Inhibition of endothelium-dependent arterial relaxation by oxidized
phosphatidylcholine
Yoshiyuki Rikitake
a, Ken-ichi Hirata
a, Seinosuke Kawashima
a, Nobutaka Inoue
a,
Hozuka Akita
a, Yuka Kawai
b, Yasuhito Nakagawa
b, Mitsuhiro Yokoyama
a,*
aThe First Department of Internal Medicine,Kobe Uni6ersity School of Medicine,7-5-1,Kusunoki-cho,Chuo-ku,Kobe 650-0017, Japan bSchool of Pharmaceutical Sciences,Kitasato Uni6ersity,Tokyo 108-0072, Japan
Received 14 January 1999; received in revised form 22 September 1999; accepted 3 November 1999
Abstract
Formation of oxidized phosphatidylcholine (ox-PC), oxidatively fragmented phosphatidylcholine (PC) containing a short-chain and/or polar oxidative residue at thesn-2 position, in the process of LDL oxidation as well as its existence in atherosclerotic lesions has been demonstrated. To clarify the pathophysiological role of ox-PC in the vascular reactivity, we investigated the effects of various ox-PCs on the isometric tensions in rabbit thoracic aortas. Ox-PCs, which were produced upon oxidation ofsn-2 polyunsaturated fatty acid (PUFA)-containing PCs, dose-dependently inhibited endothelium-dependent relaxation (EDR) evoked by acetylcholine or substance P. On the other hand, neither native PUFA-containing PCs nor an oxidative product of monounsaturated fatty acid-containing PC showed an inhibitory effect. None of ox-PCs affected endothelium-independent relaxation to nitroglycerin. The PC-headgroup fraction, but not the oxidized fatty acids fraction, was responsible for the inhibition of EDR by ox-PC. EDR was reduced by 2-(5-oxovaleroyl)-PC, one of the secondary oxidative products of PCs that contains a short chain aldehydic residue at thesn-2 position, but not by PC hydroperoxide, the primary oxidative product. Although the possibility could not be completely ruled out that lysophosphatidylcholine rather than ox-PC may be responsible for inhibitory effects on EDR, these results suggest a novel vascular activity of ox-PCs generated fromsn-2 PUFA-containing PCs which may be implicated in the pathophysiology of vascular tone. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Polyunsaturated fatty acid; Phospholipid; Oxidation; Endothelium-derived relaxing factor; Atherosclerosis
www.elsevier.com/locate/atherosclerosis
1. Introduction
The vascular endothelium releases nitric oxide (NO), which is considered as an endothelium-derived relaxing factor, and plays a key role in the regulation of vascular tone [1]. NO is considered as an antiatherogenic molecule because NO has bioactivities such as inhibi-tions of monocyte adhesion to endothelium [2], platelet aggregation [3], and smooth muscle cell proliferation [4]. In atherosclerosis and hypercholesterolemia, en-dothelial dysfunction including decreased NO release (reviewed in Ref. [5]) and NO inactivation via increases in superoxide production [6,7] has been demonstrated. The impairment of endothelium-dependent relaxation
(EDR) associated with reduced NO activity may facili-tate the atherogenic process.
Oxidized LDL has been implicated as a critical factor in atherosclerosis [8], and its presence in atherosclerotic lesions has been demonstrated [9]. Oxidative modifica-tion of LDL results in the conversion of phosphatidyl-choline (PC) to lysophosphatidylphosphatidyl-choline (LPC), which acts as an important mediator of the atherogenic effects of oxidized LDL, by intrinsic phospholipase A2(PLA2)
activity [10,11]. Oxidized LDL and LPC specifically interact with endothelial cells and promote alterations of a variety of endothelial functions including induction of gene expression of cytokines, adhesion molecules and growth factors [12 – 14]. Furthermore, we [15,16] and others [17,18] have clearly shown that oxidized LDL and LPC inhibit EDR. These observations suggest that oxidized LDL and LPC play a role in endothelial dysfunction in atherosclerotic arteries.
* Corresponding author. Tel.:+81-78-3825840; fax:+ 81-78-382-5858.
E-mail address:[email protected] (M. Yokoyama).
Most fatty acids exist in phospholipids and choles-terol esters in LDL. The first step in oxidative process of LDL is the formation of monohydroperoxy deriva-tives of fatty acids [19,20]. Peroxidation of fatty acids binding to PCs has been demonstrated in LDL oxida-tion, and hydroxy fatty acids and oxysterols have been found in human atheroma [21] and aortas of choles-terol-fed rabbits [22]. In particular, the polyunsaturated fatty acids (PUFAs) in phospholipids and cholesterol esters may be the initial sites of oxidative attack during modification of the LDL particle [23,24], and oxidation of PUFAs results in the alteration to short chain fatty acids. Recently, oxidized PC (ox-PC), oxidatively de-graded PCs containing short chain fatty acids which are formed from PUFA as a result of oxidative fragmenta-tion, has been identified in oxidized LDL [25] and human coronary atherosclerotic lesions by means of a monoclonal antibody against PC [26]. Although ox-PC has been shown to have a variety of bioactivities such as cytotoxicity [27], hypotensive effect [28,29], and platelet-activating factor (PAF)-like effects to stimulate platelets aggregation [30], neutrophil adhesion [31], and vascular smooth muscle cell proliferation [25] through the PAF receptor, the effect of ox-PC on vasomotor function of endothelium including EDR remains un-known. In the present study, we demonstrate that ox-PC inhibits EDR of rabbit thoracic aortas. This is the first report that has investigated the vascular reac-tivity of ox-PC and gives us a new insight to clarify the mechanism by which EDR is reduced in atherosclerotic arteries.
