 do not sum up to 1 because flowering in oil palm is not only influenced by environmental factors; but also by genetic factors and physiological status of the trees (Adam et al. 2005).
variable reserve compartment for carbohydrate is located in the trunk and it occasionally serves as a buffer for transitory source-sink imbalances (Mialet-Serra et al. 2008). The trunk of an 8 year old sago palm contains 93 kg of stored carbohydrates while that of a similar oil palm trunk contains 37 kg (Henson et al. 1999) up to 47.9 kg of carbohydrate (Corley1976). Hence, it is logical for the oil palm to start responding to complete defoliation only after depletion of a considerable amount of the reserve carbohydrates. This means that in this experiment, the oil palm switches on its response mechanism between day 30 and day 60 after it was completely defoliated, as suggested by the data.
Table 3.1 Correlation coefficients for selected variables that had a significant relationship with male inflorescence number.
Variable (30-60 mm) DAD (30-60 rd) DAD (0-60 mm) DAD
Mean 218.29 13.60 410.40
Correlation R = - 0.395
R2 = 0.156
R = -0.470 R2 = 0.221
R = -0.316 R2 = 0.127
Probability p < 0.01 p < 0.001 p < 0.05
(30-60 mm) DAD = Amount of precipitation registered between the 30th day and the 60th day. (30-60 rd) DAD = Number of rainy days registered between the 30th day and the 60th day; (0-60 mm) DAD = cumulative precipitation registered during the first two months after Complete defoliation.
[image:43.612.99.441.463.672.2]Total soluble sugar in leaf and inflorescence tissues
Figure 3.6 shows the different stages leading to the extraction for tissues used in total soluble sugar analysis.
Both the leaf and inflorescence tissues were obtained following the dissection the tree crown. Total soluble sugar was reduced by 55% in leaves and 21% in inflorescences on completely defoliated plants as compared to un-defoliated plants at 45 DAD.
Figure 3.7 shows the amount of total soluble sugar measured on leaves and inflorescences 45 DAD between completely defoliated plants and the control un-defoliated ones. The period of 45 DAD was chosen based on the previous findings of this present study. The depletion of total soluble sugar on severely defoliated plants by 55% on leaf tissues and 21% on flower tissues at 45 DAD, may have been an indication that the plants are already in a critical state and thus may have to acclimate in order to maintain survival. This acclimation was thus in the form of male inflorescence induction in expense of female inflorescence (Hardon and Corley 1976; Campbell et al. 2012).
[image:44.612.92.424.450.713.2]We have seen from earlier works that the oil palm has enough energy to absorb immediate shock from carbohydrate depletion caused by a sudden halt in its photosynthetic process. It is also known that the oil palm produces between two and three leaves every month (Hardon and Corley 1976; Adam et al. 2011). Therefore, enough leaves would have been produced after two months of complete defoliation stress treatment to be able to reverse the effects of complete defoliation stress on oil palm. Estimating the time period on when response mechanisms are initiated against a particular stress, gives us an indication on when genes responsible for the stress regulation are expressed in the plant. We know that the transcription of these regulatory genes is time and site specific. For further genetic and molecular studies where tissue extraction and RNA isolation are needed, it would be very important to know when and where to extract tissue for the studies because regulatory genes may be temporally and spatially expressed and the mRNA may be degraded rapidly.
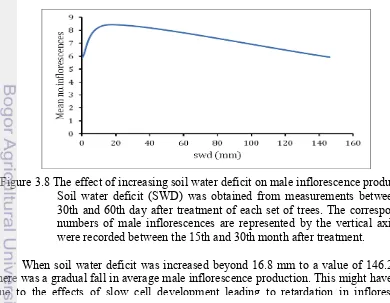
Effect of increasing soil water deficit on male inflorescence production We showed in Figure 3.4 that complete defoliation as a single factor, increased male inflorescence induction at field capacity. Increasing values of SWD measured during the second month after defoliation was tested against male inflorescence production (Figure 3.8). There was a sharp increase in the number of male inflorescences produced when SWD increased from field capacity to 16.8 mm. Then there was a decreasing trend of inflorescence production as SWD increased to 146.2 mm. Also, a two sample T-test at 95% confidence interval assuming equal variance confirmed that a significant higher number of male inflorescences were produced when complete defoliation was done during the dry periods (M = 7.03, SD = 4.387) than that when at field capacity (M = 4.88, SD = 2.790), t(57) = 2.16, p = 0.035.
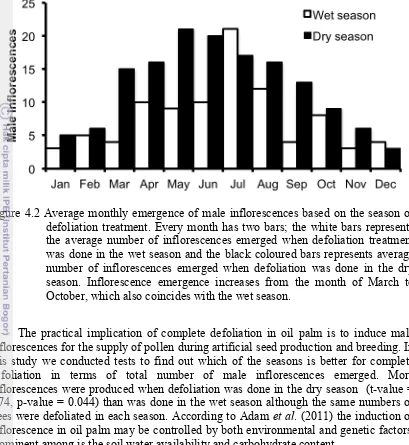
[image:45.612.100.490.457.760.2]Leaf defoliation singularly increased male inflorescence production to an average of 6.1 inflorescences at field capacity. This gives supporting evidence that the effects of complete defoliation contributed to the induction of male inflorescences. When complete defoliation was accompanied by a SWD of 16.8 mm during the second month after complete defoliation, there was an additional and significant increase in the average number of male inflorescences produced to 9.1, corresponding to an increase of 33%. The additional effects of water deficit must have contributed to this increase as observed on Figure 3.8. This could be understood because the effects of complete defoliation carried out during the dry season was more important than when carried out during the rainy season in Ivory Coast (Durand-Gasselin et al. 1999). In this study, the specific amount of SWD needed to significantly increase male inflorescence induction in oil palm and the time range for which it is effective has been determined. In a related study, it was proven that water deficit or availability was the most determining factor in oil palm production (Nouy et al. 1996).
Figure 3.8 The effect of increasing soil water deficit on male inflorescence production. Soil water deficit (SWD) was obtained from measurements between the 30th and 60th day after treatment of each set of trees. The corresponding numbers of male inflorescences are represented by the vertical axis and were recorded between the 15th and 30th month after treatment.
evocation. A similar scenario was observed in Chrysanthemum in which slow development in the capitulum of the shoot apical meristem resulted in retardation of inflorescence (Nakano et al. 2013). There was also a massive decrease in transcript abundance of cell division genes in the drought-stressed ovary of maize (Kakumanu et al. 2012).
Most of the phenological processes in plants are controlled by genes, which are predominantly regulatory in nature. This simply means