Alcohol consumption and its relation to lipid-based cardiovascular
risk factors among middle-aged women: the role of HDL
3cholesterol
Pekka Sillanaukee
a,b,c,*, Timo Koivula
a, Hannu Jokela
a, Timo Pitka¨ja¨rvi
d,
Kaija Seppa¨
aaDepartments of Clinical Chemistry and Psychiatry,Uni6ersity of Tampere,Medical School and Tampere Uni6ersity Hospital,Tampere,Finland bPharmacia & Upjohn Diagnostics AB,Alcohol Related Diseases,Uppsala,Sweden
cDepartment of Clinical Neuroscience,Karolinska Institute,Stockholm,Sweden dTampere City Health Center,Tampere,Finland
Received 12 May 1999; received in revised form 8 November 1999; accepted 16 December 1999
Abstract
To study the association of alcohol consumption and lipid-based cardiovascular risk factors among middle-age women, cross-sectional analysis among 274 middle-aged healthy women with different drinking habits and a follow-up analysis of alcoholic women during abstinence was performed. Serum total cholesterol, low and high-density lipoprotein cholesterol (LDL and HDL cholesterol), triglycerides (TG), apolipoproteins A1 (Apo A1) and B (Apo B), and HDL-cholesterol subfractions 2 (HDL2) and 3 (HDL3) were measured. All lipid values except LDL cholesterol positively correlated with self-reported alcohol
consumption. When alcoholics were excluded the correlation was significant only for HDL cholesterol, HDL3, and Apo A1. The
increasing trend of HDL cholesterol, HDL3and Apo A1 were clearly seen first in women consuming \20 – 40 g/day of absolute
alcohol. Alcohol consumption \40 g/day increased all lipid values except LDL cholesterol. Abstinence for 2 weeks caused a significant decrease in HDL3 cholesterol, and an increase in LDL cholesterol and Apo B. The results indicate that among
middle-aged women the Apo A1 and HDL cholesterol via its HDL3but not HDL2subfraction might play a role in the beneficial
coronary consequences associated with moderate alcohol consumption. However, the increasing beneficial trend first appears when daily drinking exceeds 20 g/day. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Alcohol consumption; Apo A1; Apo B; Cardiovascular risk; Cholesterol; HDL3cholesterol; HDL2cholesterol
www.elsevier.com/locate/atherosclerosis
1. Introduction
Epidemiological studies have shown a negative asso-ciation between alcohol consumption and the risk of developing coronary artery disease (CAD) [1 – 4]. Over a broad range of alcohol consumption the relationship to all-cause mortality seems to be J-shaped [5 – 7]. This issue is not only due to individuals at high risk becom-ing nondrinkers [8,9] but because studies have sug-gested a beneficial association between moderate alcohol consumption and atherosclerosis progression
and incidence events [10 – 13]. Furthermore, it has been shown that alcohol consumption may protect against severe coronary atherosclerosis [14]. Excessive alcohol use, however, has obvious detrimental effects on the cardiovascular system [12,13,15,16].
The mechanism through which alcohol might exert its protective effect remains unclear. Population studies have shown that moderate alcohol consumption usually does not change proatherogenic low-density lipoprotein (LDL) levels but high consumption may even decrease the level [17]. High-density lipoproteins (HDL) choles-terol, however, unlike other lipids show a dose-depen-dent relationship to alcohol intake [18 – 20]. Because HDL cholesterol is thought to play an important role in preventing atherosclerosis [21 – 23], the main
hypoth-* Corresponding author. Present address: Oy Finnish Immunotech-nology Ltd., Lenkkeilija¨nkatu 8, 33 520 Tampere, Finland. Tel. +358-3-31387000; fax+358-3-31387050.
E-mail address:finnish-immunotech.com (P. Sillanaukee).
esis has been that alcohol protects via increasing HDL cholesterol [23].
Total HDL cholesterol offers useful but incomplete information about HDL’s clinical significance [24]. Subfraction quantitation may not only provide data of the degree of risk of coronary artery disease, but may also yield new information about the mecha-nisms for the apparent antiatherogenic effects of HDL. Initially only HDL2 was considered protective
against CAD [25,26], but several studies show that both, HDL2 and HDL3, may be inversely related to
coronary artery disease [25 – 28].
The effect of alcohol consumption on HDL sub-fractions among males is not clear. Some studies show the effect of alcohol on HDL3 [29,30] and some
also on HDL2 [31,32]. It has also been hypothesized
that heavy drinking preferentially increases HDL2 and
moderate drinking augments HDL3 [33]. Results also
show that Apo A1 levels rise with alcohol consump-tion [34] and that they may protect against atherosclerosis even better than HDL cholesterol does [35].
