ISOLATION AND IDENTIFICATION OF CELLULASE-PRODUCING
THERMOPHILIC BACTERIA FROM OIL PALM (
Elaeis guineensis
)
RIRIN MASRINA
DEPARTMENT OF BIOLOGY
FACULTY OF MATHEMATICS AND NATURAL SCIENCES
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
ii
ABSTRACT
RIRIN MASRINA. Isolation and Identification of Cellulase-Producing Thermophilic Bacteria from Oil Palm (Elaeis guineensis). Supervised by ANTONIUS SUWANTO and ESTI PUSPITASARI.
Cellulase is an enzyme catalyzing hydrolysis of cellulose which usually consists of endoglucanases (EC.3.2.1.4), exoglucanases (EC.3.2.1.91) and β-glucosidases (EC.3.2.1.21). Cellulase is a very important enzyme due to its numerous industrial applications. The aim of this research were to isolate and identify cellulase-producing thermophilic bacteria that can be used to increase value added in oil palm industries. Samples of soil, empty fruit bunch compost and palm kernel meal were collected from oil palm plantation for obtained thermophilic bacteria. The samples were screened of cellulase-producing thermophilic bacteria by using a Congo Red method were made on carboxymethyl cellulose (CMC) agar plates. The bacterial cultures were incubated in a shaking incubator (140 rpm) at 50oC for 24 hours. The assay for the enzymatic activity was based on the release of glucose that was detected using 3,5-dinitrosalicylic acid (DNS). In this research, 19 isolates of bacteria were isolated. It was found that 11 isolates of bacteria showed positive results with clear zone around the cultures by using Congo Red method. The result showed that isolates for CK1, EM4 and CK3 possesed the highest enzyme activity of 13.56, 12.50 and 10.49 U mL-1 for cellulase was detected at pH 7, respectively. The results of strain identification based on 16S rRNA showed that strain CK1, EM4 and CK3 were identified as Bacillus subtilis.
Key words: isolation, identification, cellulase, thermophilic bacteria, oil palm
ABSTRAK
RIRIN MASRINA. Isolasi dan Identifikasi Bakteri Termofilik Penghasil Selulase dari Kelapa Sawit (Elaeis guineensis). Dibimbing oleh ANTONIUS SUWANTO dan ESTI PUSPITASARI.
Selulase merupakan enzim untuk mengkatalisasi hidrolisis selulosa yang pada umumnya terdiri atas endoglukanase (EC.3.2.1.4), eksoglukanase (EC.3.2.1.91), dan β-glukosidase (EC.3.2.1.21). Selulase sangat penting untuk diterapkan pada banyak industri. Tujuan dari penelitian ini adalah untuk mengisolasi dan mengidentifikasi bakteri termofilik penghasil selulase yang dapat digunakan untuk meningkatkan nilai tambah pada industri kelapa sawit. Sampel tanah, kompos tandan kosong, dan bungkil inti sawit diperoleh dari perkebunan kelapa sawit untuk memperoleh bakteri. Sampel ditapis untuk memperoleh isolat selulolitik dengan menggunakan metode Congo Red pada media padat carboxymethyl cellulose (CMC). Kultur bakteri diinkubasi pada inkubator bergoyang (140 rpm) pada suhu 50oC selama 24 jam. Assay aktivitas enzim didasarkan atas pelepasan glukosa yang dideteksi dengan menggunakan 3,5-dinitrosalicylic acid (DNS). Di dalam penelitian ini, 19 isolat bakteri telah diisolasi. Ditemukan 11 isolat bakteri yang menunjukkan hasil yang positif dengan adanya zona bening disekitar koloni dengan menggunakan metode Congo Red. Hasil menunjukkan bahwa isolat CK1, EM4, dan CK3 memiliki aktivitas enzim tertinggi, yaitu 13.56, 12.50, dan 10.49 U mL-1 untuk selulase yang dideteksi pada pH7 untuk masing-masing isolat. Hasil identifikasi strain berdasarkan 16S rRNA menunjukkan bahwa strain CK1, EM4, dan CK 3 telah diidentifikasi sebagai Bacillus subtilis.
ISOLATION AND IDENTIFICATION OF CELLULASE-PRODUCING
THERMOPHILIC BACTERIA FROM OIL PALM (
Elaeis guineensis
)
RIRIN MASRINA
Minithesis
In partial fulfillment of the requirement for Bachelor Degree of Science in
Department of Biology Faculty of Mathematics and Natural Sciences
Bogor Agricultural University
DEPARTMENT OF BIOLOGY
FACULTY OF MATHEMATICS AND NATURAL SCIENCES
BOGOR AGRICULTURAL UNIVERSITY
BOGOR
viii Title : Isolation and Identification of Cellulase-Producing Thermophilic Bacteria from Oil
Palm (Elaeis guineensis) Name : Ririn Masrina
NIS : G34070018
Approved by,
Endorsed by,
Head of Department of Biology
Bogor Agricultural University
Dr. Ir. Ence Darmo Jaya Supena, M.Si.
