www.elsevier.comrlocateranireprosci
What is stress, and how does it affect reproduction?
Hilary Dobson
), R.F. Smith
Department of Veterinary Clinical Science and Animal Husbandry, UniÕersity of LiÕerpool, Leahurst, Neston,
Wirral, CH64 7TE, UK
Abstract
Stress is revealed by the inability of an animal to cope with its environment, a phenomenon that is often reflected in a failure to achieve genetic potential. Field data from dairy cows show that stressors such as milk fever or lameness increase the calving to conception interval by 13–14 days, and an extra 0.5 inseminations are required per conception. We suggest that a variety of endocrine regulatory points exist whereby stress limits the efficiency of reproduction. Transport
Ž .
produces an immediate constant increase in arginine vasopressin AVP and
corticotrophin-releas-Ž . Ž .
ing hormone CRH secretion in ewes, but adrenocorticotrophic hormone ACTH reaches a maximum in the first hour while cortisol is highest during the second hour. In contrast, after an
Ž .
insulin injection, the hypothalamo–pituitary–adrenal HPA response is delayed occurring only after glucose decreases below a threshold. Changes in AVP, CRH and ACTH each follow a similar time course, but eventually the secretion of AVP and CRH decreases while glucose is still at a nadir. Negative feedback effects appear to operate mainly at the pituitary level during transport but at the hypothalamus during hypoglycaemia.
We also have endocrine evidence to show that stressors interfere with precise timings of reproductive hormone release within the follicular phase. Transport, or insulin, reduce the frequency and amplitude of gonadotrophin-releasing hormone and LH pulses, suggesting that these stressors exert effects at the hypothalamus or higher centres in the brain. Both stressors also
Ž .
delay the onset of the luteinising hormone LH surge. Preliminary results suggest that opioids mediate these effects but progesteronerglucocorticoid receptors are not involved because the antagonist, RU486, is unable to reverse insulin-induced delays in the LH surge. There is also evidence to support effects at pituitary level because exogenous ACTH, or transport, reduce the amount of LH released by challenges with GnRH. The reduction in endogenous GnRHrLH secretion ultimately deprives the ovarian follicle of adequate gonadotrophin support leading to reduced oestradiol production by slower growing follicles. Thus, there is a level of interference by stressors at the ovary. Reproduction is such an important physiological system that animals have
)Corresponding author.
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved.
Ž .
to ensure that they can respond to their surroundings; thus, it is advantageous to have several protein mechanisms, i.e. at higher brain, hypothalamus, pituitary and target gland levels. However, when pushed too far, subfertility occurs.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Stress; Reproduction; Subfertility
1. Introduction
‘‘Stress’’ is responsible for many things, including subfertility. Many agricultural advisers and veterinarians are very familiar with those intangible factors that reduce fertility on farms but often they are unable to pinpoint precise contributory causes — and hence blame ‘‘stress’’.
This, in itself, provides a definition of ‘‘stress’’, that is, the inability of an animal to cope with its environment, a phenomenon that is revealed by a failure to achieve genetic potential, e.g. for growth rate, milk yield, disease resistance, or fertility.
2. Field observations
Strong evidence that stressors affect reproductive efficiency in dairy cattle has been gained by comparing fertility data of normal cows and herd-mates suffering from
Ž .
various stressful clinical conditions Table 1 .
Furthermore, evidence of a social stressor affecting fertility has been provided by a behavioural study, which identified cows that changed social position in the herd hierarchy within the breeding period. Those cows that increased social status were more fertile and had better milk production figures than those with a lowering of social status
ŽTable 2; Dobson, unpublished data . They also had a different lameness score on a 0–5.
point scale, with 5.0 representing a very lame animal.
Unfortunately, the clinical conditions in Table 1 and social interactions exemplified in Table 2 are very common in the dairy industry. Worse than that, these factors are
Table 1
Summary of fertility parameters of dairy cows with clinical disease conditions diagnosed and treated in the
Ž .
postpartum period data selected from Borsberry and Dobson, 1989; Collick et al., 1989 . Each diseased cow was compared with a similar untreated healthy herd-mate
Ž . Ž .
Pairs Ca — 1st service days Ca — conception days Inseminations per conception Control Diseased Control Diseased Control Diseased
)
Lameness 427 68 72 86 100 1.7 2.1
Table 2
Summary of fertility and milk production figures for 45 pairs of cows that displayed increasing or decreasing
Ž .
social status based on dominance and submissiveness during the breeding period in three commercial dairy herds
Change in social status
Increase Decrease
) Ž .