Little is known about alcohol consumption and its relation to lipid-based cardiovascular risk factors among women. In large cohorts of women, HDL cholesterol is the lipoprotein most strongly associated with CAD [36,37]. However, women answering sur-veys are particularly likely to underestimate their al-cohol intake [38], and few studies have included either alcoholics or methods for measuring alcohol use that are adequate for obtaining reliable data. The present population based cross sectional study focuses on lipid values among women, employs a detailed history of alcohol use and includes also women with high alcohol consumption. The effect of 2 weeks cessation of alcohol consumption on lipids was studied, too.
2. Subjects and methods
The study protocol was approved by the Ethics Committees of Tampere University Hospital, the A-Clinic Foundation, and the Tampere Health Center. The study was performed according to the Helsinki Declaration on human experimentation. The sample size was powerful enough to give significance (PB 0.05) if the difference in HDL cholesterol was 0.2 mmol/l.
2.1. Subjects
The study was based on 274 consecutive representa-tive non-menopausal women participating in a volun-tary health screening aimed at all women in the population between 40 and 45 years (n=3116). The participation rate was 84.5%. Experienced nurses
recorded the alcohol consumption by asking the type and amount of alcohol drunk during the average week. The alcohol amounts were calculated from the self-reported number of standard drinks (14 g), equat-ing one bottle of beer (33 cl), one glass of red or white wine (12 cl), one small glass of strong wine (8 cl) or one shot (4 cl) of spirits. The women also filled in two structured alcohol questionnaires: the Malmo¨ Modified Michigan Alcoholism Screening Test (Mm-MAST) [39] and the CAGE [40]. According to the results the women were divided in three groups [41].
2.1.1. Moderate drinkers
Moderate drinkers (n=139) if they had less than two positive answers in the CAGE, less than four positive answers in the Mm-MAST, and a self-re-ported weekly consumption of less than 140 g abso-lute alcohol during last 2 months. Thus, this group included also light drinkers and abstainers. Two women in the social drinker group had diabetes but no other chronic diseases were found in this group.
2.1.2. Hea6y drinkers
Heavy drinkers (n=62), if they had at least two positive answers in the CAGE, or at least four posi-tive answers in the Mm-MAST or their self-reported weekly alcohol consumption was at least 140 g of absolute alcohol. No alcoholics were found to be in-cluded in the heavy drinker group.
2.1.3. Alcoholics
Alcoholics (n=73) were consecutive, actively drink-ing women who were admitted for treatment at the detoxification clinic of A-Clinic Foundation in Tam-pere. All had a well-documented history of chronic alcoholism. None of them had previous history of liver disease or showed clinical signs of liver diseases or other organic complications of alcohol abuse at the time of the interview. None of them had diabetes. The effect of abstinence on lipid values of 12 female alcoholics was analyzed after 1 and 2 weeks without drinking.
The characteristics of the women in the different groups are presented in Table 1. Body mass index (BMI) was calculated as weight/height2. Information
2.2. Analysis
The sera were obtained after a 12-h fast. Serum total cholesterol levels were measured by the enzymatic method [42]. The total concentration of HDL choles-terol and the cholescholes-terol subfractions were measured by the dextran sulfate method [43] as previously described [44].
The precipitation reagent for total HDL cholesterol contained 10 g/l Dextralipid 50 (Sochibo, Boulogne, France) and 101.6 g/l MgCl2– 6H2O and for HDL319.1
g/l Dextralipid 50 and 397.4 g/l MgCl2– 6H2O. Then
250 ml serum was added to 25 ml of the appropriate reagent and after a 15-min incubation at ambient tem-perature, supernatants were separated by centrifugation for 20 min at 12 000×g (Heraeus Biofuge 15, os-terodeamtlarz, Germany). The HDL cholesterol con-tents of the supernatants were analyzed enzymatically on a Monarch 2000 Analyzer (Instrumentation Labora-tory, Lexington, USA) using Chod-Pap cholesterol reagents (Cat No 237574); Boehringer Mannheim, Ger-many), and a primary cholesterol standard (Cat No 530, Orion, Espoo, Finland). The HDL2 subfraction
was calculated by subtraction of HDL3 from total
HDL. Triglyceride levels were measured by the enzy-matic, kinetic method. Apo A1 and B levels were determined immunoturbidimetrically [45]. Serum LDL levels were calculated as (cholesterol – HDL choles-terol – 0.45×triglycerides) [46]. None of the women had triglyceride values \4.0 mmol/l and thus none of them were excluded from this calculation. The percentages of HDL2 and HDL3of total cholesterol and HDL
choles-terol were calculated, and the values were compared between the groups.