Graduation Date:
Prof. Dr. Ir. Antonius Suwanto, M.Sc.
Supervisor I
Esti Puspitasari, M.Si.
ACKNOWLEDGMENTS
All praises and thanks to Allah SWT the Almighty for His bless and Who is entire source
of knowledge that I’m able to finish my minithesis entitled Isolation and Identification of
Cellulase-Producing Thermophilic Bacteria from Oil Palm (Elaeis guineensis).
I would like to express my appreciation to Prof. Dr. Ir. Antonius Suwanto, M.Sc. and Esti Puspitasari, M.Si. for their advices, knowledges and supports during my research. My sincere thanks to Dr. Ir. Ence Darmo Jaya Supena, M.Si. as the head of Biology Department, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University. My gratitude to PT Wilmar Benih Indonesia that served all my research, also all staff in there, especially for Ludovika Jessica Virginia, S.Si. and Griselda Herman Natadiputri, S.Si. for helping me completed my research.
Deppest thanks to my parents, my sisters (Teteh Yani ang Umi), and my brother (Alfi) for all prays, loves, supports and sacrifices. I also take this opportunity to say thanks to all of my friends specially for LASPATI, TANDA BACA community (Mas Eko, Mas Jay, Agra), IKC, FORCES (Mba Sari, Tiko, Riska, Ayu, Amin), my housmate Ar-Riyadh (Gita, Mba Ria, Zia, Pito, Tari, Maya, Lili, Laswi, Vyras, Indi, Putri, Arum, Achi, Nisa, Fira) and Biology 44 IPB for the encouragement, especially to Rita Handayani, Gita Kusuma Rahayu, Irwanto Adhi Nugroho, Faizal Kurnia Syavitri and Muhammad Irfan for the supports, cheerfulness and friendships that they given to me this far.
Life is not measured in the number of breathes we take, but in the moments that take our breaths away, this minithesis is a small tribute to all of the moments. Hope this minithesis will be usefull.
Bogor, November 2011
viii
CURRICULUM VITAE
Author was born in Cirebon, February 9th1989as the second child of Salim and Suaebah. Author was graduated from SMAN 1 Sumber (Cirebon) in 2007 and accepted in Department of Biology, Faculty of Mathematics and Natural Sciences, Bogor Agricultural University (IPB) through Undangan Seleksi Masuk IPB (USMI).
Author did the field study entitled Isolation of Soil Bacteria-Producing Mannanase and Indol Acetic Acid (IAA) and field work entitled Process of Carp Hatchery in Technical Implementing Fish Seed Unit Department of Marine and Fishery in Cirebon.
During the college, author assisted the practical class for Biology in 2009 and Phisiology of Procaryot in 2010. Author was also active in many organization, such as Forum for Scientific Studies in 2007-2010, IPB Debating Community in 2007-2010, Madani Foundation 2008-now, Tanda Baca Community as secretary 2008-now, Techno Magazine as Secretary 2009-2010, writer of book Modal Guyur Air Untung Besar Mengalir with Dr. Elang Ilik Martawijaya and member of Ikatan Kekeluargaan Cirebon (IKC) in 2007-2011.
Author was also active in many event, such as are Gebyar Inovasi Pemuda Indonesia, Pesta Sains, IPB goes to School, Kompetisi dan Inovasi Agroteknologi, Seminar Ilmiah Nasional and many others.
vi
CONTENT
FIGURES...
APENDIXES...
INTRODUCTION
Background ... Objectives ...
MATERIALS AND METHODS
Time and Place... Materials ... Methods...
Isolation of cellulase-producing thermophilic bacteria... Screening of cellulase producers... Cellulase production... Preparation of crude enzyme... Enzyme assays... Strain identification...
RESULTS AND DISCUSSIONS
Isolation and selection of cellulase-producing thermophilic bacteria... Enzyme assay... Identification of cellulase-producing thermophilic bacteria...
CONCLUSION...
REFERENCES...
APENDIXES... vii
viii
1 1
1 2 2 2 2 2 2 2 2
3 4 4
5
5
9
vii
FIGURES
Figure 1 Screening for cellulase-producing thermophilic bacteria by clear zone detection using Congo Red... Figure 2 Cellulase activity results using DNS method after 24 h incubation time in cellulase production medium at 50oC on rotary shaker 140 rpm ( : 546 nm)... Figure 3 Agarose gel anlaysis of PCR amplified partial 16S rRNA . Line 1 : 1kb DNA size
marker (Promega). Line 2 1800 bp PCR product with a. CK3, b. EM4 and c. CK1 are three the best isolate that producing highest cellulase crude enzyme with DNS method...
Page
3
4
viii
APPENDIXES
Appendix 1 Screening for cellulase-producing bacteria using agar medium containing 0,5% (w/v) CMC. Samples were incubated at 50oC for 3 days. The plates were stained with Congo Red and destained with 1M NaCl solution. Clear zone indicated the hydrolysis of CMC as a result of cellulases production...
