www.elsevier.comrlocateranireprosci
Epidemiologic concerns relative to in vivo and
in vitro production of livestock embryos
D.A. Stringfellow
), M.D. Givens
Department of Pathobiology, College of Veterinary Medicine, 166 Greene Hall, Auburn UniÕersity, Auburn,
AL 36849-5519, USA
Abstract
Evidence indicates low potential for transmission of pathogens with in vivo-derived embryos of cattle when appropriate precautions are taken. In apparent contrast, results of research with in vivo-derived embryos of small ruminants and swine and with in vitro-derived embryos of cattle suggest a greater tendency for their association with pathogens after artificial exposure. However, regardless of donor species, investigations involving collection of embryos from artificially or naturally infected animals and assessment of health of recipients and offspring after transfer of these embryos have indicated low potential for transmitting disease.
In this paper, results of embryo–pathogen research are summarized, emphasizing potential for spread of pathogens under natural circumstances. Also, safe embryo handling practices and their application to multiple species are discussed.q2000 Elsevier Science B.V. All rights reserved.
Keywords: Livestock embryo; Embryo–pathogen interaction; Embryo washing
1. Introduction
Methods used to produce specific-pathogen-free embryos from livestock have in-cluded testing of donor animals before and after embryo collection, treatment of embryos after collection, or a combination of donor testing and embryo treatment
ŽGivens and Stringfellow, 1999 . Donor testing is a conservative approach that provides. Ž
considerable assurance that donors are free of specific infectious agents Stringfellow,
.
1985 . However, this approach is expensive and time consuming compared to embryo
Ž .
treatment. Embryo treatments i.e., washing with or without trypsin that are
recom-)Corresponding author: Tel:q1-334-844-2667; fax:q1-334-844-4955.
Ž .
E-mail address: [email protected] D.A. Stringfellow .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
Ž .
mended by the International Embryo Transfer Society Stringfellow, 1998 are simple, relatively inexpensive procedures that can be applied routinely in embryo production. Furthermore, embryo washing for health certification of in vivo-derived bovine embryos was validated in numerous studies that have been summarized in detail elsewhere
ŽSingh, 1987; Stringfellow et al., 1991; Anonymous, 1998a . In comparison, research on.
the epidemiology of in vivo-derived embryo production in small ruminants and swine and in vitro-derived embryo production in cattle has been less complete, and results indicate a greater tendency for association of pathogens with these embryos after
Ž .
artificial exposure Anonymous, 1998a; Guerin et al., 1997 . In this paper, results of embryo–pathogen research with in vivo-derived embryos of cattle, small ruminants and swine and with in vitro-derived embryos of cattle are summarized and compared, emphasizing potential for pathogen transmission under natural circumstances. In addi-tion, safe embryo handling practices and their application to multiple species and embryo production circumstances are discussed.
2. Bovine embryo–pathogen research
2.1. InÕiÕo-deriÕed embryos of cattle
Much of the initial research on interactions between bovine embryos and pathogens was designed to test a worst-case scenario through artificial exposure of zona pellucida-intact embryos to high concentrations of infectious agents. After exposure, embryos generally were washed 10 times to dilute unattached agent. Then embryos were ground or sonicated and assayed in vitro to determine if any infective agent remained associated with them after the washing procedure. Results of this research have been summarized
Žsee reviews: Anonymous, 1998a; Stringfellow and Givens, 1999 . Briefly, for eight of. Ž
nine viral pathogens akabane virus, bovine leukemia virus, bluetongue virus, bovine viral diarrhea virus, foot-and-mouth disease virus, bovine herpesvirus-1, bovine
her-.
pesvirus-4 and vesicular stomatitis virus that were used in these studies, infectious agent could not be isolated from embryos after washing or washing with trypsin . One
Ž .
virus rinderpest virus was stated in a preliminary report to associate with a small proportion of embryos after artificial exposure and washing. However, the finding was
Ž .
never confirmed. Also, for one Brucella abortus of six prokaryotic pathogens tested, washing was effective for removal while antibiotics in medium inactivated another
ŽHaemophilus somnus . Finally, four of the six prokaryotes. ŽMycoplasma boÕis, M. .
boÕigenitalium, Mycobacterium paratuberculosis and Ureaplasma diÕersum could not be removed by washing after artificial exposure, but their association with embryos after natural exposure has never been shown.
Ž . Ž .
cows infected with bovine leukemia virus 16% , bluetongue virus 40% , foot-and-mouth
Ž . Ž .
disease virus 68% and bovine herpesvirus-1 27% . However, after washing or washing with trypsin, embryos collected from cows artificially or naturally infected with bovine leukemia virus, bovine viral diarrhea virus, bluetongue virus, foot-and-mouth disease virus, bovine herpesvirus-1, rinderpest virus, Brucella abortus and Chlamydia
Ž
psittaci were all negative for infective agent see reviews: Anonymous, 1998a;
Stringfel-.
low and Givens, 1999 . Thus, pathogens were not associated with washed or trypsin washed embryos even when in vivo exposure to pathogen was confirmed by finding infective agent in the uterine recovery medium.