Calving to conception days 97 143
Inseminations per conception 1.6 2.2
Somatic cell counts ’000rml y18 q371
)
Difference in lameness score y0.21 q0.54
) P-0.05.
clearly hindering the genetic progress of one of the major domesticated species in the world. No doubt similar data can be compiled for other commercially important species. If we are to avoid paying this price for domestication of any species, it is necessary to learn more about how animals respond to stressors, and how this affects the mechanisms controlling reproductive efficiency.
3. Examination of control mechanisms
Studying the effects of stress on reproduction is beset with difficulties. The complex nature of some stressors in the modern farm environment simultaneously exposes animals to several different stimuli. Furthermore, there is considerable variability between individuals in response to a given stimulus. Added to this, is the overriding importance of the reproductive system to pass genes on to the next generation. This last issue means that animals have developed several strategies to cope with environmental problems including alternative responses to compensate for failure of any part of the protection mechanism.
In brief, we hypothesise that there are several regulatory by which stressors regulate reproductive mechanisms. Endocrine systems appear to be an ideal way of coordinating this regulation throughout the whole body. In order to unravel the complexity of stress-induced subfertility, it is necessary to study the reaction to stressors of repeatable severity, firstly by examining responses to clearly defined stimuli, and then by investi-gating the influence on reproductive mechanisms.
4. Responses to specific stressful stimuli
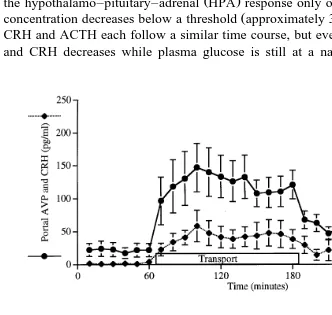
The combined physical and psychological stimulus of 2 h transport in a vehicle
Ž .
produces an immediate and constant increase in both arginine vasopressin AVP and
Ž .
corticotrophin-releasing hormone CRH concentrations in hypophyseal-portal blood of
Ž .
ewes, but the adrenocorticotrophic hormone ACTH response reaches a maximum in
Ž
the first hour while cortisol concentrations are highest during the second hour Fig. 1;
.
Smith et al., 1997 . This suggests that the input into the hypothalamus is constant during
Ž .
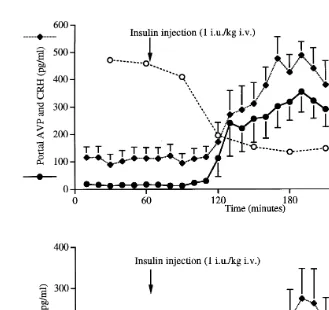
this stimulus, even though the final output measured as plasma cortisol changes during the stimulus. Insulin-induced hypoglycaemia, a physiological stimulus that does not require cognitive processing, produces a different response. After an insulin injection,
Ž .
the hypothalamo–pituitary–adrenal HPA response only occurs after the blood glucose
Ž .
concentration decreases below a threshold approximately 3 mmolrl . Changes in AVP,
CRH and ACTH each follow a similar time course, but eventually the secretion of AVP
Ž .
and CRH decreases while plasma glucose is still at a nadir Fig. 2 . Plasma cortisol
Fig. 2. HPA responses to insulin-induced hypoglycaemia. Mean and S.E.M. of data obtained from three ewes treated on four occasions at two week intervals.
concentrations remain elevated due to the long half-life of this steroid. In this case,
Ž .
during the prolonged presence of a stressful input low glucose concentration , there is a
decrease in hypothalamic AVPrCRH.
To limit over-stimulation of the stress axis and its deleterious effects, there are mechanisms to control the HPA including cortisol negative feedback effects at hypotha-lamic andror pituitary levels to restrict on-going responses to a stimulus. However, an
Ž .
opposite balancing mechanism facilitation also occurs within the HPA so that the
Ž .
responses are not totally inhibited Dallman et al., 1992 .
From the above evidence comparing responses to transport and insulin, we suggest that negative feedback effects operate mainly at the pituitary level during transport and at the hypothalamus during hypoglycaemia. However, these differences could be
inherent in the pituitary responses to different concentrations and ratios of AVPrCRH
secreted in response to different stimuli.
longer intervals, there is no reduction in total cortisol response. However, if transport is
Ž
repeated daily, some ewes have reduced responses after four trips Smith and Dobson,
.
unpublished observation . Individual differences in response may be due to prenatal or
Ž .
early life experiences Lay et al., 1997; Liu et al., 1997 or genetic background
ŽRomeyer and Bouissou, 1992 ..