2. Materials and methods
2.1. Materials
1-Palmitoyl-2-linoleoyl-sn-glycero-3-phosphocholine (linoleoyl PC) and 1-palmitoyl-2-arachidonoyl-sn -glycero-3-phosphocholine (arachidonoyl PC) were pur-chased from Avanti Polar Lipids (Alabaster, AL). 1 - Palmitoyl - 2 - oleoyl -sn- glycero - 3 - phosphocholine (oleoyl PC) was from Sigma (St Louis, MO). All other chemicals were the best grade available from commer-cial sources.
2.2. Peroxidation of PCs
PCs were peroxidized by the method previously de-scribed [26,32]. Briefly, oleoyl PC, linoleoyl PC and arachidonoyl PC in a mixture of chloroform and methanol were placed in glass tubes and solvents were dried under a stream of N2 gas. Each PC was
sus-pended in phosphate-buffered saline (PBS) with a vor-tex mixer and the suspension was sonicated in a probe type sonicator for 30 s to make a 0.4-mM suspension.
Each PC in PBS was incubated with 40 mM FeSO4
and 0.4 mM ascorbic acid for 3 h at 37°C and the reaction was stopped by butylated hydroxytoluene (final 0.2 mM). Ox-PCs in PBS were diluted by Krebs bicarbonate buffer to make 1 – 20-mM suspension, added into organ baths and used for EDR experiment. To clarify the profiles of ox-PCs, we subjected the lipid extracts from oxidative product of arachidonoyl PC (ox-arachidonoyl PC) to thin-layer chromatography (TLC) (Silica gel G; Merck, Darmstadt, Germany) as described previously [34]. Ox-PC showed a broad spot corresponding between native arachidonoyl PC and the origin, which represented a significantly different pat-tern from a narrow single spot of native arachidonoyl PC or LPC (data not shown). TLC showed that there was only a weak zone in the LPC-corresponding area, suggesting that only a small amount of LPC was pro-duced in ox-PC products. In some experiments, the lipids were extracted from the LPC-corresponding zone by the method of Folch et al. [35] and tested on EDR. Ox-PC was generated on the day of measurement of EDR and immediately used in the experiments.
2.3. Fractionation of ox-PC by column chromatography
To further investigate whether PCs were responsible for the inhibitory effect of oxidative products of PUFA-containing PCs, we fractionated ox-arachi-donoyl PC into the PC-headgroup fraction and the other oxidized fatty acids fraction by silica gel column chromatography by the modified method of Hanahan et al. [36]. Total lipids of ox-arachidonoyl PC were extracted by the method of Bligh and Dyer [33] and the chloroform layer was evaporated under a stream of N2
gas. The extract was dissolved by a small amount of chloroform and applied to a silica gel column. The column was eluted sequentially three times with a column volume of chloroform (oxidized fatty acids fraction) and then three times with a column volume of chloroform/methanol, 1:3 (v/v) (PC-headgroup frac-tion). After drying under a stream of N2gas, lipids were
redissolved in Krebs bicarbonate buffer and tested on EDR.
2.4. Synthesis of PC hydroperoxide (PC-OOH)
was purified by TLC with a mixture of chloroform, methanol and water (5:10:0.5, v/v/v) as the mobile phase and extracted by the method of Bligh and Dyer [33].
2.5. Synthesis of ox-PC containing aldehydic residue
(PC-5CHO)
1-Palmitoyl-2-(5-oxovaleroyl)-sn -glycero-3-phospho-choline (2-(5-oxovaleroyl)-PC, PC-5CHO), one of the ox-PCs that contains aldehyde residues at the sn-2 position, was prepared by ozonolysis as described previ-ously [38]. Briefly, arachidonoyl PC was ozonized and a reductive work-up with dimethyl sulfide performed. Aldehydic PC was purified by TLC with a mixture of chloroform, methanol and water (10:5:1, v/v/v) as the mobile phase. The production of PC-5CHO was quan-titated by fluorometric HPLC [38]. PC-5CHO purified by TLC was reacted with a fluorescent reagent, 4-(N,N - dimethylaminosulfonyl)-7-hydrazino-2,1,3-benzoxadia-zole (Tokyo Kasei Kogyo, Tokyo, Japan). The fluores-cent derivatives of aldehydic PC were fractionated and quantitated by reversed-phase HPLC. The ozonolysis was complete in our experiments, and the purity of PC-5CHO was 100%. There were no contaminations such as C8, C11, and C14 derivatives.
