Ž .
Animal Reproduction Science 60–61 2000 511–525
www.elsevier.comrlocateranireprosci
ž
X/
Effect of an inverse subtropical 19
8
13 N
photoperiod on ovarian activity, melatonin and
prolactin secretion in Pelibuey ewes
C. Cerna, A. Porras, M.J. Valencia, G. Perera, L. Zarco
)Departamento de Reproduccion, Facultad de Medicina Veterinaria y Zootecnia, Uni´ Õersidad Nacional
Autonoma de Mexico, Apartado Postal 22-256, Mexico, D.F. 14000, Mexico´ ´
Abstract
Twenty-one Pelibuey ewes were used from December 21, 1996 to December 21, 1998. Fourteen of them had never been exposed to artificial photoperiod, and they were maintained on natural photoperiod until March 21, 1997, when they were assigned to natural photoperiod Žcontrol group, ns8 or to inverse photoperiod n. Ž s6 . The other seven animals had been kept.
Ž .
on a long photoperiod 16L:8D from October 21, 1996 to December 21, 1996, when they entered the present study and were subjected to a gradual decrease in photoperiod, so that they reached an
Ž .
equinox photoperiod 12L:12 D on March 21, 1997. At that time, they were assigned to natural
Ž . Ž .
photoperiod ns3 or to inverse photoperiod ns4 . Blood samples for progesterone determina-tion were taken twice a week from all the animals. During the second year of the study, prolactin was measured in the samples from five animals in inverse photoperiod and from five control ewes. Hourly samples were obtained to determine the 24-h melatonin profile of five animals from each group on September 21, 1997, December 21, 1997, March 21, 1997, and June 21, 1997. Exposure to inverse photoperiod resulted in a gradual shift on the annual reproductive cycle, so that the second ovulatory season was advanced by 5 months in the ewes kept on inverse photoperiod as
Ž .
compared to the control ewes P-0.05 . There were wide variations in the dates for the onset and the end of the ovulatory season within the inverse photoperiod groups, and three animals in this groups maintained ovulatory activity for at least 18 consecutive months. The duration of
Ž .
melatonin secretion was directly related to the length of the dark period P-0.05 , and this response was not affected by the calendar date. Prolactin concentrations were directly related to daylength, however, they were also affected by calendar date, being lower in the inverse group as compared to the corresponding time of the annual photoperiodic cycle of ewes on natural photoperiod. It is concluded that reproductive activity, melatonin secretion and prolactin secretion
)Corresponding author. Tel.:q52-56-22-58-83; fax:q52-73-80-10-52.
Ž .
E-mail address: [email protected] L. Zarco .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
( ) C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525
512
of Pelibuey ewes respond to the small variations in photoperiod that are present at 19813XN, and that under natural conditions, photoperiod appears to be the main regulator of ovarian activity at this latitude. However, other factors such as temperature or humidity may act as modulators, and their relative importance could increase at more equatorial latitudes.q2000 Elsevier Science B.V.
All rights reserved.
Keywords: Pelibuey sheep; Reproductive seasonality; Photoperiod; Subtropics
1. Introduction
Photoperiod is the main environmental factor that regulates seasonal breeding in Ž
sheep and goats from northern latitudes Yeates, 1949; Thwaites, 1965; Karsch, 1984; .
Chemineau et al., 1988 . It has been considered that the effects of photoperiod on
Ž .
reproductive activity decrease in lower latitudes Hafez, 1952 , so that seasonal repro-Ž
duction is absent or greatly reduced in tropical or subtropical regions Carles and .
Kipngeno, 1986; Eloy et al., 1990 , or that if present, it is mainly due to seasonal
Ž .
variations in food availability Gonzalez et al., 1991; Chemineau et al., 1995
´
In the case of Pelibuey sheep, a breed of hair sheep of African origin, initial studies Ž
conducted in Mexico suggested that reproductive seasonality was absent Castillo et al., .
1972; Valencia et al., 1975 . Some degree of seasonality was later accepted, but it was
Ž .
attributed to variations in food availability Gonzalez et al., 1991; Cruz et al., 1994 ,
´
Ž .
temperature or humidity Gonzalez et al., 1992 . However, evidences were soon found
´
that the reduced ovarian activity that occurs during spring is independent from
nutri-Ž .
tional status Rodrıguez-Maltos et al., 1992; Velazquez et al., 1995; Martınez, 1998 .