Our working hypothesis is that the time-course of a response varies at each level of the system and depends on the nature of the stimulus. The corresponding time-course of the stress effect on the reproductive system may indicate which precise components of
Ž .
the HPA are important in the interaction with the hypothalamo–pituitary–ovarian HPO axis.
5. How do stressful stimuli affect reproduction?
In the follicular phase of a normal oestrous cycle, the correct pattern of
go-Ž .
nadotrophin-releasing hormone GnRH secretion from the hypothalamus leads to
Ž . Ž
increased pulsatile release of luteinising hormone LH from the pituitary gland Moenter
.
et al., 1990 . In concert with follicle stimulating hormone, this dictates the rate of follicular growth and oestradiol production, ultimately leading to a preovulatory LH
Ž .
surge and ovulation McNeilly et al., 1991 .
In order to achieve a perfectly timed LH surge, a series of closely controlled events must occur within the hypothalamus and pituitary gland. After removal of the
suppres-Ž .
sive effects of progesterone during luteolysis, GnRH and thus LH pulses are secreted with increasing frequency, to culminate eventually in continuous secretion at the onset
Ž
of the LH surge in response to the positive-feedback effects of oestradiol Evans et al.,
.
1995 .
In view of the complications incurred with repeatability, habituation and duration of stressors as already high-lighted, these aspects have to be standardised as much as possible when examining the influence of stress responses on physiological mechanisms such as reproduction. Furthermore, the effects of more than one stressor must be investigated in order to avoid the dangers inherent with stressor-specific artefacts. However, in spite of some differences between stress responses discussed above, there are some quite surprisingly consistent effects on reproductive endocrinology.
From a series of experiments conducted over the past 5 years, we suggest that stressors reduce fertility by interfering with the mechanisms that regulate the precise
Ž
timings of events within the follicular phase. Acute stressors either transport or
.
hypoglycaemia imposed at precisely defined times have been investigated for effects on different parts of the reproductive control mechanism.
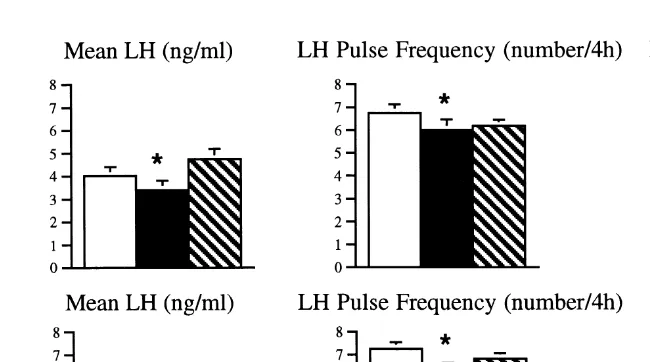
Transport for 4 or 8 h reduces the frequency and amplitude of LH pulses especially within the first few hours in ovariectomised ewes or intact animals in the late follicular
Ž .
phase Dobson et al., 1999b; Phogat et al., 1999b . Similar effects have been observed
Ž .
Fig. 3. Mean LH concentration, LH pulse frequency and amplitude in 10 ovariectomised ewes for 4-h periods
Ž . Ž . Ž . Ž . Ž
before open bars , during filled bars and after hatched bars insulin 2 IUrkg; top panels or transport 4 h;
. ) Ž .
lower panels . denotes significantly different P-0.05 from pretreatment value.
amplitude could either be mediated by the hypothalamus, or at pituitary level. Direct proof of the suppressive effects of an acute stressor on GnRH secretion has been
Ž .
provided by Battaglia et al. 1997 after endotoxin administration.
In addition, there is evidence from both in vitro perifusions and in vivo experiments to show that exogenously increased ACTH concentrations or transport reduce the
Ž
amount of LH released by challenges with small doses of GnRH Phogat et al., 1997,
.
1999a,b . This provides support for additional effects at pituitary level.
Clearly, activation of the hypothalamus–pituitary–adrenal axis by stressors reduces
the pulsatility of GnRHrLH by actions at both the hypothalamus and pituitary gland,
ultimately depriving the ovarian follicle of adequate LH support. This will lead to reduced oestradiol production by slower growing follicles. Such a hypothesis is sup-ported by the marked decrease in oestradiol secretion observed after reducing the frequency of exogenous LH pulses driving follicular growth in an ovarian autotransplant
Ž .
model Dobson et al., 1999a .