Finally, research attempting to simulate natural circumstances was conducted. In these studies, embryos were collected from artificially or naturally infected cows, washed or washed with trypsin and transferred to uninfected recipients. Subsequently, recipients and offspring were monitored for disease. Such studies were conducted with donor cows that were infected with or seropositive to bovine leukemia virus, bluetongue virus, foot-and-mouth disease virus, bovine herpesvirus-1 and B. abortus. None of these
Ž
agents were transferred from donors to recipients or their embryo transfer offspring see
.
reviews: Anonymous, 1998a; Stringfellow and Givens, 1999 .
Thus, based on assessment of general epidemiological factors associated with embryo
Ž .
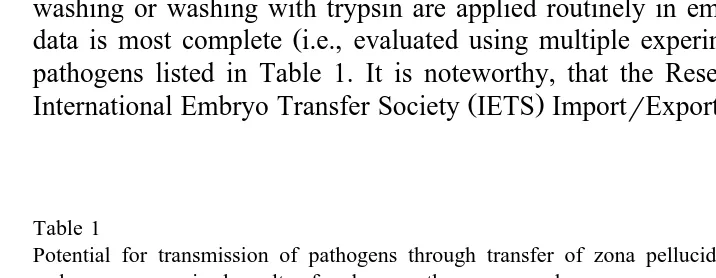
transfer Stringfellow, 1985 and the results of specific investigations that are summa-rized above, it was clear that in vivo-derived, zona pellucida-intact bovine embryos are not likely to serve as vectors for transmission of disease if simple precautions such as washing or washing with trypsin are applied routinely in embryo processing. Research
Ž .
data is most complete i.e., evaluated using multiple experimental designs for the six pathogens listed in Table 1. It is noteworthy, that the Research Subcommittee of the
Ž .
International Embryo Transfer Society IETS ImportrExport Committee has concluded
Table 1
Potential for transmission of pathogens through transfer of zona pellucida-intact, in vivo-derived, bovine embryos: summarized results of embryo–pathogen research
Pathogen Potential for Pathogen associated with Recipientr
natural exposure washed embryos calf infected
to pathogen After artificial After natural after transfer from infected
exposure exposure
donor cows
Bluetongue virus Possible No No No
a a
Bovine herpesvirus-1 Possible No No No
Bovine leukemia virus Possible No No No
Bovine viral diarrhea Possible No No No
virus
Foot-and-mouth disease Possible No No No
virus
Brucella abortus Unlikely No No No
a
Washing included treatment with trypsin. Washing procedures conformed to guidelines suggested by the
Ž .
Ž
that five of the pathogens bluetongue, bovine herpesvirus-1, bovine leukemia virus,
.
foot-and-mouth disease virus and B. abortus in Table 1 are ‘‘disease agents for which sufficient evidence has accrued to show that the risk of transmission is negligible provided that the embryos are properly handled between collection and transfer’’
ŽAnonymous, 1998b . The proper handling to which this statement refers is the use of.
standardized methods for washing and trypsin treatment of embryos that are described in
Ž .
the Manual of the International Embryo Transfer Society Stringfellow, 1998 .
2.2. InÕitro-deriÕed embryos of cattle
There are reasonable concerns about transmission of pathogens with in vitro-pro-duced embryos of cattle, yet research on the topic has been limited compared to that done with in vivo-derived embryos. Topics of investigation have included exposure
Ž
potential i.e., possibility of introducing pathogens with raw materials used during in
.
vitro fertilization , the nature of embryo–pathogen associations and infectivity of embryo-associated pathogen.
Ž . Ž .
Bovine herpesvirus-1 BHV-1 and bovine viral diarrhea virus BVDV have been at the center of consideration because they are ever present in cattle populations and are
Ž
known to occur in serum and reproductive tissues Baker, 1995; Brock, 1998; Rossi et
.
al., 1980 .
Cells and fluids from cattle infected with BHV-1 have been examined for virus. Cumulus–oocyte complexes, follicular fluid, granulosa cells, corpora lutea and uterine tubal cells all contained infective BHV-1 when collected from cattle acutely infected
Ž .
with the virus Bielanski and Dubuc, 1994; Guerin et al., 1989 . Further, the virus was isolated from in vitro-derived embryos produced with gametes and somatic cells from
Ž .
BHV-1-infected cattle Bielanski et al., 1998a , and when BHV-1 was artificially
Ž
introduced at various stages of in vitro embryo production systems Bielanski and
.
Dubuc, 1993; Bielanski et al., 1997 .