2.6. Measurement of EDR
Strip rings were prepared from rabbits’ aortas as described previously [39], and all procedures were con-ducted according to the ‘Guidelines for Animal Experi-mentations at Kobe University School of Medicine’. Japanese white rabbits (2.0 – 2.5 kg) were anesthetized with pentobarbital sodium (30 mg/kg body wt i.v.), and the descending thoracic aortas were isolated and cleaned of surrounding tissue. Aortic rings approxi-mately 2-mm wide were cut and opened. Care was taken not to damage the endothelial surface. For iso-metric force measurements, transverse aortic strips were suspended in 30-ml organ baths containing Kreb’s bi-carbonate buffer of following composition (mM): NaCl 118, KCl 4.0, CaCl2 1.5, MgSO4 1.2, NaH2PO4 1.2,
NaHCO3 25 and glucose 5, and equilibrated at 37°C
with a 95% O2-5% CO2 gas mixture. One end of the
strip was attached to the bottom of the chamber, and the other end was attached to a transducer (Nihon-Ko-hden, Tokyo, Japan), which was connected to an am-plifier (Nihon-Kohden)/recorder (Nippon Denshi Kagaku, Kyoto, Japan) system. An initial preload of 1.5 g was applied, and the strips were allowed to stabilize for 90 min. A test contraction was induced by raising KCl concentration to 40 mM. When the devel-oped tension attained its peak value, the strips were relaxed by rinsing with Krebs buffer. Then the strips were contracted with phenylephrine (PE, 0.3 mM) and
subsequently relaxed by cumulative additions of acetyl-choline (ACh, final concentration 1 nM – 3 mM), sub-stance P (SP, 10 pM – 10 nM), or nitroglycerin (NTG, 0.1 nM – 3 mM). After wash-out and equilibration, the strips were incubated with selected concentrations of ox-PC for 30 min, and then the contraction-relaxation cycle was repeated as above. In all the experiments, butylated hydroxytoluene was added in a buffer (final 10 mM). In certain experiments, the endothelium was removed mechanically by rubbing the intimal surface with filter paper moistened with the buffer. Relaxation values were expressed as percent decreases of the PE-in-duced constrictor tone.
2.7. Statistical analysis
Data are expressed as mean9S.E.M. The signifi-cance of the difference between group means was ana-lyzed by one-way ANOVA and the Bonferroni test for samples. Values of PB0.05 were considered statisti-cally significant.
3. Results
3.1. Effects of ox-PCs on ACh-induced EDR
Representative tracings of responses to ACh in rabbit aortic strips incubated with or without ox-PCs are shown in Fig. 1A. We confirmed that ox-PCs by them-selves altered neither the resting tension nor PE-elicited contraction (1.4890.05 vs. 1.4790.03 g, mean9
S.E.M., n=6, before versus after exposure to 20 mM ox-arachidonoyl PC). The tracing showed reduced re-laxation to ACh after incubation with ox-linoleoyl PC (center), but not with native linoleoyl-PC (right). As shown in Fig. 1B, the inhibitory effects of oxidative products of PUFA-containing PCs were reversible, as wash-out of oxidative products of PUFA-containing PCs with Krebs buffer containing 0.1% bovine serum albumin restored ACh-induced EDR (maximum relax-ation was 73.892.0 vs. 63.793.2%, mean9S.E.M., n=5, before exposure to ox-PCs versus after wash-out), indicating that the functional integrity of endothe-lium was preserved after exposure to ox-PCs.
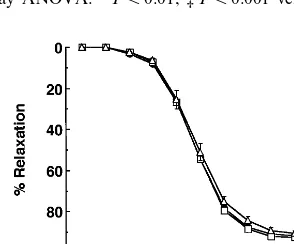
The incubation of the strips with oxidative residues of PUFA-containing PCs, namely ox-linoleoyl PC (Fig. 2A) and ox-arachidonoyl PC (Fig. 2B), significantly inhibited EDR to ACh in a dose-dependent manner. Relaxation to ACh (1 mM) was 71.193.2% in control, whereas it was reduced to 22.193.4 and 14.592.9% by ox-linoleoyl PC and ox-arachidonoyl PC (mean9
Fig. 1. (A) Representative tracings of responses to ACh in rabbit aortic strips incubated with or without ox-PC. The strips were contracted with phenylephrine (0.3mM), and subsequently EDR was induced by cumulative additions of ACh (1 nM – 3mM). In comparison with control (left),
the strip showed reduced relaxation to ACh after the incubation with 20mM ox-linoleoyl PC (center), but not with 20mM native linoleoyl PC
(right). (B) Representative tracings of responses to ACh in rabbit aortic strips incubated with or after wash-out of ox-PC. After the contraction-relaxation cycle (left), the strip was incubated with 20mM ox-arachidonoyl PC (center), and then the contraction-relaxation cycle was
repeated. After extensive washing of the strip with Krebs buffer containing 0.1% bovine serum albumin, the contraction-relaxation cycle was repeated (right). PE, phenylephrine; W, wash-out.