2.3. Statistical methods
BMDP software programs were used in the statistical analyses of the material [47]. The correlations of
self-re-ported alcohol consumption and lipid values were stud-ied using bivariate plots for linear regression. The lipid values were compared between the groups, by using analyses of variance. When variances were different, the Welch approximation was used. To compare the results with earlier studies, the women were divided also into five groups according to self-reported alcohol consump-tion. In both cases the lipid values that were signifi-cantly different using PANOVA were also compared
using pairwise t-test between the groups; separate, when the variances between the groups differed and pooled when they did not differ. Nonnumeric parame-ters between the groups were compared using cross-tabulations.
3. Results
Among 274 women all lipid values except LDL cholesterol significantly correlated with self-reported al-cohol consumption (Table 2). When alal-coholics were excluded, only Apo A1, HDL cholesterol and HDL3
cholesterol correlated significantly with the self-re-ported alcohol consumption (Table 2). A strong signifi-cant positive correlation was found also between Apo A1 and HDL2 (r=0.62, PB0.001) and Apo A1 and
HDL3 (r=0.71, PB0.001).
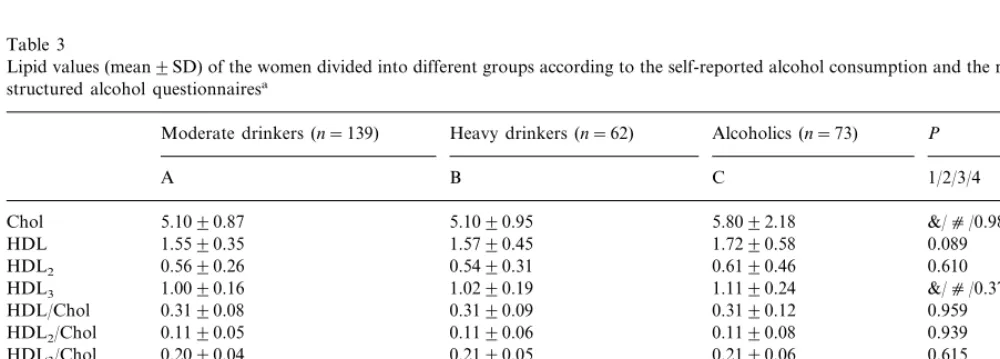
Lipid values were compared between different groups based on self-report and results from two question-naires (Table 3). PANOVA was significant for total
cholesterol, HDL3 cholesterol, triglycerides, Apo AI
and Apo B. Differences in lipid values using t-tests were found between the moderated drinkers and the alcoholics and between heavy drinkers and alcoholics. No differences were found between the groups in the percentage of abnormally low HDL cholesterol/total cholesterol (B0.20), ApoA1 (B0.86 g/l), HDL3
cholesterol (B0.8 mmol/l), and HDL2 cholesterol (B
0.3 mmol/l).
Table 1
Characteristics of the women divided into different groups according to the self-reported alcohol consumption and the results from structured alcohol questionnaires
Moderate drinkers (n=139) Heavy drinkers (n=62) Alcoholics (n=73) PANOVAa
Alcohol consumption (g/week) 39.0 160.6 934.3 B0.001
38.3
42.4 B0.001
Age (years) 42.3
24.3 25.3
BMIb(kg/m2) 23.5 0.062
Smokers (%) 33.3 46.8 85.0 B0.001
7
Anxiolytes (%) 1 33 B0.001
Gonadal hormones (%) 6 6 1 \0.05
12 16
Other medication (%) 16 \0.05
Table 2
Correlations between the self-reported alcohol consumption and dif-ferent lipid measures among women
Serum lipids All women All except alcoholics (n=201)
HDL2 0.107 B0.001 0.209
cholesterol
HDL3 0.284 B0.001 0.227 B0.001
cholesterol
Apo B 0.240 B0.001 −0.005 0.946
\40 g/day (PB0.01,P-value not shown in table) and those drinking 0 – 10 g/day.
The relative changes of total HDL cholesterol, its density based subtractions and apolipoprotein A1 in the group drinking \10 – 20 g/day (compared to those drinking 0 – 10 g/day) indicate constant increasing trend for HDL3 but significant reduction of HDL2 resulting
in decreasing or plateau trend in total HDL cholesterol and apolipoprotein A1 (Fig. 1). In-groups with higher consumption the trend for both HDL subfractions was increasing, resulting in increase in total HDL choles-terol and apolipoprotein A1.