A combination of the above effects on LH pulsatility at hypothalamic and pituitary levels no doubt contributes to the delay and reduced magnitude of the LH surge observed after transport or insulin administration in the follicular phase just prior to the
Ž .
expected LH surge Dobson et al., 1999c; Table 3 . This effect on the LH surge control mechanism could be exerted directly via an influence of GnRH on production of its own receptors, or indirectly by the induced reduction in oestradiol which, in turn, will alter the balance of systems controlling LH surge release. Thus, another level of interference
Žat the ovary has been revealed to play a part in the multi-centred effects of stress on.
reproductive control mechanisms.
Table 3
Time of onset of the LH surge relative to progesterone withdrawal in intact control ewes and those treated with insulin, with or without additional naloxone or RU486
Ž .
Treatment Onset of LH surge hours after P withdrawal
a
Significantly different from insulin alone group P-0.05 .
b Ž .
Significantly different from control group P-0.05 .
of two neurotransmitter regulators, and the preliminary results are very interesting. Infusion of the opioid antagonist, naloxone just before insulin administration prevented
Ž .
the delay in the onset of the LH surge observed after insulin alone Table 3 . This clearly implicates opioids in the mediation of stress-induced changes in LH secretion.
Furthermore, it would appear that progesteronerglucocorticoid receptors are not
in-volved in the interaction between the stress and reproductive axes because the antagonist
Ž .
RU486 was unable to reverse the insulin-induced delays in the LH surge Table 3 .
6. The link between stress-induced low LH pulse frequency and cases of subfertility
Within the growing follicle, the oocyte maintains direct contact with granulosa cells
Ž .
by means of cellular projections through the zona pellucida Moor et al., 1980 . Thus, events influencing the integrity of follicular function can have direct effects on oocyte viability. These effects are not always immediately obvious, for example, it is known that mRNAs are laid down in the oocyte nucleus but not translated until the 8-cell stage
Ž .
of conceptus development Staigmiller and Moor, 1984 . Consequently, any event that changes granulosa cell activity may influence pregnancy rates. Indeed, Mihm et al.
Ž1994 have provided evidence for reduced pregnancy rates after prolonging the duration.
of the follicular phase by artificially delaying the onset of the LH surge.
It is envisaged that in some situations, such as during the chronic stress of more
severe lameness or fever, the pulse GnRHrLH frequency will be so slow that initial
follicular growth will occur but will be unable to continue in to the later stages that depend on faster pulse frequencies. Thus, the animal fails to maintain oestrous cycles and the consequent anoestrus is easily recognised by veterinarians.
In slightly less stressful situations, GnRHrLH pulse frequency may be just fast
enough to support follicular growth, but because it is on a knife-edge, it will be susceptible to interruption or variation by otherwise innocuous stimuli. In this case, the integrity of granulosa cells and thus the oocyte may be compromised, and although oestrus and fertilisation may occur, the conceptus will fail to develop into a pregnancy. This is reflected in the insidious idiopathic subfertility recognised by agricultural advisers and veterinarians.
GnRH priming of the pituitary andror adequate oestradiol production. Hence, an inappropriate LH surge is generated and, as it is unable to cause ovulation and luteinisation, the follicle persists to produce the clinically recognised cystic ovarian syndrome.
7. Conclusion
In evolutionary terms, progression from single cell organisms to the complexity of mammals has required the development of communication systems throughout the body via which regulatory mechanisms are exerted. Intracellular mechanisms provide a basis for contact within individual cells, an endocrine system working through the blood circulatory system provides another level of control in more complex animals, and a further level of control exists via the nervous system, masterminded by centres in the brain.
Reproduction is a very important physiological system for the furtherance of a species, and this has to succeed despite the imposition of sometimes detrimental environmental stimuli. To ensure that an animal can respond to its surroundings, it is advantageous to have several lines of defence as exemplified above by the different levels of stress response, i.e. higher brain, hypothalamus, pituitary and adrenal glands. Likewise each of these responses has influence on the different levels of the reproduc-tive organisation, i.e. higher brain, hypothalamus, pituitary and gonads. Such diverse methods of control to ensure the success of a species might also have the advantage of energy conservation, so that less detrimental stressors may be dealt with at one level,
Ž .
while more severe circumstances or several additive situations may require responses at many levels.
The continued genetic development of several species, carried out to meet Man’s needs, especially for food production, is now meeting a stumbling block in that fertility appears to be reducing. For example, conception rates in high-producing dairy cattle are thought to be declining. Similarly, in some human situations, increased levels of ‘‘stress’’ lead to an inability to reproduce. Are all these animals trying to tell us something?