Fig. 2. Effects of oxidative products of sn-2 PUFA-containing PCs, sn-2 saturated fatty acid-containing PCs, or monounsaturated fatty acid-containing PCs on ACh-induced EDR. Rabbit aortic strips were incubated either with ox-linoleoyl PC (A), ox-arachidonoyl PC (B), or ox-oleoyl PC (C) at concentrations of 1mM (open square), 10mM (open triangle) or 20mM (open circle), or without PC (control, closed circle)
for 30 min in Krebs buffer. The strips were contracted with 0.3mM PE, and subsequently EDR was induced by cumulative additions of ACh (1
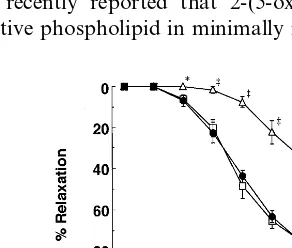
Fig. 3. Effects of ox-PCs on SP-induced EDR. Rabbit aortic strips were incubated with 20mM ox-oleoyl PC (open triangle), ox-linoleoyl
PC (open circle), or ox-arachidonoyl PC (open square), or without PC (control, closed circle) for 30 min in Krebs buffer. The strips were contracted with 0.3mM PE, and subsequently EDR was induced by
cumulative additions of SP (10 pM – 10 nM). Symbols represent the mean percent of relaxation9S.E.M. (n=6 – 8). Data were analyzed using one-way ANOVA: *PB0.01, ‡PB0.001 versus control.
3.3. Effects of ox-PCs on endothelium-independent relaxation to NTG
In an additional series of experiments, to determine whether the dilator capacity of smooth muscle was altered after exposure to ox-PCs, we studied the effects of oxidative products of PUFA-containing PCs on en-dothelium-independent relaxation induced by NTG (0.1 nM – 3mM). In these series of experiments, the endothe-lium was removed mechanically by rubbing the intimal surface with filter paper moistened with the buffer. As shown in Fig. 4, NTG-induced relaxation was not impaired in arteries after incubation with oxidative products of PUFA-containing PCs, indicating that the dilator function of smooth muscle was not influenced by ox-PCs.
3.4. Effects of fractions of oxidati6e product of
arachidonoyl PC on ACh-induced EDR
During oxidation of PC, a PUFA at thesn-2 position of PC is degraded to a short-chain fatty acid, resulting in the production of oxidized free fatty acids which are known to have some bioactivity. For example, F2
-iso-prostanes, a family of prostaglandins produced by free radical-catalyzed peroxidation of arachidonoyl PC, have been reported to act as a vasoconstrictor [40]. Therefore, to examine whether degraded PCs or oxi-dized fatty acids were responsible for the inhibitory effects of oxidative products of PUFA-containing PCs, we fractionated ox-arachidonoyl PC into the PC-head-group fraction and the other oxidized fatty acids frac-tion by silica gel column chromatography. As shown in Fig. 5, incubation with the PC-headgroup fraction, but not with the oxidized fatty acids fraction, resulted in the inhibition of EDR. Furthermore, lipids from the LPC-corresponding zone of a silica gel plate with appli-cation of 20 mM ox-arachidonoyl PC or 2 – 20mM LPC which have inhibitory effects on EDR, were extracted [15]. The fraction obtained after TLC of LPC inhibited ACh-induced EDR, whereas ACh-induced EDR was not inhibited by the LPC fraction obtained after TLC of ox-arachidonoyl PC (data not shown). The findings indicated that the amount of LPC synthesis in our experiments was not enough to inhibit EDR. Thus, the impairment of EDR by oxidative products of PUFA-containing PCs was conceivably caused by degraded PCs, but not oxidized fatty acids or LPC.
3.5. Effects of PC-OOH and PC-5CHO on
ACh-induced EDR
Peroxidation of PUFA-containing PCs generates PC hydroperoxide, a primary oxidative product of PC [41]. Oxidative breakdown of PC hydroperoxide provides various ox-PCs that contain short-chain carbonyl,
alde-Fig. 4. Effects of oxidative products of PUFA-containing PCs on endothelium-independent relaxation to NTG. Rabbit aortic strips whose endothelium was removed, were preincubated with 20 mM
ox-linoleoyl PC (open square), or ox-arachidonoyl PC (open trian-gle), or without PC (control, closed circle) for 30 min in Krebs buffer. The strips were contracted with 0.3mM PE, and subsequently
en-dothelium-independent relaxation was induced by cumulative addi-tions of NTG (0.1 nM – 3mM). Symbols represent the mean percent of relaxation9S.E.M. for five independent experiments. Data were analyzed using one-way ANOVA:P=NS versus control.
monounsaturated fatty acid-containing PC, have an inhibitory effect on EDR.
3.2. Effects of ox-PCs on SP-induced EDR
Fig. 5. Effects of fractions of ox-arachidonoyl PC on ACh-induced EDR. Ox-arachidonoyl PC was subjected to silica gel column chromatography and fractionated into the PC-headgroup fraction and the oxidized fatty acids fraction as described in Section 2. After incubation with the PC-headgroup fraction or the oxidized fatty acids fraction, the strips were contracted with 0.3mM PE, and subsequently EDR was induced by
cumulative additions of ACh (1 nM – 3mM). In comparison with control (left), representative tracings showed reduced relaxation to ACh after
incubation with the PC-headgroup fraction (center), but not with the oxidized fatty acids fraction (right) of ox-arachidonoyl PC. PE, phenylephrine; W, wash-out.
hydic, or carboxyl moieties as secondary products. To further investigate whether the inhibition of EDR by ox-PCs is caused by the primary oxidative product or the secondary short-chain ox-PC, we tested the effects of incubations of the strips with 5OOH and PC-5CHO on EDR evoked by ACh. PC-PC-5CHO (2-(5-oxo-valeroyl)-PC), which contains a short chain aldehydic residues at the sn-2 position and has been reported to exist in ox-arachidonoyl PC [25,30,31], reduced EDR (Fig. 6), however, PC-OOH did not inhibit EDR. These results demonstrated for the first time that ox-PC con-tainingsn-2 short chain fatty acid, a secondary oxida-tive product, impaired EDR, whereas EDR was not changed by PC-OOH, a primary product. This result suggests that the secondary oxidative products includ-ing aldehydic ox-PCs rather than PC hydroperoxide, the primary product, may play a role in the inhibition of EDR by oxidized PUFA-containing PCs.