Appendix 2 Glucose standard curve...
Appendix 3 Cellulase activity results using DNS method after 24 h incubation time in cellulase production medium at 50oC on rotary shaker 140 rpm at 546 nm... Appendix 4 Result of spectrophotometric measurment of absorbance by DNS method ( : 546 nm)...
Page
9
9
10
1
INTRODUCTION Background
The oil palm (Elaeis guineensis) has become the most important economic plantation crop in the world. Based on the data from National Agriculture Department of Indonesia in 2009, Indonesia palm oil production reached 18,64 billion tons for oil palm and 3,47 billion tons for palm kernel most abundant renewable bioresource produced in the biosphere (Jarvis 2003; Zhang & Lynd 2004). Cellulose is a linear polymer consisting of D-anhydrogluco pyranose molecules joined together by β-1,4 glicosidic bond with a degree of polymerization (Lynd et al. 2005; Zhang et al. 2006).
Cellulose is commonly degraded by an enzyme called cellulase (Kotchoni et al. 2006). Cellulases are inducible enzymes synthesized by microorganisms during their growth on cellulosic materials (Cai et al. 1999; Lee & Koo 2001). The complete enzymatic hydrolysis of cellulosic materials needs at least three different types of cellulase, there are endoglucanase (Carboxymethylcellulase or CMCase; EC 3.2.1.4), exoglucanase (EC 3.2.1.91) and β -glucosidase (EC 3.2.1.21) (Yi et al. 1999; Saha 2000; Bhat 2000; Holker et al. 2004).
The endoglucanase randomly hydrolized
the β-1,4 bonds in the cellulose molecule and the exoglucanase in most cases release a cellobiose unit showing a recurrent reaction from chain extremity. Lastly, the cellobiose
is converted to glucose by β-glucosidase (Beguin & Aubert 1994; Ibrahim & El-diwany 2007).
With the recent development of biotechnology, there has been vast interest to use cellulose digestive microorganisms to convert cellulosic biomass to glucose that can be used in different applications such as production of fuel ethanol, use in waste treatment (Thambirajah et al. 1995), brewing industry, for bio-polishing of fabrics and producing stonewashed look of denims, animal feeds for improving the nutritional quality and digestibility (Kasana et al. 2008), for improving fabric softness,
brightness and anti-deposition (Ibrahim & El-diwany 2007).
Many mesophilic and thermophilic bacteria and fungi have been investigated with respect to the bioconversion of agricultural and forest biomass into fuels and valuable chemicals (Tomme et al. 1988; Meinke et al. 1991; Wang et al. 1993; Wittmann et al. 1994). The most common producer is fungi (Lee & Koo 2001; Ariffin et al. 2006). But, bacteria, which has high produced highly thermostable, alkalistable enzyme complement and may serve as highly potent sources of industrially important enzyme. One of the prerequisite for the enzymes to be employed for industrial applications is that they must be robust enough and highly stable under hostile conditions of industrial processes like extremes of temperature and pH. For instance, for the successfull application of cellulases in detergent industry, enzymes must have alkaline pH optima, similarly for lignocellulose transformation, in pulp and paper industry or in feed industry, highly thermostable cellulases with acid or alkalistability are desirable. Many resesearchers have documented production of thermostable and alkalistable cellulases from different microorganism (Bhat 2000).
There are limited studies on bacteria that reported as cellulase producers e.g. Ruminococcus albus (Wood et al. 1982; Ohara et al. 2000; Schwarz 2001), Bacillus (Robson & Chambliss 1984), Clostridium thermocellum (Lamed & Bayer 1988), Clostridium cellulyticum (Belaich et al. 2002), Thermoactinomycetes sp. (Amritkar 2002), a mutant of Bacillus pumilus BpCR16 and Bacillus pumilus EB3(Ariffin et al. 2006).
Objectives
The objectives of this research were to isolate and identify cellulase-producing thermophilic bacteria from oil palm.
.
MATERALS AND METHODS Time and Place
2 Wilmar Benih Indonesia-Cikarang,
Indonesia.
Materials
Materials that were used for this research were soil, empty fruit bunch compost (EFBC), palm kernel meal (PKM) of oil palm, carboxymethyl cellulose (CMC), Congo Red (CR), Gram staining, 3.5-dinitro salicylic acid (DNS) reagent and other materials for routine laboratory analysis and identification.
Methods
Isolation and screening of cellulase-producing thermophilic bacteria carboxymethyl cellulose (CMC) agar medium (Ruijssenaars & Hartman 2001) with some modification. CMC agar medium containing 0.1% (NH4)2SO4, 0.5% KCl, and purified by transferring them several times onto CMC agar plates. The isolated colonies were further incubated at 50°C for 3 days to allow for the secretion of cellulase. At the end of the incubation, to visualized 1 M NaCl for 15 minutes. The formation of a clear zone of hydrolysis indicated cellulose degradation by microorganism (Lee 2007; Baharudin et al. 2010).