´
´
´
In the first direct studies of the effects of artificial photoperiod on Pelibuey sheep, we
Ž .
found that it strongly influenced ovarian activity in intact ewes Porras et al., 1998 and
Ž .
LH profiles in ovariectomized ewes implanted with oestradiol Porras, 1999 . However,
Ž .
the alternate artificial photoperiods used in those studies were classic long 16L:8D or
Ž .
short 8L:16D ones, which are extremes that are not naturally present at the latitudes where Pelibuey sheep are raised. Thus, the objective of this study was to evaluate if the ovarian activity of Pelibuey ewes is affected by variations in photoperiod that mimic those found in tropical and subtropical regions of Mexico, where the difference between the longest and the shortest day of the year is only about 2 h. In addition, 24-h melatonin profiles were characterized at different times of the year in order to evaluate the pineal response to small changes in photoperiod, and the annual profiles of prolactin were characterized in order to evaluate the transduction of the photoperiodic signal to other physiological end points.
2. Materials and methods
The study was conducted from December 21, 1996 to December 21, 1998, on a farm
( )
C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525 513
been exposed to artificial photoperiod, and they were maintained on natural photoperiod Ž
until March 21, 1997, when they were assigned to natural photoperiod control group,
. Ž .
ns8 or to inverse photoperiod ns6 . The other seven ewes had been on a previous
Ž . Ž .
experiment Porras et al., 1999 , and had been kept on long photoperiod 16L:8D from October 21 to December 21, 1996, when they entered the present study and were exposed to a gradual decrease in photoperiod until reaching an equinox photoperiod
Ž12L:12D on March 21, 1997, when they were assigned to natural n. Ž s3 or inverse.
Ž .
photoperiod for the rest of the study ns4 .
Independently of their previous photoperiodic history, starting on March 21, 1997, all the ewes assigned to natural photoperiod were exposed to the photoperiod prevalent at
19813XN, while those on inverse photoperiod were exposed to a daily ligth:dark relation
Ž .
opposite to that occurring naturally Yeates, 1949 . The photoperiodic treatments were
given in well ventilated, 7=3 m chambers isolated from natural light and provided with
Ž
artificial light at an intensity of 350 lx at the height of the animal’s head Legan and
. Ž
Karsch, 1980 . Lights were turned on and off as required by a digital switch Tork EW .
101, Mexico City that allowed for daily adjustments. In both groups, the length of the longest day of the year was 13 h 6 min, and that of the shortest one was 10 h 54 min, as
Ž .
occurs naturally at this latitude Muhlia and Chavez, 1980 . The difference between the
´
longest and the shortest day was 2 h 12 min.
The animals were isolated from males throughout the experiment, and they were maintained on a constant plane of nutrition using oat hay, alfalfa hay, corn silage and concentrate. Heparinized blood samples were obtained from all the ewes twice a week. The samples were immediately centrifuged and the plasma was separated and kept
frozen aty208C until assayed for progesterone using a solid-phase radioimmunoassay
ŽPulido et al., 1991 . Sensitivity of the assay was 0.15 ng. rml; intra- and inter-assay
variation coefficients were 5.0% and 9.5%, respectively. Prolactin concentrations were determined in the samples from the second year of the study of five ewes on natural photoperiod and five ewes on inverse photoperiod. A homologous radioimmunoassay
Ž .
was used Porras, 1999 . The sensitivity of the assay was 0.25 ngrml, the intra- and
inter-assay coefficients were 5.8% and 12.5%, respectively.
Blood samples for melatonin determination were obtained from five ewes on natural photoperiod and five ewes on inverse photoperiod on September 21, 1997, December 21, 1997, March 21, 1998, and June 21, 1998,. Each time the sampling protocol consisted of a 24-h period during which samples were obtained every hour, except 2 h before and after the lights were turned on or off, when the intervals were reduced to 30
Ž . Ž .
min. Red light 1 lx was used for sampling during dark periods Malpaux et al., 1987 .
Ž .
The assay used Malpaux et al., 1987 had a sensitivity of 4 pgrml; intra- and
inter-assay variation coefficients were 7.5% and 13.7%. The duration of the nightly melatonin elevation was defined as the period during which its concentrations were
above 16 pgrml, i.e. four times the sensitivity of the assay.
Ovulation was assumed to occur 4 days before the first of two or more consecutive
Ž .
samples with progesterone concentrations over 1 ngrml Rodrıguez-Maltos et al., 1992 .