4. Discussion
The present study demonstrates for the first time that oxidative products of sn-2 PUFA-containing PCs, namely ox-linoleoyl PC and ox-arachidonoyl PC, in-hibit EDR in rabbit aortic strips. However, EDR was reduced by neither ox-oleoyl PC nor native PCs. In addition, this inhibitory effect of oxidative products of PUFA-containing PCs was caused by the lipids in PC fraction, but not oxidized fatty acids or LPC existing in the oxidative products of PUFA-containing PCs in our experiments. Moreover, ox-PC with ansn-2 short chain aldehyde, but not PC hydroperoxide, attenuated EDR. Therefore, we conclude that oxidatively degraded PCs reduce EDR and may contribute to the impaired vaso-motion in atherosclerotic arteries.
During oxidative modification of LDL, PUFA-con-taining PCs change to PC hydroperoxide, a primary product, and further oxidative modification causes the
alteration of PUFAs to short chain fatty acids. This modification results in formation of four kinds of PCs with a short-chain dicarboxylate, dicarboxylate semi-aldehyde, monocarboxylate or v
-hydroxymonocar-boxylate moiety, as secondary oxidative products [34]. For instance, previous reports showed that 2-azelaoyl PC [27], ansn-2 dicarboxylate group, is generated from 2-linoleoyl PC, and 2-(4-hydroxynonenal)PC [42] and (5-oxovaleroyl)PC [43 – 45] are generated from 2-arachidonoyl PC. 2-(5-oxovaleroyl)PC (PC-5CHO), used in our experiment as a secondary oxidative product, has been reported to exist in oxidized LDL [25] and to have atherogenic effects to stimulate platelets aggregation [30], neutrophil adhesion [31], and vascular smooth muscle cell proliferation [25]. Ho¨rkko¨ et al. [46] have recently demonstrated that 2-(5-oxo-valeroyl)PC is an important ligand for the recognition of oxidized LDL by macrophages. Watson et al. [47] have recently reported that 2-(5-oxovaleroyl)PC is a bioactive phospholipid in minimally modified LDL and
Fig. 6. Effects of PC-OOH and PC-5CHO on ACh-induced EDR. PC-OOH and PC-5CHO were prepared as described in Section 2. Rabbit aortic strips were incubated with 20 mM PC-OOH (open
square), PC-5CHO (open triangle), or without PC (control, closed circle) for 30 min in Krebs buffer. Relaxations were assessed as described above. Symbols represent the mean percent of relaxation9
S.E.M. (n=5). Data were analyzed using one-way ANOVA: *PB
is identified in aortas of atherogenic diet-fed rabbits. In the present study, oxidative products of sn-2 PUFA-containing PCs as well as PC-5CHO inhibited EDR, whereas neither native PUFA-containing PCs nor PC-OOH, a primary oxidative product, reduced EDR. We also confirmed that the presence of PC-5CHO in ox-arachidonoyl PC by fluorometric HPLC (data not shown), suggesting that PUFAs at the sn-2 position changed to short chain residues during oxida-tive modification of PCs. However, PC-5CHO occu-pies a relatively small constituent of ox-arachidonoyl PC products. In the present study, ox-arachidonoyl PC is more potent than PC-5CHO (Fig. 2B vs. Fig. 6). Since ox-arachidonoyl PC contains diverse degraded PCs other than PC-5CHO, this result suggests that oxidatively degraded products which are more potent than PC-5CHO may be generated in ox-arachidonoyl PC. In addition, it is also suggested that these de-graded PCs may also have weak inhibitory effects individually, but they may additionally or synergisti-cally augment the effect of PC-5CHO or themselves. Because oxidatively modified PCs besides PC-5CHO may have diverse profound effects on vascular en-dothelium, it is unlikely that only PC-5CHO is respon-sible for the inhibitory effects of ox-PCs. Therefore, although PC-5CHO is likely to be a participant in reduced EDR by oxidizedsn-2 PUFA-containing PCs, other secondary oxidative products including PCs with a short-chain dicarboxylate or monocarboxylate may also play a role in the ox-PC-induced inhibition of EDR. The reason why ox-oleoyl PC did not inhibit EDR may be due to the low late of conversion of a monounsaturated fatty acid to a short chain residue during oxidative modification in our experiment.
We consider that LPC is unlikely to be responsible for the impaired EDR by oxidative products of PUFA-containing PCs because only a small amount of LPC seemed to exist in the oxidative products of PUFA-containing PCs and the LPC fraction obtained after TLC of ox-arachidonoyl PC did not inhibit ACh-induced EDR (data not shown). However, the possi-bility that ox-PCs may be hydrolyzed to LPC by PLA2
or PAF acetylhydrolase activities of the endothelium in situ could not be excluded in the present study.