Acknowledgements
The authors are grateful for the collaboration of many colleagues who have worked in the Reproduction-Stress Research Group in the University of Liverpool Faculty of Veterinary Science. We are also grateful for discussions with Gerald Lincoln from Edinburgh.
References
Borsberry, S., Dobson, H., 1989. Periparturient diseases and their effect on reproductive performance in five dairy herds. Vet. Rec. 124, 217–219.
Collick, D.W., Ward, W.R., Dobson, H., 1989. Associations between types of lameness and fertility. Vet. Rec. 125, 103–106.
Dallman, M.F., Akana, S.F., Scribner, K.A., Bradbury, M.J., Walker, C.-D., Strack, A.M., Cascio, C.S., 1992. Stress, feedback and facilitation in the hypothalamo–pituitary–adrenal axis. J. Neuroendocrinol. 4, 517–526.
Dobson, H., Campbell, B.K., Scaramuzzi, R.J., Baird, D.T., 1999a. Effect of reducing LH pulse frequency and amplitude on ovarian oestradiol production in the ewe. In: 5th International Symposium on Reproduction
Ž .
in Domestic Ruminants. J. Reprod. Fertil., Suppl. 54, in press .
Ž .
Dobson, H., Tebble, J.E., Ozturk, M., Smith, R.F., 1999b. J. Reprod. Fertil., in press .
Dobson, H., Tebble, J.E., Phogat, J.B., Smith, R.F., 1999c. Effect of transport on pulsatile and surge secretion of LH in ewes in the breeding season. J. Reprod. Fertil. 116, 1–8.
Evans, N.P., Dahl, G.E., Mauger, D., Karsch, F.J., 1995. Estradiol induces both qualitative and quantitative changes in the pattern of gonadotropin-releasing hormone secretion during the presurge period in the ewe. Endocrinology 136, 1603–1609.
Lay, D.C., Randel, R.D., Friend, T.H., Carroll, J.A., Welsh, T.H., Jenkins, O.C., Neuendorff, D.A., Bushong, D.M., 1997. Effects of prenatal stress on the fetal calf. Domest. Anim. Endocrinol. 14, 73–80.
Liu, D., Diorio, J., Tannenbaum, B., Caldji, C., Francis, D., Freedman, A., Sharma, S., Pearson, D., 1997. Maternal care, hippocampal glucocorticoid receptors, and hypothalamic–pituitary–adrenal responses to stress. Science 277, 1659–1662.
McNeilly, A.S., Picton, H.M., Campbell, B.K., Baird, D.T., 1991. Gonadotrophic control of follicle growth in the ewe. J. Reprod. Fertil. 92, 177–186.
Mihm, M., Baguisi, A., Boland, M.P., Roche, J.F., 1994. Association between the duration of dominance of the ovulatory follicle and pregnancy rate in beef heifers. J. Reprod. Fertil. 102, 123–130.
Moenter, S.M., Caraty, A., Karsch, F.J., 1990. The estradiol-induced surge of gonadotropin-releasing hormone in the ewe. Endocrinology 127, 1375–1384.
Moor, R.M., Smith, M.W., Dawson, R.M.C., 1980. Measurement of intercellular coupling between oocytes and cumulus cells using intracellular markers. Exp. Cell Res. 126, 15–29.
Phogat, J.B., Smith, R.F., Dobson, H., 1997. Effect of adrenocorticotrophic hormone on gonadotrophin-releas-ing hormone-induced luteinizgonadotrophin-releas-ing hormone secretion in vitro. Anim. Reprod. Sci. 48, 53–65.
Ž Ž ..
Phogat, J.B., Smith, R.F., Dobson, H., 1999a. Effect of adrenocorticotrophic hormone ACTH 1–24 on ovine pituitary gland responsiveness to exogenous pulsatile GnRH and oestradiol-induced LH release in vivo. Anim. Reprod. Sci. 55, 193–203.
Phogat, J.B., Smith, R.F., Dobson, H., 1999b. Effect of transport on pituitary responsiveness to exogenous pulsatile GnRH and oestradiol-induced LH release in intact ewes. J. Reprod. Fertil. 116, 9–18.
Romeyer, A., Bouissou, M.F., 1992. Assessment of fear reactions in domestic sheep, and influence of breed and rearing conditions. Appl. Anim. Behav. Sci. 34, 1–2.
Smith, R.F., Gore, S.W., Phogat, P.B., Dobson, H., 1997. The psychological stress of transport stimulates both CRF and AVP secretion into hypophysial portal blood. J. Endocrinol. 152, P172, Suppl.