´
A luteal phase was comprised by all the consecutive samples above 1 ngrml. An
()
Mean dates for the onset and the end of the first ovulatory season and for the onset of the second ovulatory season in Pelibuey ewes maintained under natural or inverse photoperiod, according to their previous photoperiodic history
Values are mean date"standard error.
Ž . Ž . Ž .
For a given variable row , values with different literal superscripts a, b, c are statistically different P-0.05 .
Ewes without previous exposure to artificial photoperiod Ewes with previous exposure to artificial photoperiod
Ž . Ž . Ž .
Natural photoperiod Inverse photoperiod ns6 Natural photoperiod ns3 Inverse photoperiod ns4
Žcontrol group, ns8. First oÕulatory season
c b a a
Ž . Ž . Ž . Ž . Ž .
Onset date July 31, 1997 "5.8 days June 16, 1997 "7.0 days April 3, 1997 "7.5 days March 22, 1997 "13.5 days
b a ) c a ) )
Ž . Ž . Ž . Ž . Ž .
End date February 21, 1998 "6.7 days November 19, 1997 "31.4 days March 15, 1998 "2.3 days December 25, 1997 "26.5 days
Second oÕulatory season
b a ) b a ) )
Ž . Ž . Ž . Ž . Ž .
Onset date July 19, 1998 "7.0 days March 4, 1998 "7.6 days July 27, 1998 "17.2 days February 8, 1998 "32.3 days )
Excludes two ewes with a first ovulatory season that extended until the end of the study. ) )
()
Interval from longest day to first ovulation, from shortest day to last ovulation, and duration of the ovulatory and anovulatory season in Pelibuey ewes exposed to different photoperiodic treatments
Values are mean"standard error.
Ž . Ž . Ž .
For a given variable row , values that do not share at least one literal superscripts a, b, c are different P-0.05 .
Ewes without previous exposure Ewes with previous exposure to artificial photoperiod to artificial photoperiod
Natural photoperiod Inverse photoperiod Natural photoperiod Inverse photoperiod
Žcontrol group, ns8. Žns6. Žns3. Žns4.
Interval day from longest day to first ovulation 40"6 87"6 83"7 71"13
Ž .
Length day of first ovulatory season 204"9 )289"88 345"10 286"32
b ab ) b a ) )
Does not include two animals that continued ovulating until the end of the study. ) )
Does not include one ewe that continued ovulating until it died. ) ) )
( ) C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525
516
the day of the first ovulation and to finish with the end of the last luteal phase. An anovulatory season was defined as a period of 20 or more days with basal progesterone concentrations.
Ž .
An analysis of variance that considered the effects of photoperiod natural or inverse
Ž .
and the previous history with or without previous exposure to artificial photoperiod
( )
C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525 517
was used to compare the dates for the onset and end of each ovulatory season, the intervals from the longest day to first ovulation and from the shortest day to the last ovulation, as well as the duration of the first ovulatory season, and that of the anovulatory season. The duration of melatonin elevations was compared by a two-way
Ž .
analysis of variance treatment and date . Prolactin concentrations were compared using an analysis of variance that considered the effects of treatment and month, with the animal nested within the treatment.
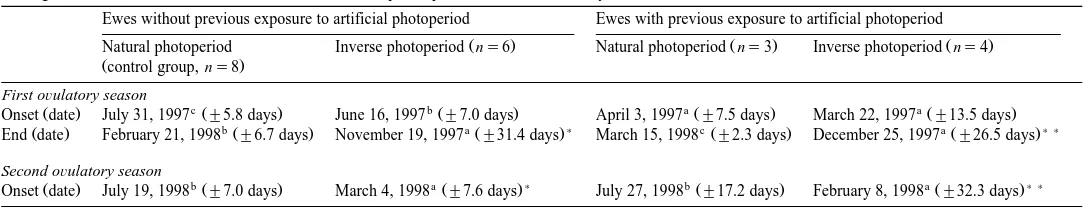
Fig. 2. Individual ovulatory seasons in Pelibuey ewes that had been previously exposed to 3 months of long
Ž16L:8D photoperiod before gradually reducing it starting on December 22, 1996 in order to reach an equinox. Ž X . photoperiod on March 21, 1997. Thereafter, the ewes were assigned to natural or inverse 19813 N photoperiod. Each bar represents an ovulatory season for the ewe identified by the number at the left. Ewe N8
( ) C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525
518
3. Results
Table 1 shows that the ewes that were kept on natural photoperiod but had been previously exposed to a long photoperiod initiated their first ovulatory season at a
Ž . Ž .
significantly earlier date April 3 than those in the control group July 31 . However, as shown on Table 2, they required a significantly longer period of exposure to decreasing
Ž .
photoperiod before their first ovulation 83"7.5 days than the ewes in the control
Ž .
group 40"5.8 . The first ovulatory season of the ewes that were transferred from long
to natural photoperiod ended only 3 weeks after that of control ewes, despite having
Fig. 3. Mean melatonin concentrations during 24-h periods on four different dates in Pelibuey ewes maintained
Ž X .