Because Apo A1, HDL cholesterol and HDL3
sub-fraction correlated significantly with self-reported alco-hol consumption even when the alcoalco-holics were excluded, we studied these values further. The mean HDL cholesterol value among alcoholics was 0.17 mmol/l higher than among moderated drinkers (95% confidence interval from 0.02 to 0.32 mmol/l), and Apo A1 values 0.19 mmol/l higher (95% confidence interval from 0.12 to 0.26 mmol/l). HDL3 cholesterol values
were 0.11 mmol/l higher among alcoholics than in moderated drinkers (95% confidence interval from 0.05 to 0.17 mmol/l). The concentrations of HDL choles-terol, HDL3 cholesterol, and Apo A1 in alcoholic
women were 12% (PB0.05), 11% (PB0.001), and 13% (PB0.001) higher than in the moderated drinkers, respectively.
Among the 12 alcoholic females who were followed for 2 weeks during abstinence, HDL3 cholesterol
de-creased (P=0.004), whereas LDL cholesterol (P= 0.02), and Apo B (P=0.02) significantly increased. To further confirm the above findings, we studied
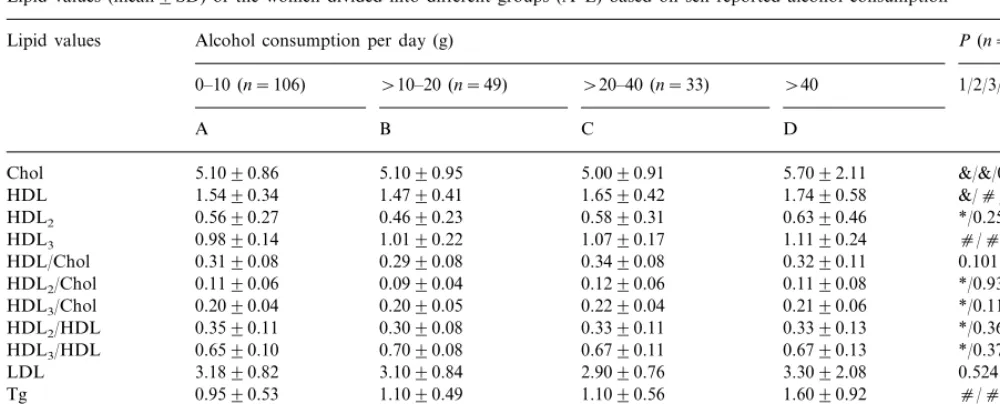
lipid values based only on the self-reported alcohol consumption (Table 4). Here PANOVA was significant
concerning total HDL, Apo A1, HDL3 cholesterol,
HDL2, HDL2/total cholesterol, HDL3/total cholesterol,
HDL2/HDL cholesterol, and HDL3/HDL cholesterol.
An increasing trend of HDL cholesterol (PB0.05, P -value not shown in table), HDL3/cholesterol and Apo
A1 (PB0.05,P-value not shown in table) was first seen among those women drinking \20 – 40 g/day as com-pared to those drinking \10 – 20 g/day. HDL2
choles-terol values were significantly lower among those drinking \10 – 20 g/day as compared to those drinking
Table 3
Lipid values (mean9SD) of the women divided into different groups according to the self-reported alcohol consumption and the results from two structured alcohol questionnairesa
Moderate drinkers (n=139) Heavy drinkers (n=62) Alcoholics (n=73) P
A B C 1/2/3/4
5.1090.87
Chol 5.1090.95 5.8092.18 &/c/0.983/&
1.5590.35 1.5790.45 1.7290.58 0.089
HDL
0.5690.26 0.5490.31
HDL2 0.6190.46 0.610
HDL3 1.0090.16 1.0290.19 1.1190.24 &/c/0.375/*
0.3190.12 0.3190.09
0.3190.08
HDL/Chol 0.959
HDL2/Chol 0.1190.05 0.1190.06 0.1190.08 0.939
HDL3/Chol 0.2090.04 0.2190.05 0.2190.06 0.615
HDL2/HDL 0.3490.10 0.3290.11 0.3290.13 0.384
0.6690.10 0.6890.11
HDL3/HDL 0.6890.14 0.399
3.1090.80 3.1090.88
LDL 3.3092.15 0.730
1.0090.51 1.0090.47
Tg 1.7090.93 c/c/0.541/c
ApoA1 1.4090.18 1.5090.23 1.6090.30 c/c/0.070/& ApoB 0.7090.18 0.7090.18 0.9090.32 &/c/0.496/&
aP
1 (ANOVA),P2(C vs. A),P3 (B vs. A),P4(C vs. B). Chol, Cholesterol; LDL, LDL-cholesterol; Tg, Triglycerides. *PB0.05, &PB0.01, c
Table 4
Lipid values (mean9SD) of the women divided into different groups (A–E) based on self-reported alcohol consumptiona
Lipid values Alcohol consumption per day (g) P(n=76)
\10–20 (n=49) \20–40 (n=33) \40 1/2/3/4 0–10 (n=106)
B C D
A
5.1090.95 5.0090.91
Chol 5.1090.86 5.7092.11 &/&/0.612/0.764
HDL 1.5490.34 1.4790.41 1.6590.42 1.7490.58 &/c/0.158/0.268 0.4690.23 0.5890.31
0.5690.27 0.6390.46
HDL2 */0.256/0.788/*
1.0190.22 1.0790.17
HDL3 0.9890.14 1.1190.24 c/c/&/0.33
0.2990.08 0.3490.08
0.3190.08 0.3290.11
HDL/Chol 0.101
0.1190.06
HDL2/Chol 0.0990.04 0.1290.06 0.1190.08 */0.939/0.729/&
0.2090.05 0.2290.04
0.2090.04 0.2190.06
HDL3/Chol */0.117/&/0.254
0.3590.11
HDL2/HDL 0.3090.08 0.3390.11 0.3390.13 */0.360/0.441/&
HDL3/HDL 0.6590.10 0.7090.08 0.6790.11 0.6790.13 */0.371/0.451/& 3.1090.84 2.9090.76