Cellulase production
The medium used for production of the cellulase contained the following components 0.25% yeast extract, 0.5% K2HPO4, 0.1% NaCl, 0.02% MgSO4.7H2O,
0.06% (NH4)2.SO4 and 2% CMC was used
as carbon source. The pH was adjusted to initial pH 7.0 by 1M NaOH. These medium was inoculated with one single colony from CMC agar plates into 5 ml CMC broth production medium and reinoculated with 1 ml bacterial suspensions in 25 ml CMC
broth production medium. Two replicates were used for each bacteria isolates and the standard strain Escherichia coli BL 21 for negative control. The inoculated flasks were incubated at 50oC on rotary shaker at 140 rpm under aerobic condition as stationary culture for 24 hours (Kim et al. 2009). After determination of enzyme activities (Kotchoni et al. 2003; Immanuel et al. 2006). Then, the crude enzyme or free cell supernatant was obtained and cellulase was assayed. The supernatans were used for determination of reducing sugars (Samira et al. 2011).
Enzyme assays
Cellulase activity was assayed using a modified method described by Wood and Bhat (1998) with some modifications. The cellulase activtity was measured by mixing 0,1 mL of the crude enzyme supernatant incubated with 0,1 ml of 1% (w/v) CMC in 0,01M sodium phosphate buffer solution pH 7.0 at 50oC for 60 minutes. The reaction was terminated by adding 1 ml DNS reagent. The mixture was boiled for 10 minutes and cooled in ice, then its optical density at 546 nm was determined (Samira et al. 2011). The cellulase activity was measured by using a calibrationed curve for glucose. One unit of cellulase was defined as the amount
Bacteria strain molecular identification used pure culture DNA sequencing method
3 cycle, while pre-denaturation and
post-elongation passed off for one cycle. PCR purified by EXOSAP-IT® PCR Purification Kit (USB Corporation, Ohio, USA). Sequencing cycle used BigDye® X-Terminator Cycle Sequencing Kit (Applied Biosystem, Foster City, California) and amplified by Bio Rad C1000TM Thermal at 95oC (5), denaturation at 95oC (30), annealing at 55oC (30), elongation at 60oC (130) and post-elongation for 5 min. Denaturation, annealing and elongation passed off for 25 cycles, while pre-denaturation and post-elongation passed of for one cycle. Result from sequencing cycle purified with Big Dye® X-Terminator Purification Kit (Applied Biosystem, Foster City, California) and sequenced by ABI PRISMTM 3130 Genetic analyzer (Applied Biosystem, Foster City, California). Then, the 16S rRNA sequences of the isolates obtained were compared directly with sequences in the NCBI (National Centre for Biotechnology Information) database using Basic Local Alignment Search Tool (BLAST) (http://ncbi.nlm.gov/BLAST). Then, the best isolates were stained with Gram staining procedure.
RESULTS AND DISCUSSION
Isolation and selection of cellulase-producing thermophilic bacteria
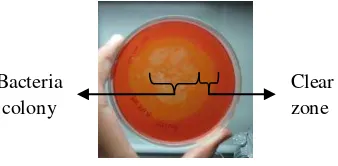
The results indicated after 24 h incubation, from 19 bacterial isolates grew on CMC agar plates. There were four isolates from palm kernel meal (PKM), ten isolates from empty fruit bunch compost (EFBC), and five isolates from soil of oil palm. In this research, screening of bacteria was conducted by using the CR method as a preliminary study for identifying cellulase producers. Eleven isolates exibited clearing zone around their colonies on CMC agar primary isolation and screening of cellulolytic bacteria, but the clear zone width was not implied the amount of cellulase activity. In 2000, a report showed that among 77 thermotolerant bacterial isolates grown on CMC agar, an isolate CMU4.4 exhibited the highest enzyme activity whereas its clear zone was smaller than others isolates (Krootdilaganandh 2000).
Fig 1 Screening for cellulase-producing thermophilic bacteria by clear zone detection using Congo Red.
4
Enzyme assay
The results of enzyme assay indicated eleven isolates as the isolates were able to decompose CMC detection by CR method showed enzyme activity. The measurement of enzyme activity in this research also support Wood and Bhat (1998) and Miller (1959). The mentioned researchers, by using colorimetery method with DNS reagent and drawing calibration curve by D-glucose, investigated cellulolytic isolates activities.
The enzymatic hydrolysis produced sugar with reducing ends that reacted to 3,5-dinitrosalicylic acid showing the high absorbance in 546 nm (Samira et al. 2011). In order to show cellulase activity, it is necessary to measure the glucose concentration which released by enzymatic hydrolysis. Therefore, calibration glucose curve was drawn. Then, enzymatic activity based on U mL-1 stated.