( )
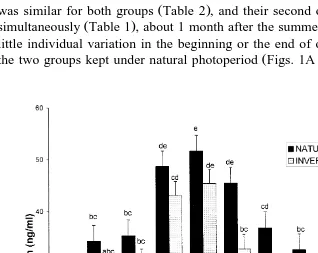
C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525 519 Table 3
Duration of night-time melatonin elevations on the dates of the natural equinoxes and solstices in Pelibuey ewes kept under natural or inverse photoperiod
Values are mean date"standard error.
Ž . Ž .
Different literals superscripts a, b, c indicate significant differences P-0.05 .
Ž .
Date Duration of melatonin elevation h
Natural photoperiod Inverse photoperiod
b b
September 21–22, 1997 11.3"0.4 11.7"0.2
c a
December 21–22, 1997 12.3"0.3 9.5"0.4
b b
March 21–22, 1998 11.0"0.1 11.1"0.1
a c
June 21–22, 1998 9.7"0.2 12.3"0.1
Ž .
started with a difference of 4 months Table 1 . The duration of the anovulatory season
Ž .
was similar for both groups Table 2 , and their second ovulatory season began almost
Ž . Ž .
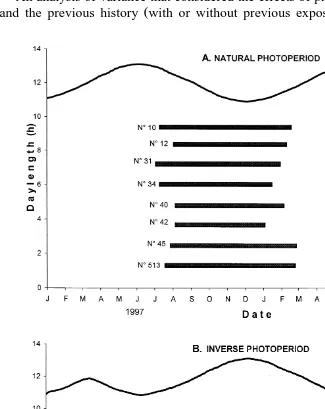
simultaneously Table 1 , about 1 month after the summer solstice Table 2 . There was little individual variation in the beginning or the end of ovarian activity within each of
Ž .
the two groups kept under natural photoperiod Figs. 1A and 2A .
Fig. 4. Average monthly prolactin concentrations in Pelibuey ewes kept under normal or inverse photoperiod
Ž X .
19813 N . Data are normalized to the longest day of the year for each treatment. Month 0 is June for the natural photoperiod group and December for the inverse photoperiod group. Values are mean"standard error.
Ž .
( ) C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525
520
Exposure to inverse photoperiod caused a gradual shift on the annual reproductive Ž
cycle, so that in each inverse group with or without previous exposure to artificial .
photoperiod , the first ovulatory season ended about 3 months before that in its corresponding group kept on natural photoperiod, while the second ovulatory season was
Ž .
advanced about 5 months in the inverse groups Table 1 . There was little individual variation in the starting dates of the first period of ovarian activity within each of the two groups kept under inverse photoperiod. However, both the end of the first ovulatory season and the start of the second one showed ample variation within each of these
Ž .
groups Figs. 1B and 2B . Two ewes kept on inverse photoperiod continued ovulating throughout the experimental period, at the end of which they had been continuously
Ž .
cyclic for more than 18 months Fig. 1B . Another animal was continuously cyclic for
Ž .
19 months before it died from causes not related to the study Fig. 2B .
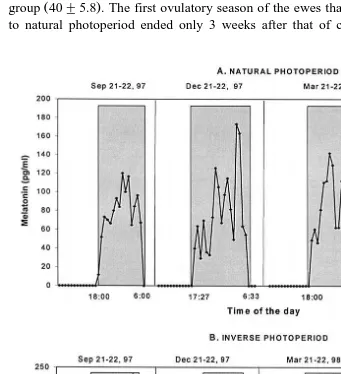
Fig. 3 shows the profiles of melatonin on four different dates in the ewes kept on natural or inverse photoperiod. Table 3 shows that the duration of the night-time
Ž .
elevations were significantly different P-0.05 between groups during the solstices
ŽDecember and June , but not during the equinoxes September and March . Mean. Ž .
melatonin concentration during the night-time elevations was 101.3"18.2 pgrml, with
no differences between groups or dates.