3.1890.82 3.3092.08
LDL 0.524
0.9590.53
Tg 1.1090.49 1.1090.56 1.6090.92 c/c/0.338/0.166
1.4090.24 1.5090.19 1.6090.30 c/c/&/0.868 Apo A1 1.4090.16
0.7090.18 0.7090.18 0.8090.32 */c/0.643/0.491
Apo B 0.7090.19
aP
1 (PAnova), P2 (D vs. A),P3 (C vs. A), P4 (B vs A). Chol, cholesterol; LDL, LDL-cholesterol; Tg, triglycerides,
cPB0.001; &PB0.01, *PB0.05.
4. Discussion
Our data agree with previous surveys in showing that alcohol consumption is associated with higher concen-trations of total high-density lipoprotein cholesterol [18 – 20]. The favourable effect of alcohol consumption on total HDL is reached only among alcoholic women and women drinking \40 g/day. This finding does not parallel large epidemiological studies: e.g. the Framing-ham study [18], where already a consumption of 4 – 16 g/day increased HDL cholesterol in women by 0.16 mmol/l (6 mg/dl), indicating a decrease in CAD mortal-ity of about 25%. However, the beneficial effects ob-served in the present study would be counteracted by detrimental factors caused by high alcohol consumption [48].
The present study gave indication that the alcohol induced increase in total HDL cholesterol and apolipo-protein A1 is based on HDL3 cholesterol and that the
association seems to be linear (Table 4 and Fig. 1). HDL2 was increased just after high alcohol
consump-tion levels and in fact it was reduced significantly among moderate drinking women (\10 – 20 g/day) as compared to women with light drinking (0 – 10 g/day) or women drinking more than 20 g/day. The phe-nomenon that moderate alcohol consumption increases only HDL3and excessive consumption both HDL3and
HDL2 cholesterol, noticed in the present, is parallel
with a corresponding study among Finnish men [30]. Apolipoprotein A1 has earlier been shown to be alcohol-induced [49] and has been described to be an even better discriminator of angiographically docu-mented CAD than HDL cholesterol [35]. In the present study, Apo A1 as well as HDL cholesterol and its
HDL3 subfraction correlated significantly with
self-re-ported alcohol consumption among social and heavy drinking women, but not in alcoholics. Overall the level of consumption at which HDL cholesterol, HDL3
sub-fraction, and in Apo A1 first increased was higher in the present study than in earlier epidemiological stud-ies. Possible bias was thus considered. Age, smoking, use of oral hormones and obesity are known to affect lipid values. The social and heavy drinker groups were age-matched, and the alcoholics were significantly younger than the other groups, which alone would have caused lower, not higher, lipid values in the alcoholics. Smoking is known to decrease HDL cholesterol values [50], and alcohol consumption correlates positively to smoking. This may partly explain the fact, that in the present study, where smoking also strongly correlated
Fig. 1. Relative changes (% of the values among women drinking 0 – 10 g/day) on serum HDL3 cholesterol, HDL2 cholesterol, total HDL cholesterol, and Apolipoprotein A1 values among women in four different drinking categories; 0 – 10 g/day (n=116), \10 – 20 g/day (n=49), \20 – 40 g/day (n=33), \40 g/day (n=76). ***
with drinking, the alcohol-induced changes in HDL cholesterol values appeared with higher consumption amounts than in earlier studies. Additionally, no dif-ferences in BMI or in the use of hormones were ob-served between the groups.