Figure 2 illustrated cellulase activity in the broth for cellulase production in 100 ml Erlenmeyer flasks. Cellulase activity for CK1, EM4 and CK3 was obtained after 24 h incubation with 13.56, 12.50 and 10.49 U mL-1, as three the best isolates, respectively.
Fig 2 Cellulase activity results using DNS method after 24 h incubation time in cellulase production medium at 50oC on rotary shaker 140 rpm ( : 546 nm).
These results are in agreement with those of Narashima et al. (2006) and Niranjane et al. (2007) who found that carboxymethyl cellulose was the best carbon source followed by cellulose for cellulase production. A higher production of cellulase when CMC served as substrate may be as a result of induction of the enzyme since cellulose is known to be a universal inducer of cellulase synthesis. Then, This results is higher than results from Meryandini et al. (2009). Meryandini et al. (2009) isolated 4 isolates of C4-4, C5-1, C5-3 and C11-1. The researchers observations showed that maximum cellulase activity were 3.17, 1.50,
0.17, and 3.33 U mL-1 at 50oC (pH 7.0)after 24h incubation on CMC production medium.
A duplicate of experiment was performed to verify the optimization result in order to validate the developed optimized medium. To be able to control whether CMC production media works or not, positive control with commercial cellulase (0,2% concentration), blank sample and negative control with E.coli BL 21 known as cellulase-negative.
The colorimetric assay used for the determination of cellulase activity was the dinitrosalicylic acid (DNS) method (Miller 1959). The amount of enzyme production stage of the organism largely depends upon the type of microbial strains and their genetic make up and on cultural and enviromental conditions employed during growth of the organism (Bajaj et al. 2009). Many microorganisms are capable of degrading and utilizing cellulose and hemicellulose as carbon and energy sources (Baharudin et al. 2010).
Cellulases yields appear to depend on a complex relationship involving a variety of factors like inoculum size, pH value, temperature, presence of inducers, medium additive, aeration, growth time, etc (Immanuel et al. 2006).
Identification of the cellulase-producing thermophilic bacteria
To identify the experimental strain exactly according to 16S rRNA sequence analysis as well as taxonomical studies, genomic DNA of the strain was used as template to amplify partial 16S rRNA using 63f primer and 1387r primer. The result from gel electrophoresis obtained the expected length of fragment (~1800 bp) was observed in 1.5% gel electrophoresis (fig 3).
5 isolated from palm kernel meal and empty
fruit bunch compost of oil palm and all of them are Gram positive bacteria.
Fig 3 Agarose gel anlysis of PCR amplified partial 16S rRNA . Line 1 : 1kb DNA size marker (Promega, USA). Line 2 1800 bp PCR product with a. CK3, b. EM4 and c. CK1 are three the best isolate that producing highest cellulase crude enzyme with DNS method.
Since most industrial processes are carried out at high temperature, there is a
Bacillus subtilis species are commonly found both in soil and water and they have great scientific and technological importance. Bacillus subtilis species have the ability to use various simple and complex organic compounds so they are involved in biodegradation of natural or man-made chemical compounds. Moreover; the bacterial genus Bacillus is the most important producer of extracellular enzymes like cellulases. Thermophilic bacterial cellulases have been frequently reported from Bacillus sp. (Hala & Priset 1994; Mawazda et al. 2000). Obtained data confirmed the findings reported by Ray et al. (2007) who mentioned that pH 7 more suitable for optimization of cellulase production by Bacillus subtilis. Furthermore, the cellulolytic enzyme, endoglucanase obtained from some baceria including Bacillus hydrolyzed substrate in the pH range of 4.0 to 9.0, with maximum activity transpiring at pH 7 (Immanuel et al. 2006).
CONCLUSION
A total of 19 cellulase producing thermophilic bacteria was successfully isolated from soil, palm kernel meal and empty fruit bunch compost of oil palm.
Eleven isolates showed clear zone on CMC supplemented with Congo Red. Based on DNS method, three isolates showed the highest cellulase activity and were identified as Bacillus subtilis.
REFERENCES
Acharya S, Chaudhary A. 2011. Effect of nutritional and environmental factors on cellulases activity by thermophilic bacteria isolated from hot spring. Journal of Scientific and Industrial Research 70: 142-148.
Amritkar N. 2002. Studies related to purification of industrially important microbial metabolites. PhD Thesis. Mumbai University. India.
Ariffin H, Abdullah N, Umi-Kalsom MS, Shirai Y, Hassan MA. 2006. Production and characterization of cellulose by Bacillus pumilus EB3. Int. J. Eng. Technol. 3: 47-53.
Arifin H et al. 2008. Production of bacterial endoglucanase from pretreated oil palm empty fruit bunch by Bacillus pumilus EB3. Journal of Bioscience and Bioengineering 106(3): 231-236. Baharudin AS et al. 2010. Isolation and
characterization of thermophilic cellulase-producing bacteria from empty fruit bunches-palm oil mill effluent compost. American Journal of Applied Sciences 7(1): 56-62. Baird DS, Johnson DA, Seligy VL. 1990.