Fig. 4 shows the mean prolactin concentrations during the second year of the study, normalized to the longest day, month 0 being June for the natural photoperiod group and December for the inverse group. The prolactin profile of ewes kept on inverse photoperiod was 6 months out-of-phase in relation to that of the control group. In both
Ž .
groups, the concentrations of the hormone were highly correlated P-0.01 with the
duration of the day, but this correlation was higher for the ewes kept on natural
Ž . Ž .
photoperiod rs0.61 than for those on inverse photoperiod rs0.48 . Prolactin
concentrations always tended to be higher in the ewes on natural photoperiod than in
Ž .
those on inverse photoperiod. However, the differences were only significant P-0.05
during the month with the longest day, and 1 and 5 months after the longest day.
4. Discussion
The present study is the first one to demonstrate that the relatively small variation in
Ž . X
day length 2 h and 12 min that occurs during the year at 19813 N, can be translated by
Pelibuey sheep into significant changes in the duration of melatonin secretion, strongly affecting the breeding season and prolactin profiles of ewes kept on inverse photoperiod. In ewes without a previous history of exposure to artificial photoperiod, the mean dates for both the onset and the end of ovarian activity were significantly different in the animals exposed to inverse photoperiod than in those kept on natural photoperiod. Although the difference in the date of onset of ovarian activity between these groups
Ž .
was much larger during the second year than during the first one Table 1 , this was due to the fact that in 1997, the photoperiod in the inverse group started to decrease on
Ž .
March 22, whereas in 1998 the reduction started on December 22 Fig. 1 .
Reproductive seasonality in sheep from temperate regions is regulated by the change Ž
( )
C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525 521
. Ž
and Karsch, 1987; Malpaux et al., 1989 , or by the rate of daily change Chemineau et .
al., 1992, 1995 . This appears to be the case also in Pelibuey ewes, since in all the groups exposed to artificial photoperiod, either before or during the experiment, ovulatory activity started on average after 2 or 3 months of exposure to a decreasing photoperiod, independently of the length of the longest day from which this reduction
Ž .
started Table 2 or of the daylength that was present on the date of the first ovulation.
Ž .
Furthermore, Porras 1999 found a similar period of latency when Pelibuey ewes were
Ž .
abruptly transferred to a short 8L:16D photoperiod after 3 months of exposure to a
Ž .
long one 16L:8D .
The 50 to 90 days that elapsed from the start of a gradual photoperiod reduction to the onset of ovarian activity in the ewes kept on inverse photoperiod, as well as during the first year in those animals transferred from an artificially long photoperiod to natural photoperiod, is similar to the interval from the summer solstice to the onset of
Ž reproductive activity that occurs in many breeds of sheep in temperate regions Hafez,
.
1952; Robinson et al., 1992 . However, this was not the case in the Pelibuey ewes that
Ž .
were always kept on natural photoperiod control group , whose ovarian activity on both
Ž .
years started quite rapidly average of 30 to 40 days after the longest day, with some individuals ovulating few days after the summer solstice, especially during the second
Ž .
year Fig. 1A . This ability to start their natural ovulatory season around the time of the summer solstice, or even slightly before it, appears to be usual in Pelibuey ewes
ŽMartınez, 1998; Porras, 1999 , and since it does not conform to the norm for other
´
.types of sheep, it has been used to suggest that the reproductive seasonality of Pelibuey sheep is not driven by photoperiod, but rather by the increased forage availability derived from the rainy season, which in Mexico starts on late May or early June
ŽGonzalez et al., 1991; Cruz et al., 1994 . However, since the direct effect of photope-
´
.riod on reproductive activity of Pelibuey ewes has been clearly demonstrated in the
Ž .
present study, as well as by Porras 1999 , it would seem likely that under natural photoperiod the rapid onset of ovarian activity after the summer solstice in Pelibuey ewes is due to refractoriness to long days, rather than by exposure to short days
ŽRobinson et al., 1985 . If this is the case, refractoriness would naturally occur in.
Pelibuey sheep after 5 to 6 months of exposure to increasing photoperiod. This would
Ž .
explain why the animals exposed to only 3 months of long 16L:8D photoperiod needed
Ž . Ž .
more time 60 to 90 days to start ovulating after an abrupt Porras, 1999 or gradual
Žanimals with previous exposure to artificial photoperiod in this study shift to short.
photoperiod than the time required by the ewes kept on natural photoperiod.