Several mechanisms by which ox-PC inhibited EDR are considered. It may be possible that peroxy radicals or other reactive oxygen intermediates are induced by ox-PCs in endothelial cells. However, n-acetylcysteine (1 mM), an antioxidant, did not block the inhibitory effects of ox-PCs on EDR (data not shown), suggest-ing that reactive oxygen intermediates are unlikely to be involved in the impairment of EDR by ox-PC. In some cell types, ox-PC may function via the PAF receptor since the bioactivities of ox-PC including 2-(5-oxovaleroyl)PC are blocked by the PAF receptor antagonist or diminished by the PAF acetylhydrolase
[25,31]. A previous study showed that PAF attenuates EDR in canine coronary artery both in vivo and in vitro at the concentrations of 0.1 – 1 nM [48]. Thus, it appears that actions via the PAF receptor may be one potential mechanism of the impairment of EDR by ox-PC. However, this possibility is unlikely in the present study on the basis of the following observa-tions. PAF inhibited EDR at rather high concentra-tions of at least several micromoles per liter, and CV-6209 (10 nM), a specific and powerful PAF recep-tor antagonist, did not block ox-PC-induced impair-ment of EDR (data not shown). These results suggest that the inhibition of EDR by ox-PC is not mediated via the PAF receptor in rabbit thoracic aortas.
The third possibility is that ox-PC may cause the impairment of receptor-mediated intracellular signaling like LPC. Flavahan has shown that LPC inhibits a pertussis toxin-sensitive G protein-dependent signaling pathway in porcine endothelial cells [5]. We have pre-viously demonstrated that LPC inhibits the increase in G protein coupled receptor-mediated calcium mobi-lization in cultured and intact endothelial cells [49,50]. Amphiphilic compounds such as LPC may modulate the activity of membrane-bound enzymes through the increased membrane fluidity and permeability as a re-sult of the alteration of lipid composition of the cell membrane. It is speculated that the inhibitory effect of ox-PC may be related to changes in the endothelial membrane fluidity, which may alter the kinetics of membrane-bound proteins and lead to the impairment of membrane-associated signaling. However, further investigations about the effects of ox-PC on intracellu-lar signal transduction and NO production in endothe-lial cells are required to clarify the mechanisms of the inhibitory effect of ox-PC on EDR.
In conclusion, ox-PCs generated from PCs contain-ing PUFA at the sn-2 position as well as ox-PC with an sn-2 short chain fatty acid inhibited EDR. It is likely that, in addition to LPC, ox-PC is one of the plausible causes of the impaired EDR in atheroscle-rotic arteries. In addition to stimulation of smooth muscle cell growth and neutrophil activation, ox-PC may play a role in the progression and pathophysio-logical conditions in atherosclerosis through the inhibi-tion of bioactivity of endothelium-derived relaxing factor.
Acknowledgements
References
[1] Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature 1980;288:373 – 6.
[2] Bath PMW, Hassall DG, Gladwin A-M, Palmer RMJ, Martin JF. Nitric oxide and prostacyclin: divergence of inhibitory effects on monocyte chemotaxis and adhesion to endothelium in vitro. Arterioscler Thromb 1991;11:254 – 60.
[3] Yao S, Ober JC, Krishnaswami AK, Ferguson JJ, Anderson HV, Golino P, et al. Endogenous nitric oxide protects against platelet aggregation and cyclic flow variations in stenosed and endothelium-injured arteries. Circulation 1992;86:1302 – 9. [4] Garg UC, Hassid A. Nitric oxide-generating vasodilators and
8-bromo-cyclic guanosine monophosphate inhibit mitogenesis and proliferation of cultured rat vascular smooth muscle cells. J Clin Invest 1989;83:1774 – 7.
[5] Flavahan NA. Atherosclerosis or lipoprotein-induced endothelial dysfunction: possible mechanisms underlying reduction in EDRF/nitric oxide activity. Circulation 1992;85:1927 – 38. [6] Ohara Y, Peterson TE, Sayegh HS, Subramanian RR, Wilcox
JN, Harrison DG. Dietary correction of hypercholesterolemia in the rabbit normalizes endothelial superoxide anion production. Circulation 1995;92:898 – 903.
[7] Ohara Y, Peterson TE, Harrison DG. Hypercholesterolemia increases endothelial superoxide anion production. J Clin Invest 1993;91:2546 – 51.
[8] Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL. Beyond cholesterol: modifications of low-density lipoprotein that increase its atherogenicity. New Engl J Med 1989;320:915 – 24. [9] Yla¨-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S,
Carew TE, Butler S, et al. Evidence for the presence of oxida-tively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest 1989;84:1086 – 95.
[10] Steinbrecher UP, Parthasarathy S, Leake DS, Witztum JL, Steinberg D. Modification of low density lipoprotein by endothe-lial cells involves lipid peroxidation and degradation of low density lipoprotein phospholipids. Proc Natl Acad Sci USA 1984;81:3883 – 7.