Molecular cloning, expression and characterization of endo-β -1,4-glucanase genes from Bacillus polymixa and Bacillus circulans. J. Bacteriol 172: 1576-1586.
Bajaj BK, Wani MA, Sharma A, Pangotra H. 2009. Partial purification and characterization of a highly
thermostable pH stable
endoglucanase from a newly isolated Bacillus strain M.9. Ind. J. Chem. Technol 16: 382-387.
Bakar NKA, Aziz SA, Hassan MA, Ghazali FM. 2010. Isolation and selection of appropriate cellulolytic mixed microbial cultures for cellulases production from oil palm empty fruit bunc. Biotechnology 9(1): 73-78. Beguin P, Aubert JP. 1994. The biological
6 9 cellulase of the Clostridium
cellulyticum. J. Bacteriol 184: 1387-1384.
Bhat MK. 2000. Cellulases and related enzymes in biotechnology. Biotech Adv 18: 355-383.
Cai YJ, Chapman SJ, Buswell JA, Chang ST. 1999. Production and distribution of endoglucanase, cellobiohydrolase
and β-glucoside components of the cellulolytic system of Volvariella volvacea, the edible straw mushroom. Applied Environ. Microbiol 65: 553-559.
[Deptan] Departemen Pertanian. 2010.
Outlook Komoditas Pertanian
Perkebunan. Jakarta: Pusat Data dan Informasi Pertanian.
Deraman M. 1993. Carbon pellets prepared from fibres of oil palm empty fruit bunches: a comparison of weight loss and dimensional shrinkage with carbon from phenolic resin. Solid State Science and Technology 1(1): 41-49.
El-Sersy NA et al. 2010. Optimization, economization and characterization of cellulase produced by marine Streptomyces ruber. African Journal of Biotechnology 9(38): 6355-6364. Frothingham R, Allen RL, Wilson KH.
1991. Rapid 16S ribosomal DNA sequencing from a single colony without DNA extraction or purification. Biotechniques 11: 40-44. Haki GD, Rakshit SK. 2003. Development in industrially important thermostable enzymes. Biores Technol 89: 17-34. Hala KH, Priset FG. 1994. Thermotholerant
varieties of Bacillus licheniformis isolated from desert environments. J. Appl Bacteriol 77: 392-400.
Holker U, Hofer M, Lenz J. 2004. Biotechnological advantages of laboratory-scale solid-state fermentation with fungi. Appl. Microbiol. Biotechnol 64: 175-186. Ibrahim ASS, El-diwany A. 2007. Isolation
and identification of new cellulases producing thermophilic bacteria from an egyptian hot spring and some properties of the crude enzyme. Australian Journal of Basic and Applied Sciences 1(4): 473-478. Immanuel G, Dhanusa R, Prema P,
Palavesan A. 2006. Effect of different growth parameters on endoglucanase enzyme activity by bacteria isolated
from coir retting effluents of estuarine environment. Int. J. Environ. Sci. Tech. 3: 25-34.
Jarvis M. 2003. Cellulose stacks up. Nature 426: 611-622.
Kasana RC, Salwan R, Dhar H, Dutt S, Gulati A. 2008. A rapid and easy method for the detection of microbial cellulases on agar plates using gram’s iodine. Curr. Microbiol. 57: 503-507. Kim BK, Lee BH, Lee YJ, Jin IIH, Chung
CH, Lee JW. 2009. Purification and characterization of carboxymethyl cellulase isolated from a marine bacterium, Bacillus subtilis sp. subtilis A-53. Enzyme Microbial. Technol. 44: 411-416.
Kotchoni OS, Gachomo WE, Omafuvbe B, Shonukan OO. 2006. Purification and biochemical characterization of carboxymethyl cellulase (CMCase) from a catabolite repression insensitive mutant of Bacillus pumilus. Int. J. Agric Biol 8: 286-292. Kotchoni OS, Shonukan OO, Gachomo WE. 2003. Bacillus pumilus BpCR1 6, a promising candidate for cellulase production under conditions of catabolite repression. African Journal of Biotechnology 2(6): 140-146. Krootdilaganandh J. 2000. Isolation and
selection of thermotolerant bacteria capable of producing cellulose. Chiang Mai: Chiang Mai University Press.
Lamed R, Bayer EA. 1988. Cellulosomes from Chlostridium thermocellum. Methods. Enzymol. 160: 472-481. Lee NS. 2007. The production of fungal
mannanase, cellulase and xylanase using palm kernel meal as a substrate. Walailak J Sci & Tech 4(1): 67-82. Lee SM, Koo YM. 2001. Pilot scale
production of cellulose using Tricodherma reesei rut C-30 in fed batch mode. J. Microbiol. Biotechnol 11: 229-233.