Refractoriness to long days appears to have acted in all ewes kept on natural
Ž .
photoperiod for several months before the onset of ovarian activity Figs. 1A and 2A . In contrast, the ovulatory season did not start shortly after the longest day in most of the ewes exposed to inverse photoperiod, and some of them only ovulated after several
Ž .
months of decreasing photoperiod Figs. 1B and 2B , suggesting that there is an
Ž .
endogenous circannual rhythm Malpaux et al., 1989 that needs more than 1 year to adjust to an inverse photoperiod, or that there are other factors that may modulate the effects of photoperiod.
( ) C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525
522
Ž .
more had very short anovulatory periods around 35 days , situations that never occurred in the animals kept on natural photoperiod. Furthermore, the variation on the dates at which the anovulatory periods started and ended was much wider among the ewes on inverse photoperiod than among those on natural photoperiod, which suggests a conflict between the information conveyed by the inverse photoperiod and that derived from other sources. This conflict does not appear to be important when Pelibuey ewes are
Ž . Ž . Ž .
exposed to extreme long 16L:8D or short 8L:16D photoperiods, since Porras 1999 found uniform responses after each abrupt change in photoperiodic regime, regardless of the time of the year at which the change was provided. This would suggest that Pelibuey ewes can respond primarily to photoperiodic information when the variations are large enough, but when the annual variation is small, as in the present study, the response to photoperiod could be modulated by other factors. Thus, the relative importance of photoperiod would decrease, and that of other factors would increase, as the animals are closer to the equator.
The importance of food availability as a modulating factor does not appear to be of great significance, since it was kept constant both in the present study and in that of
Ž .
Porras 1999 . On the other hand, temperature and humidity were not controlled in the present experiment, and they could have provided information about the time of the year
Ž
that conflicted with that provided by inverse photoperiod Pevet, 1987; Bronson and .
Heideman, 1994 .
It is clear than any difference in the reproductive response to photoperiod between the control and the inverse groups was not accounted for by a different pineal responsive-ness to photoperiod, since both groups responded similarly, with an almost immediate increase after the lights were turned off and an equally rapid decrease when the lights
Ž .
were on Fig. 3 . Despite a 6-month difference in calendar time, and thus in other variables that depend on it, Table 3 shows that the duration of melatonin elevation was
Ž .
identical on the longest day of both groups 12.3 h , and almost identical on the shortest
Ž .
day 9.7 vs. 9.5 h for the natural and inverse groups . The changes in melatonin secretion in response to the light:dark cycle in Pelibuey sheep conform to the classic
Ž .
pattern for other types of sheep Lincoln, 1992; Malpaux et al., 1993 , thus confirming that African hair sheep can perceive photoperiodic information and translate it into a melatonin signal in a similar way as sheep from temperate regions do.
The prolactin profile under natural photoperiod and its response to inverse
photope-Ž . Ž
riod Fig. 4 conform to those found on other types of sheep Pelletier, 1973; Poulton et .
al., 1987; Curlewis, 1992 , and may indicate that the transduction of photoperiodic information through melatonin to other physiological processes in Pelibuey sheep is
Ž
similar to that in breeds from temperate regions Daveau et al., 1994; Lincoln and .
Clarke, 1994 . However, the response of prolactin to a given daylength was slightly affected in the ewes exposed to inverse photoperiod, since the correlation between daylength and prolactin concentrations was lower in these ewes than in the control animals. Also, concentrations of the hormone were always lower in the inverse group than at the corresponding point, in terms of photoperiodic cycle, of the control group. This effect of the time of the year on the prolactin response to photoperiod was not
Ž . Ž .
observed when Pelibuey ewes were exposed to long 16L:8D or short 8L:16D
Ž .
( )
C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525 523
applied at different times of the year. This difference between our results and those of
Ž .
Porras 1999 again suggests that the relative importance of photoperiodic information with respect to other modulating factors may be reduced as Pelibuey ewes are kept closer to the equator.
5. Conclusion
It is concluded that the reproductive activity, melatonin secretion and prolactin secretion of Pelibuey ewes respond to the small variation in photoperiod that is present
at 19813XN, and that under natural conditions, photoperiod appears to be the main
regulator of ovarian activity at this latitude. However, other factors such as temperature or humidity may act as modulators, and their relative importance could increase at more equatorial latitudes. Since under natural conditions Pelibuey ewes start ovulating around, or even before the summer solstice, studies are needed to understand the processing of photoperiodic information in this type of sheep.
Acknowledgements
This study was supported by research grants PAPIIT IN-222798 and PAEP 9302 from the National University of Mexico.