[11] Steinbrecher UP. Oxidation of human low density lipoproteins results in derivatization of lysine residues of apolipoproteins B by lipid peroxide decomposition products. J Biol Chem 1987;262:3603 – 8.
[12] Rajavashisth TA, Andalibi A, Territo MC, Berliner JA, Navab M, Fogelman MA, et al. Induction of endothelial cell expression of granulocyte and macrophage colony-stimulating factors by modified low-density lipoproteins. Nature 1990;344:160 – 2. [13] Kume N, Cybulsky MI, Gimbrone MA Jr.
Lysophosphatidyl-choline, a component of atherogenic lipoproteins, induces mononuclear leukocyte adhesion molecules in cultured human and rabbit arterial endothelial cells. J Clin Invest 1992;90:1138 – 44.
[14] Kume N, Gimbrone MA Jr. Lysophosphatidylcholine transcrip-tionally induces growth factor gene expression in cultured hu-man endothelial cells. J Clin Invest 1994;93:907 – 11.
[15] Yokoyama M, Hirata K, Miyake R, Akita H, Ishikawa Y, Fukuzaki H. Lysophosphatidylcholine: essential role in the inhi-bition of endothelium-dependent vasorelaxation by oxidized low density lipoprotein. Biochem Biophys Res Commun 1990;168:301 – 8.
[16] Matsuda Y, Hirata K, Inoue N, Suematsu M, Kawashima S, Akita H, et al. High density lipoprotein reverses inhibitory effect of oxidized low density lipoprotein on endothelium-dependent arterial relaxation. Circ Res 1993;72:1103 – 9.
[17] Kugiyama M, Kerns SA, Morrisett JD, Roberts R, Henry PD. Impairment of endothelium-dependent arterial relaxation by
lysolecithin in modified low-density lipoproteins. Nature 1990;344:160 – 2.
[18] Mangin EL Jr, Kugiyama K, Nguy JH, Kerns SA, Henry PD. Effects of lysolipids and oxidatively modified low density lipo-protein on endothelium-dependent relaxation of rabbit aorta. Circ Res 1993;72:161 – 6.
[19] Lenz ML, Hughes H, Mitchell JR, Uia DP, Guyton JR, Taylor AA, et al. Lipid hydroperoxy and hydroxy derivatives in copper-catalyzed oxidation of low density lipoprotein. J Lipid Res 1990;31:1043 – 50.
[20] Wang T, Yu W-G, Powell WS. Formation of monohydroxy derivatives of arachidonic acid, linoleic acid, and oleic acid during oxidation of low density lipoprotein by copper ions and endothelial cells. J Lipid Res 1992;33:525 – 37.
[21] Carpenter KL, Taylor SE, Ballantine JA, Fussell B, Halliwell B, Mitchinson MJ. Lipids and oxidised lipids in human atheroma and normal aorta. Biochim Biophys Acta 1993;1167:121 – 30. [22] Wang T, Powell WS. Increased levels of monohydroxy
metabo-lites of arachidonic acid and linoleic acid in LDL and aorta from atherosclerotic rabbits. Biochim Biophys Acta 1991;1084:129 – 38.
[23] Steinbrecher UP, Lougheed UPM, Kwan WC, Dirks M. Recog-nition of oxidized low density lipoprotein by the scavenger receptor of macrophages results from derivatization of apolipo-protein B by products of fatty acid peroxidation. J Biol Chem 1989;264:15216 – 23.
[24] Esterbauer H, Ju¨rgens G, Quehenberger O, Kollar E. Autoxida-tion of human low density lipoprotein: loss of polyunsaturated fatty acids and vitamin E and generation of aldehydes. J Lipid Res 1987;28:495 – 509.
[25] Heery JM, Kozak M, Stafforini DM, Jones DA, Zimmerman GA, McIntyre TM, et al. Oxidatively modified LDL contains phospholipids with platelet-activating factor-like activity and stimulates the growth of smooth muscle cells. J Clin Invest 1995;96:2322 – 30.
[26] Itabe H, Takeshima E, Iwasaki H, Kimura J, Yoshida Y, Imanaka T, et al. A monoclonal antibody against oxidized lipoprotein recognizes foam cells in atherosclerotic lesions. J Biol Chem 1994;269:15274 – 9.
[27] Itabe H, Kushi Y, Handa S, Inoue K. Identification of 2-aze-laoylphosphatidylcholine as one of the cytotoxic products gener-ated during oxyhemoglobin-induced peroxidation of phosphatidylcholine. Biochim Biophys Acta 1988;962:8 – 15. [28] Tsukatani H, Yamada S, Takauchi K, Tokumura A, Tatsumichi
H, Kumegawa K. Physico-chemical characteristics of D-I, a hypotensive factor occurring in acetone extract of the bovine brain. Chem Pharm Bull Tokyo 1978;26:3281 – 8.
[29] Yoshida J, Tokumura A, Fukuzawa K, Terao M, Takauchi K, Tsukatani H. A platelet-aggregating and hypotensive phospho-lipid isolated from bovine brain. J Pharm Pharmacol 1986;38:878 – 82.
[30] Tanaka T, Iimori M, Tsukatani H, Tokumura A. Platelet-aggre-gating effects of platelet-activating factor-like phospholipids formed by oxidation of phosphatidylcholines containing an sn-2-polyunsaturated fatty acyl group. Biochim Biophys Acta 1994;1210:202 – 8.