Lynd LR, Van Zyl WH, McBride JE, Laser M. 2005. Consolidated bioprocessing of cellulosic biomass. An Update Curr Opin Biotechnol 16: 577-583. Marchesi JR et al. 1998. Design and
evaluation of useful bacterium specific PCR primer that amplify genes coding for bacterial 16S-rRNA. Appl Environ Microbiol 64: 795-799. Mawazda C, Hatti KR, Zvauya R,
7 characterization of cellulase produced
by two Bacillus strains. J. Biotechnol 83: 177-187.
Meinke A et al. 1991. Unusual sequence organization in Cen B, an inverting endoglucanase from Cellulomonas fimi. Journal of Bacteriology 171: 308-314.
Meryandini A et al. 2009. Isolasi bakteri selulolitik dan karakterisasi enzimnya. Makara Sains 1: 33-38. Miller GL. 1959. Use of dinitrosalicylic acid
reagent for determintaion of reducing sugar. Anal Chem 3: 426-428.
Narashima GA, Sridevi A, Viswanath B, Chandra SM, Reddy RB. 2006. Nutrient effect on production of cellulolytic enzymes by Aspergillus
niger. African Journal of Enzyme and Microbial Technology 40(1): 1464-1468.
Ohara H, Karita S, Kimura T, Sakka K, Ohmiya K. 2000. Characterization of the cellulolytic complex (cellulosome) from Ruminoccoccus albus. Biosc. Biotechno, Biochem 64: 254-260.
Ray AK, Bairagi A, Ghos KS, Sen SK. 2007. Optimization of fermentation conditions for cellulase production by Bacillus subtilis CY5 and Bacillus circulans TP3 isolated from fish gut. Acta Ichthyologica ET Piscatoria 37(1): 47-53.
Robson LM, Chambliss GH. 1984. Characterization of the cellulolytic activity of a Bacillus isolate. Appl. Environ. Microbiol 47(5) 1039-1046. Ruijssenaars HJ, Hartsman S. 2001. Plate
screening methods for the detection of polysaccharase-producing microorganism. Appl. Microbiol Biotechnol 55: 143-149.
Saha BC. 2000.
Alpha-L-arabinofuranosidases-biochemistry, molecular biology and application in biotechnology. Biotech Adv 18: 403-423.
Samira M, Mohammad R, Gholamreza G. 2011. Carboxymethyl-cellulase and filter-paperase activity of new strains isolated from persian gulf. Microbiology Journal 1(1): 8-16.
Schwarz WH. 2001. The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Biotechnol 56: 634-649.
Sirisena, DM, Manamendra TP. 1995. Isolation and characterization of cellulolytic bacteria from decomposing rice straw. J. Nat. Sci. Country Sri Lanka 23: 25-30.
Thambirajah JJ, Zulkali MD, Hashim MA. 1995. Microbiological and biochemical changes during the composting of oil palm empty fruit bunches-effect of nitrogen supplementation on the substrates. Bioresource Technology 52: 133-144. Tomme P, Tilbeugh VH, Petterson G, Damme VM. 1988. Studies of the cellulolytic system of Trichoderma reeseii QM9414-analysis of domain function in two cellobiohydrolases by limited proteolysis. European Journal of Biochemistry 170: 575-581.
Wang W, Reid SJ, Thomson JA. 1993. Transcription regulation of an endoglucanase and cellodextrinase gene in Ruminococcus flavefaciens FD-1. Journal of General Microbiology 139: 1219-1226. Wittmann S, Shareck F, Kluepfel D,
Morosol R. 1994. Purification and characterization of the CelB endoglucanase from Streptomyces lividans 66 and DNA sequence of the encoding gene. Applied and
Enviromental Microbiology 60:
1701-1703.
Wood TM, Bhat KM. 1998. Method for Measuring Cellulose Activities. In: Methods in Enzymology Cellulose
and Hemicellulose. New York:
Academic Press.
Wood TM, Wilson CA, Stewart CS. 1982. Preparation of the cellulase from the cellulolytic anaerobic rumen bacterium Ruminococcus albus and its release from the bacterial cell wall. Biochem J 205: 129-137. Yi JC, Sandra JC, John AB, Shu-ting C.