Corpus Cerna was supported by an scholarship from the W.K. Kellog Foundation. We are grateful to Dr. B. Malpaux for providing antibodies and reagents for melatonin assay.
References
Bronson, F.H., Heideman, P.D., 1994. Seasonal regulation of reproduction in mammals. In: Knobil, E., Neill,
Ž .
J.D. Eds. , The Physiology of Reproduction. Raven Press, New York, pp. 541–583.
Carles, A.B., Kipngeno, W.A.K., 1986. The effect of season and the introduction of rams on oestrous activity in Somali, Nandi, Merino, Karakul and New Zealand Romney Marsch ewes in Kenya. Anim. Prod. 43, 447–457.
Castillo, R.H., Valencia, Z.M., Berruecos, V.J.M., 1972. Comportamiento reproductivo del borrego Tabasco mantenido en clima tropical y subtropical: I. Indices de fertilidad. Tec. Pecu. Mex. 20, 52–56.
Chemineau, P., Pelletier, J., Guerin, Y., Colas, G., Ravault, J.P., Almeida, G., Thimonier, J., Ortavant, R.,´
1988. Photoperiodic and melatonin treatments for the control of seasonal reproduction in sheep and goats. Reprod. Nutr. Dev. 28, 409–422.
Chemineau, P., Malpaux, B., Delgadillo, J.A., Guerin, Y., Ravault, J.P., Thimonier, J., Pelletier, J., 1992.´
Control of sheep and goat reproduction: use of light and melatonin. Anim. Reprod. Sci. 30, 157–184. Chemineau, P., Malpaux, B., Thiery, J.C., Viguie, C., Morello, H., Zarazaga, L., Pelletier, J., 1995. The´ ´
control of seasonality: a challenge to small ruminant breeding. In: Enne, G., Greppi, G.F., Lauria, A.
( ) C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525
524
Cruz, L.C., Fernandez-Baca, S., Alvarez, L.J.A., Perez, R.H., 1994. Variaciones estacionales en la presentacion´ ´ ´
de ovulacion, fertilizacion y sobrevivencia embrionaria en ovejas Tabasco en el tropico humedo. Vet. Mex.´ ´ ´ ´
25, 23–27.
Curlewis, J.D., 1992. Seasonal prolactin secretion and its role in seasonal reproduction. A review. Reprod. Fertil. Dev. 4, 1–23.
Daveau, A., Malpaux, B., Tillet, Y., Roblot, G., Wylde, R., Chemineau, P., 1994. Active immunization against melatonin in Ile-de-France ewes and photoperiodic control of prolactin secretion and ovulatory activity. J. Reprod. Fertil. 102, 285–292.
Eloy, A.M.X., Simplicio, A.A., Foote, W.C., 1990. Reproduction in sheep. In: Shelton, W., Figuereido, E.A.P.
ŽEds. , Hair Sheep Production in Tropical and Subtropical Regions. United States Agency for International.
Development, Davis, CA, pp. 97–111.
Gonzalez, R.A., Valencia, M.J., Foote, W.C., Murphy, B.D., 1991. Hair sheep in Mexico: reproduction in´
Pelibuey sheep. Anim. Breed. Abstr. 59, 509–524.
Gonzalez, A., Murphy, B.D., Foote, W.C., Ortega, E., 1992. Circannual estrous variations and ovulation rate´
in Pelibuey ewes. Small Rum. Res. 8, 225–232.
Hafez, E.S.E., 1952. Studies on the breeding season and reproduction of the ewe. J. Agric. Sci. 42, 189–265. Karsch, F.J., 1984. Endocrine and environmental control of oestrous cyclicity in sheep. In: Lindsay, D.R.,
Ž .
Pearce, D.T. Eds. , Reproduction in Sheep. Cambridge Univ. Press, New York, pp. 10–15.
Legan, S.J., Karsch, F.J., 1980. Photoperiodic control of seasonal breeding in ewes. Modulation of the negative feedback action of estradiol. Biol. Reprod. 23, 1061–1068.
Lincoln, G.A., 1992. Photoperiod–pineal–hypothalamic relay in sheep. Anim. Reprod. Sci. 28, 203–217. Lincoln, G.A., Clarke, J.J., 1994. Photoperiodically-induced cycles in the secretion of prolactin in
hypotha-lamo-pituitary disconnected rams: evidence for translation of the melatonin signal in the pituitary gland. J. Neuroendocrinol. 6, 251–260.