The present study did not indicate any beneficial changes in total cholesterol, in LDL cholesterol, in triglycerides or in triglycerides rich apolipoprotein B. In contrast, triglycerides, apo B, and total cholesterol were significantly elevated among women drinking \ 40 g/day as compared to women drinking 0 – 10 g/ day. These results also indicate that although alcohol consumption increases HDL cholesterol, its ratio to total cholesterol (HDL/Chol) does not change among women. The fact that alcohol intake apparently did not change total cholesterol or its ratio to HDL cholesterol, LDL cholesterol or triglyceride values be-tween those drinking 0 – 10 versus \10 – 20 g/day is in good agreement with an earlier corresponding study among Finnish men [20]. However, beneficial changes in total cholesterol levels (increased HDL/ Chol ratio, and reduced total cholesterol and Apo B) earlier detected among male alcoholics [17,20] were not observed in the present study among female alco-holics, indicating possible gender difference.
It is known that alcohol consumption is underesti-mated, especially among heavily drinking women [38]. In our study well-experienced nurses interviewed all the subjects personally, and in addition, two question-naires were used. Thus, these self-reports are likely to be more reliable than those obtained with less de-manding techniques. Earlier epidemiological studies found that women reporting a consumption of 10 g/ day might, actually drank 20 g/day. On the other hand, some differences in drinking pattern among Finnish women may cause some differences in lipid values when compared to studies made in other coun-tries. For example, daily drinking is rare in Finland; most of the moderate and heavy drinkers only drink during weekends, and there is hitherto little evidence of the consequences of different drinking habits on lipid values.
The main hypothesis for the protective effect of alcohol on CAD, that it is mediated by HDL terol, is based on the hypothesis that reverse choles-terol transport is the principal protective mechanism [51]. However, HDL may have other antiatherogenic properties, such as an antioxidant effect and the abil-ity to reduce LDL uptake by endothelial cells, pre-vent LDL aggregates from forming, counteract the platelet-activating effect of LDL, increase the solubil-ity of cholesterol in bile and promote fibrinolysis [52]. Other suggested mechanisms include changes in in-sulin resistance caused by alcohol [53 – 55], involve-ment of platelet aggregation [56], and finally, an effect not from alcohol at all but from the
antioxi-dant in red wine [57].
In conclusion, the present data support the possi-bility that among moderately drinking women, the al-cohol-induced changes in HDL3 cholesterol and Apo
A1, have a role in the beneficial consequences of al-cohol on CAD. The alal-cohol-caused increase in HDL3
is emphasized by the fact that abstinence decreased this value. Clear positive changes in the level of HDL cholesterol metabolism based risk factors were seen primarily among women when daily drinking exceeds 40 g/day. Thus, taking into account the harm and risk associated with heavy drinking and alcoholism, alcohol cannot be recommended as a means to im-prove lipid values in order to avoid CAD for non-postmenopausal middle-aged women.
Acknowledgements
Our thanks are due to the nurses of the Tampere Health Center for conducting the interviews, to Anne Kaipoma¨ki, Aira Heine, Arja Mikkelinen and Helena Myllyharju M.Sc. for technical assistance and to Dr Rauno Ma¨kela¨, M.D. for allowing us to examine pa-tients from his clinic. This study was partially sup-ported by the Finnish Foundation for Alcohol Studies.
References
[1] Yano K, Rhoads GG, Kagan A. Coffee, alcohol and risk of coronary heart disease among Japanese men living in Hawaii. N Engl J Med 1977;297:405 – 9.
[2] Friedman LA, Kimball AW. Coronary heart disease mortality and alcohol consumption in Framingham. Am J Epidemiol 1986;124:481 – 9.
[3] Kannel WB. Alcohol and the cardiovascular system. Proc Nutr Soc 1988;47:99 – 110.
[4] Stampfer MJ, Colditz GA, Willett WC, Speizer CE, Henrickins CH. A prospective study of moderate alcohol consumption and the risk of coronary artery disease and stroke in women. N Engl J Med 1988;319:267 – 73.
[5] Marmot MG, Rose G, Shipley MJ, Thomas BJ. Alcohol and mortality: a U-shaped curve. Lancet 1981;I:580 – 3.
[6] Shaper AG, Wannamethee G, Walker M. Alcohol and mortality: explaining the U-shaped curve. Lancet 1988;2:1268 – 73. [7] Bofetta P, Garfinkel L. Alcohol drinking and mortality among
men enrolled in an American cancer society prospective study. Epidemiology 1990;1:342 – 8.
[8] Rimm EB, Giovannucci EL, Willett WC, et al. Prospective study of alcohol consumption and risk of coronary artery disease in men. Lancet 1991;338:464 – 8.