1999. Production and distribution of endoglucanase, cellobiohydrolase and
8 Zhang YHP, Himmel ME, Mielenz JR.
2006. Outlook for cellulase improvement: screening and selection strategies. Biotech Advances 24: 452-481.
9
APPENDIXES
Appendix 1 Screening for cellulase-production bacteria using agar medium containing 0,5% (w/v) CMC. Samples were incubated at 50oC for 3 days. The plates were stained with Congo Red and destained with 1M NaCl solution. Clear zone indicated the hydrolysis of CMC as a result of cellulases production
NP
TH OP
P4 TC5 TF2
CK5 CK1
CK3 CK4
10
Appendix 3 Cellulase activity results using DNS method after 24 h incubation time in cellulase production medium at 50oC on rotary shaker 140 rpm at 546 nm
Appendix 2 Glucose standard curve
Concentration (w/v %) Absorbance
0,025 0,007
0,050 0,117
0,075 0,302
0,100 0,474
0,125 0,633
0,150 0,771
0,175 0,909
Sample y x (%) gr/ml µgr/ml µmol/ml µmol/ml/menit
(U/ml)
Positive
Control 0,108 4,3338% 0,0433 43338,17 240,77 4,01
Negative
Control 0,007 2,7066% 0,0271 27066,22 150,37 2,51
CK 1 0,748 14,6448% 0,1464 146447,56 813,60 13,56
EM 4 0,677 13,5009% 0,1350 135008,86 750,05 12,50
CK 3 0,542 11,3259% 0,1133 113259,22 629,22 10,49
TH 0,504 10,7137% 0,1071 107137,10 595,21 9,92
P4 0,500 10,6493% 0,1065 106492,67 591,63 9,86
CK 4 0,489 10,4720% 0,1047 104720,48 581,78 9,70
TC 4 0,445 9,7632% 0,0976 97631,71 542,40 9,04
TC 5 0,418 9,3282% 0,0933 93281,78 518,23 8,64
OP 0,399 9,0221% 0,0902 90220,72 501,23 8,35
NP 0,067 3,6733% 0,0367 36732,72 204,07 3,40
11 Appendix 4 Result of spectrophotometric measurment of absorbance by DNS method ( : 546 nm)
Sample Part 1 Part 2 Stdev Average
Average per Isolate
1 2 3 1 2
Positive
control 0,109 0,085 0,104 0,115 0,121 0,01 0,107 0,108
0,076 0,097 0,122 0,130 0,121 0,02 0,109
Negative
control 0,021 0,040 0,000 0,000 0,000 0,02 0,012 0,007 0,000 0,010 0,000 0,000 0,000 0,00 0,002
CK1 0,756 0,772 0,788 0,758 0,771 0,01 0,769 0,748
0,759 0,763 0,725 0,765 0,771 0,02 0,757
0,719 0,718 0,728 0,755 0,786 0,03 0,741
0,712 0,729 0,705 0,757 0,727 0,02 0,726
EM4 0,719 0,691 0,711 0,684 0,701 0,01 0,701 0,677
0,699 0,640 0,639 0,702 0,696 0,03 0,675 0,654 0,658 0,652 0,651 0,637 0,01 0,650 0,714 0,723 0,726 0,627 0,623 0,05 0,683
CK3 0,530 0,513 0,530 0,601 0,570 0,04 0,549 0,542
0,519 0,620 0,540 0,557 0,570 0,04 0,561
0,570 0,530 0,601 0,493 0,513 0,04 0,541
0,479 0,550 0,479 0,545 0,530 0,04 0,517
TH 0,478 0,486 0,538 0,491 0,535 0,03 0,506 0,504
0,445 0,517 0,565 0,453 0,597 0,07 0,515
0,481 0,522 0,477 0,528 0,503 0,02 0,502 0,481 0,529 0,501 0,416 0,533 0,05 0,492
P4 0,415 0,431 0,487 0,522 0,519 0,05 0,475 0,500
0,407 0,401 0,452 0,486 0,471 0,04 0,443
0,535 0,605 0,576 0,475 0,491 0,06 0,536
0,550 0,563 0,605 0,518 0,483 0,05 0,544
CK4 0,555 0,479 0,480 0,506 0,506 0,03 0,505 0,489
0,529 0,495 0,470 0,519 0,466 0,03 0,496 0,515 0,436 0,479 0,466 0,481 0,03 0,475 0,527 0,424 0,451 0,481 0,519 0,04 0,480
TC4 0,502 0,453 0,374 0,668 0,336 0,13 0,467 0,445
0,482 0,452 0,365 0,496 0,441 0,05 0,447
0,487 0,422 0,365 0,419 0,408 0,04 0,420
0,490 0,387 0,381 0,409 0,571 0,08 0,448
TC5 0,385 0,384 0,405 0,466 0,497 0,05 0,427 0,418
0,386 0,341 0,342 0,46 0,529 0,08 0,412 0,34 0,379 0,34 0,414 0,496 0,06 0,394 0,372 0,413 0,393 0,516 0,506 0,07 0,440
OP 0,317 0,293 0,313 0,528 0,578 0,14 0,406 0,399
12
0,224 0,350 0,344 0,578 0,509 0,14 0,401
0,220 0,335 0,337 0,548 0,521 0,14 0,392
NP 0,023 0,087 0,097 0,040 0,03 0,03 0,055 0,067
0,033 0,089 0,129 0,04 0,074 0,04 0,073
0,028 0,113 0,123 0,049 0,033 0,05 0,069 0,047 0,113 0,123 0,038 0,040 0,04 0,072
TF2 0,074 0,063 0,088 0,043 0,069 0,02 0,067 0,064
0,072 0,076 0,064 0,031 0,105 0,03 0,070