Malpaux, B., Robinson, J.E., Brown, M.B., Karsch, F.J., 1987. Reproductive refractoriness of the ewe to inductive photoperiod is not caused by inappropriate secretion of melatonin. Biol. Reprod. 36, 1333–1341. Malpaux, B., Robinson, J.E., Wayne, N.L., Karsch, F.J., 1989. Regulation of the onset of the breeding season of the ewe: importance of long days and of an endogenous reproductive rhythm. J. Endocrinol. 122, 269–278.
Malpaux, B., Chemineau, P., Pelletier, J., 1993. Melatonin and reproduction in sheep and goats. In: Yu, H.S.,
Ž .
Reiter, R.J. Eds. , Melatonin: Biosynthesis, Physiological Effects and Clinical Applications. CRC Press, Boca Raton, FL, pp. 253–287.
Martınez, R.R.D., 1998. Estudios sobre la estacionalidad reproductiva de la oveja Pelibuey del tropico humedo´ ´ ´
mexicano. D. Vet. Sci. Thesis. Universidad Nacional Autonoma de Mexico, Mexico City. Mexico.´ ´ ´ ´
Muhlia, A., Chavez, A., 1980. Insolacion y radiacion solar en el tope de la atmosfera para las latitudes que´ ´ ´ ´
cubre la Republica Mexicana. Ann. Inst. Geofıs. 26, 127–129.´ ´
Ž .
Pevet, P., 1987. Environmental control of the annual reproductive cycle in mammals. In: Pevet, P. Ed. , Comparative Physiology of Environmental Adaptations Vol. 3 Karger, Basel, Switzerland, pp. 82–89. Pelletier, J., 1973. Evidence for photoperiodic control of prolactin release in rams. J. Reprod. Fertil. 35,
143–147.
Porras, A.A., 1999. Efectos del fotoperiodo artificial sobre la actividad reproductiva de la oveja Pelibuey. D. Vet. Sci. Thesis, Universidad Nacional Autonoma de Mexico Mexico City, Mexico.´ ´ ´ ´
Porras, A.A., Zarco, Q.L., Valencia, M.J., Rojas, M.S., 1998. Efectos del fotoperiodo artificial sobre la actividad ovarica de la oveja Pelibuey. In: Proc. 16th Panamerican Congress of Veterinary Sciences.´
PANVET, Santa Cruz, Bolivia, p. 308, abstr.
Poulton, A.L., English, J., Symons, A.M., Arendt, J., 1987. Changes in plasma concentrations of LH, FSH and prolactin in ewes receiving melatonin and short-photoperiod treatments to induce early onset of breeding activity. J. Endocrinol. 112, 103–111.
Pulido, A., Zarco, L., Galina, C.S., Murcia, C., Flores, G., Posadas, E., 1991. Progesterone metabolism during storage of blood samples from Gyr cattle: effects of anticoagulant, time and temperature of incubation. Theriogenology 35, 965–975.
( )
C. Cerna et al.rAnimal Reproduction Science 60–61 2000 511–525 525 Robinson, J.E., Wayne, L.N., Karsch, F.J., 1985. Refractoriness to inhibitory daylengths initiates the breeding
season of the Suffolk ewe. Biol. Reprod. 32, 1024–1030.
Robinson, J.J., Wigzell, S., Aitken, R.P., Wallace, J.M., Ireland, S., Robertson, I.S., 1992. Daily oral administration of melatonin from March onwards advances by 4 months the breeding season of ewes maintained under the ambient photoperiod at 578N. Anim. Reprod. Sci. 27, 141–160.
Rodrıguez-Maltos, R., Zarco, L., Cruz, C., 1992. In: Effects of Different Levels of Supplementation on Age´
and Weight at Puberty Onset in Pelibuey Ewes Born during the Autumn. Proc. 12th Int. Congr. Anim. Reprod., 23–27 August 1992, The Hague, The Netherlands, Serie 616. pp. 2096–2098.
Thwaites, C.J., 1965. Photoperiod control of breeding activity in the Southdown ewe with particular reference to the effects of an equatorial light regime. J. Agric. Sci. Cambridge 65, 57–64.
Valencia, Z.M., Castillo, R.H., Berruecos, V.J., 1975. Reproduccion y manejo del borrego Tabasco o Peliguey.´
Tec. Pecu. Mex. 29, 66–72.
Velazquez, I.A., Cruz, L.C., Alvarez, L.J.A., 1995. Efecto del nivel de suplementacion sobre la presentacion´ ´ ´
del primer estro en ovejas Tabasco nacidas en verano. Vet. Mex. 26, 107–111.