[9] Jackson R, Scragg R, Beaglehole R. Alcohol consumption and risk of coronary heart disease. Br Med J 1991;303:211 – 5. [10] Kreisberg RA. A Votre Sante´. Arch Int Med 1992;152:263 – 5. [11] Doll R. One for the heart. Br Med J 1997;315:1664 – 8. [12] Keil U, Chambless LE, Doring A, Filipiak B, Stieber J. The
[13] Kannel WB, Ellison RC. Alcohol and coronary heart disease: the evidence for a protective effect. Clin Chim Acta 1996;246:59 – 76.
[14] Handa K, Sasaki J, Saku K, Kono S, Arakawa K. Alcohol consumption, serum lipids and severity of angiographically de-termined coronary artery disease. Am J Cardiol 1990;65:287 – 9.
[15] Rosengren A, Wilhelmsen L, Pennert K, Berglund G, Elmfeldt D. Alcoholic intemperance, coronary heart disease and mortality in middle-aged Swedish men. Acta Medica Scand 1987;222:201 – 13.
[16] Lands WEM, Zakhari S. Alcohol and cardiovascular disease. Alcohol Health Res World 1990;14:304 – 12.
[17] Hulley SB, Gordon S. Alcohol and high-density lipoprotein cholesterol. Causal inference from diverse study designs. Circula-tion 1981;64:57 – 63.
[18] Castelli WP, Doyle JT, Gordon T, et al. Alcohol and blood lipids: the Cooperative Lipoprotein phenotyping Study. Lancet 1977;2:153 – 5.
[19] Ekman R, Fex G, Johansson BG, Nilsson-Ehle P, Wadstein J. Changes in plasma high density lipoproteins and lipolytic en-zymes after long-term, heavy ethanol consumption. Scand J Clin Lab Invest 1981;41:709 – 15.
[20] Seppa¨ K, Sillanaukee P, Pitka¨ja¨rvi T, Nikkila¨ M, Koivula T. Moderate and heavy alcohol consumption have no favorable effect on lipid values. Arch Intern Med 1992;152:297 – 300. [21] Miller GJ, Miller NE. Plasma high-density lipoprotein
concen-tration and development of ischaemic heart disease. Lancet 1975;I:16 – 9.
[22] Gordon DJ, Probstfield JL, Garrison RJ, Neaton JD, Castelli WP, Knoke JD, Jacobs DR Jr, Bangdiwala S, Tyroler HA. High-density lipoprotein cholesterol and cardiovascular disease. Four prospective American studies. Circulation 1989;79:8 – 15. [23] Steinberg D, Pearson TA, Kuller LH. Alcohol and
atherosclero-sis. Ann Intern Med 1991;114:967 – 76.
[24] Silverman DI, Ginsburg GS, Pasternack RC. High density lipo-protein subfractions. Am J Med 1993;94:636 – 45.
[25] Miller NE, Hammet S, Saltissi S, et al. Relation of angiographi-cally defined coronary artery disease to plasma lipoprotein sub-fractions and apolipoproteins. Br Med J 1981;282:1741 – 4. [26] Ballentyne FC, Clark RS, Simpson HS, Ballentyne D. High
density and low density lipoprotein subfractions in survivors of myocardial infarction and in control subjects. Metabolism 1982;31:433 – 7.
[27] Stampfer MJ, Sacks FM, Salvini S, Willett WC, Hennekens CH. A prospective study of cholesterol, apolipoproteins, and the risk of myocardial infarction. N Engl J Med 1991;325:373 – 81. [28] Gaziano JM, Buring JE, Breslow JL, et al. Moderate alcohol
intake, increased levels of high-density lipoprotein and its sub-fractions, and decreased risk of myocardial infraction. N Eng J Med 1993;329:1829 – 34.
[29] Haskell WL, Camargo C, Williams PT, Vranizan KM, Krauss RM, Lindgren FT, Wood PD. The effect of cessation and resumption of moderate alcohol intake on serum high density lipoprotein subfractions. A controlled study. N Engl J Med 1984;310:805 – 10.
[30] Sillanaukee P, Koivula T, Jokela H, Myllyharju H, Seppa¨ K. Relationship of alcohol consumption to changes in HDL-sub-fractions. Eur J Clin Inv 1993;23:486 – 91.
[31] Taskinen M-R, Va¨lima¨ki M, Nikkila¨ EA, Kuusi T, Ehnholm C, Ylikahri R. High density lipoprotein subfractions and post hep-arin plasma lipases in alcoholic men before and after ethanol withdrawal. Metabolism 1982;31:1168 – 74.
[32] Dai WS, LaPorte RE, Hom DL, et al. Alcohol consumption and high density lipoprotein concentration among alcoholics. Am J Epidemiol 1985;122:620 – 7.