[31] Smiley PL, Stremler KE, Prescott SM, Zimmerman GA, McIn-tyre TM. Oxidatively fragmented phosphatidylcholines activate human neutrophils through the receptor for platelet-activating factor. J Biol Chem 1991;266:11104 – 10.
[32] Itabe H, Yamamoto H, Suzuki M, Kawai Y, Nakagawa Y, Suzuki A, et al. Oxidized phosphatidylcholines that modify proteins. Analysis by monoclonal antibody against oxidized low density lipoprotein. J Biol Chem 1996;271:33208 – 17.
[34] Tanaka T, Minamino H, Unezaki S, Tsukatani H, Tokumura A. Formation of platelet-activating factor-like phospholipids by Fe2+/ascorbate/EDTA-induced lipid peroxidation. Biochim Bio-phys Acta 1993;1166:264 – 74.
[35] Folch J, Lees M, Sloane Stanley GH. A simple method for the isolation and purification of total lipids from animal tissue. J Biol Chem 1957;226:497 – 509.
[36] Hanahan DJ, Dittmer JC, Warashina E. A column chromato-graphic separation of classes of phospholipids. J Biol Chem 1957;228:685 – 700.
[37] Therond P, Couturier M, Demelier JF, Lemonnier F. Simulta-neous determination of the main molecular species of soybean phosphatidylcholine or phosphatidylethanolamine and their cor-responding hydroperoxides obtained by lipoxygenase treatment. Lipids 1993;28:245 – 9.
[38] Ou Z, Ogamo A, Guo L, Konda Y, Harigaya Y, Nakagawa Y. Identification and quantitation of choline glycerophospholipids that contain aldehyde residues by fluorometric high-performance liquid chromatography. Anal Biochem 1995;227:289 – 94. [39] Yokoyama M, Akita H, Mizutani T, Fukuzaki H, Watanabe Y.
Hyperreactivity of coronary arterial smooth muscles in response to ergonovine from rabbits with hereditary hyperlipidemia. Circ Res 1983;53:63 – 71.
[40] Takahashi K, Nammour TM, Fukunaga M, Ebert J, Morrow JD, Roberts LJ II, et al. Glomerular actions of a free radical-generated novel prostaglandin, 8-epi-prostaglandin F2a, in the
rat. J Clin Invest 1992;90:136 – 41.
[41] Yamamoto Y, Niki E, Kamiya Y, Eguchi J, Shimasaki H. Oxidation of biological membranes and its inhibition. Free radi-cal chain oxidation of erythrocyte ghost membranes by oxygen. Biochim Biophys Acta 1985;819:29 – 36.
[42] Esterbauer H, Benedetti A, Lang J, Fulceri R, Fauler G, Com-porti M. Studies on the mechanism of formation of 4-hydrox-ynonenal during microsomal lipid peroxidation. Biochim
Biophys Acta 1986;876:154 – 66.
[43] Stremler KE, Stafforini DM, Prescott SM, Zimmerman GA, McIntyre TM. An oxidized derivative of phosphatidylcholine is a substrate for the platelet-activating factor acetylhydrolase from human plasma. J Biol Chem 1989;264:5331 – 4.
[44] Stremler KE, Stafforini DM, Prescott SM, Zimmerman GA, McIntyre TM. Human plasma PAF acetylhydrolase: oxidatively-fragmented phospholipids as substrate. J Biol Chem 1991;266:11095 – 103.
[45] Patel KD, Zimmerman GA, Prescott SM, McIntyre TM. Novel leukocyte agonists are released by endothelial cells exposed to peroxide. J Biol Chem 1992;267:15168 – 75.
[46] Ho¨rkko¨ S, Bird DA, Miller E, Itabe H, Leitinger N, Sub-banagounder G, et al. Monoclonal autoantibodies specific for oxidized phospholipids or oxidized phospholipid-protein adducts inhibit macrophage uptake of oxidized low-density lipoproteins. J Clin Invest 1999;103:117 – 28.
[47] Watson AD, Leitinger N, Navab M, Faull KF, Ho¨rkko¨ S, Witztum JL, et al. Structual identification by mass spectrometry of oxidized phospholipids in minimally oxidized low density lipoprotein that induce monocyte/endothelial interactions and evidence for their presence in vivo. J Biol Chem 1997;272:13597 – 607.
[48] DeFily DV, Kuo L, Chilian WM. PAF attenuates endothelium-dependent coronary arteriolar vasodilation. Am J Physiol 1996;270:H2094 – 9.
[49] Inoue N, Hirata K, Yamada M, Hamamori Y, Matsuda Y, Akita H, et al. Lysophosphatidylcholine inhibits bradykinin-in-duced phosphoinositide hydrolysis and calcium transients in cultured endothelial cells. Circ Res 1992;71:1410 – 21.
[50] Miwa Y, Hirata K, Kawashima S, Akita H, Yokoyama M. Lysophosphatidylcholine inhibits receptor-mediated Ca2+ mobi-lization in intact endothelial cells of rabbit aorta. Arterioscler Thromb Vasc Biol 1997;17:1561 – 7.