[33] Va¨lima¨ki M, Taskinen M-R, Ylikahri R, Roine R, Kuusi T, Nikkila¨ EA. Comparison of the effect of two different doses of alcohol on serum lipoproteins, HDL-subfractions and apolipo-proteins A-I and A-II: a controlled study. Eur J Clin Invest 1988;18:472 – 80.
[34] Camargo CA, Williams PT, Vranizan KM, Albers JJ, Wood PA. The effect of moderate alcohol intake on serum apolipoproteins A-I and A-II. J Am Med Assoc 1985;253:2854 – 2857.
[35] Maeiejko JJ, Holmes DR, Kottke BA, Zinsmeister AR, Dinh DM, Mao SJT. Apolipoprotein A-I as a marker of angiographi-cally assessed coronary artery disease. N Engl J Med 1983;309:385 – 9.
[36] Criqui MH, Cowan LD, Tyroler HA. Lipoproteins as med-iators for the effects of alcohol consumption and cigarette smoking on cardiovascular mortality: results from the Lipid Research Clinic’s follow-up study. Am J Epidemiol 1987;125:629 – 37.
[37] Lerner D, Kannel WP. Patterns of coronary heart disease morbidity and mortality in both sexes: a 26-year follow-up of Framingham population. Am Heart J 1986;111:383 – 90.
[38] Seppa¨ K, Lo¨f K, Sinclair D, Sillanaukee P. Hidden alcohol abuse among women. Br J Psychiatr 1994;164:544 – 6.
[39] Kristenson H, Trell E. Indicators of alcohol consumption, com-parison between a questionnaire (Mm-MAST), interviews and serum glutamyltransferase (GGT) in a health survey of middle-aged males. Br J Addict 1982;77:297 – 304.
[40] Mayfield D, McLeod G, Hall P. The CAGE questionnaire: validation of a new alcoholism screening instrument. Am J Psychiatr 1974;131:1121 – 3.
[41] Seppa¨ K, Koivula T, Sillanaukee P. Drinking habits and detec-tion of heavy drinking among middle-aged women. Br J Addict 1992;87:77 – 83.
[42] Stender S. Reference methods-with special reference to choles-terol. Scand J Lab Invest 1989;49:63 – 7.
[43] Cooper GR. Dextran sulfate-Mg2+ precipitation procedure for
quantitation of high-density-lipoprotein cholesterol. Clin Chem 1982;28:1379 – 88.
[44] Nguven T, Warnick RG. Improved method for separation of total HDL and subclasses. Clin Chem 1989;35:1086.
[45] Riepponen P, Marniemi J, Rautaoja T. Immunoturbidimetric determination of apolipoproteins A-1 and B in serum. Scand J Clin Lab Invest 1987;47:739 – 44.
[46] Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem 1972;18:499 – 502.
[47] Dixon WJ, editor. BMDP statistical software. University of California Press, Berkeley, 1996.
[48] O’Connor PG, Schottenfeld RS. Medical progree: patients with alcohol problems. N Engl J Med 1998;338:592 – 602.
[49] Huang C-M, Elin RJ, Ruddel M, Schmitz J, Linnoila M. The effect of alcohol withdrawal on serum concentration of Lp(a), apolipoproteins A1 and B, and lipids. Alcohol Clin Exp Res 1993;16:895 – 8.
[50] Criqui MH, Wallace RB, Heiss G, et al. Cigarette smoking and plasma high-density lipoprotein cholesterol. The Lipid Re-search Clinic Program Prevalence Study. Circulation 1980;62:70 – 6.
[51] Barter P. High-density lipoproteins and reverse cholesterol trans-port. Curr Opin Lipidol 1993;4:210 – 7.
[52] Ng DS, Hegele RA. High-density-lipoprotein cholesterol and atherosclerosis. Can Med Assoc J 1993;149:1807 – 8.
[53] Hein HO, Sorensen H, Suadicani P, Gyntelberg F. Alcohol consumption, Lewis phenotypes, and risk of ischaemic heart disease. Lancet 1993;341:392 – 6.
non-in-sulin-dependent diabetic and non-diabetic subjects. Am J Epi-demiol 1987;125:611 – 21.
[55] Razay G, Heaton KW, Bolton CH, Huges AO. Alcohol con-sumption and its relation to cardiovascular risk factors in British women. Br Med J 1992;304:80 – 3.
[56] Renaud S, deLorgeril M. Wine, alcohol, platelets and the French paradox for coronary heart disease. Lancet 1992;339:1523 – 6. [57] Frankel EN, Kanner J, German JB, Parks E, Kinsella JE.
Inhibition of oxidation of human low-density lipoprotein by phenolic substance in red wine. Lancet 1993;341:454 – 7.