Cells and fluids from cattle infected with BVDV have been examined for virus. Infectious BVDV was found in follicular fluid, cumulus–oocyte complexes and uterine
Ž
tubal cells from acutely infected cattle Bielanski and Dubuc, 1995; Bielanski et al.,
.
1998b and was isolated from ovarian tissue, follicular fluid, cumulus–oocyte com-plexes, granulosa cells, uterine tubal cells and semen from persistently infected cattle
ŽBielanski and Loewen, 1994; Booth et al., 1995; Tsuboi and Imada, 1998 . Further,.
using indirect immunofluorescent assay of cryosections of ovaries from persistently infected heifers, BVDV antigen was found in 6% to 20 % of oocytes in primordial,
Ž .
primary and secondary follicles Brownlie et al.,1997; Fray et al., 1998 .
Thus, potential for introduction of BHV-1 and BVDV during in vitro production of embryos and the association of these viruses with developed embryos had been
Ž
demonstrated. Even more alarming was the discovery that embryo processing i.e.,
. Ž
washing and trypsin treatment was ineffective for removing these viruses Bielanski
.
and Dubuc, 1993; Trachte et al., 1998 . Furthermore, embryo treatments were equally
Ž .
ineffective for removal of bluetongue virus Langston et al., 1999 , foot-and-mouth
Ž . Ž
disease virus Marquant-Le Guienne et al., 1998 and Leptospira Bielanski and
.
The question of how pathogens associate with in vitro-produced embryos was highlighted by the ineffectiveness of embryo processing procedures. In one study, leptospires were shown by electron microscopy to penetrate the zona pellucida and enter embryonic cells when embryos were artificially exposed in medium without antibiotics
ŽBielanski and Surujballi, 1998 . However, the hazard of this occurring under normal.
circumstances appears to be low since this bacterium also was shown to be susceptible
Ž
to antibiotics commonly used in media for in vitro fertilization Bielanski and Surujballi,
.
1996 . In experiments in which there was artificial exposure to viral pathogens, there was no evidence for penetration of the zona pellucida, but the viruses were obviously firmly attached.
Ž .
In a report by Vanroose et al. 1999 , mechanical entrapment by the zona pellucida was suggested as a mechanism for association of both BHV-1 and BVDV with the zona pellucida of in vitro-derived bovine embryos. They used confocal laser scanning microscopy to examine the location of fluorescent microspheres that were placed by positive pressure on the surface of the zona pellucida, and demonstrated that particles equivalent in size to BHV-1 and BVDV were able to penetrate through approximately 25% and 50% of the thickness of the zona pellucida, respectively. Thus, partial penetration and entrapment was possible while complete penetration and infection of embryonic cells was considered unlikely. Association of viral pathogens with the zona pellucida by entrapment had been hypothesized, but this report provided the first tangible evidence.
Ž .
A comparison of infective pathogens that associate presumably by entrapment or do not associate with either in vivo- or in vitro-derived, zona pellucida-intact, bovine embryos after exposure and washing or trypsin treatment is provided in Table 2. The trend is clear. Pathogens that were not associated with in vivo-derived embryos after
Ž
washing i.e., bluetongue virus, bovine herpesvirus-1, bovine viral diarrhea virus, and
.
foot-and-mouth disease virus did remain associated with in vitro-derived embryos
Table 2
Comparison of infective pathogens associated with in vivo- or in vitro-derived, zona pellucida-intact, bovine embryos after in vitro or in vivo exposure to the pathogens and washing
Pathogen In-vivo-derived embryos In-vitro-derived embryos
After in vitro After in vivo After in vitro After in vivo
exposure exposure exposure exposure
Bluetongue virus No No Yes ND
a a a a
Bovine herpesvirus-1 No No Yes Yes
a
Bovine viral diarrhea virus No No Yes Yes
Foot and Mouth Disease Virus No No Yes ND
b b b,c
Leptospira spp. ND No No No
a
Trypsin in washes.
b
Although infective agent was not isolated from washed embryos, some were positive by PCR assay. NDsNot determined. Washing procedures conformed to guidelines suggested by the International Embryo
Ž .
Transfer Society Stringfellow, 1998 . Information in this table summarized from reviews by Anonymous, 1998a; Bielanski, 1998; Stringfellow and Givens, 1999; and from Bielanski et al., 1998c; Langston et al., 1999; Marquant-Le Guienne et al., 1998; and Trachte et al., 1998.
c
despite processing according to procedures recommended by the International Embryo
Ž .
Transfer Society for in vivo-derived embryos Stringfellow, 1998 . A summary of available information relative to the possibility of transmission of these pathogens with transferred, in vitro-derived embryos is provided in Table 3. The known potential for
Ž
natural exposure to at least two of these pathogens bovine herpesvirus-1 and bovine
.
viral diarrhea virus and the relative ineffectiveness of embryo processing procedures
Žwashing and trypsin treatment create legitimate concerns that they might be transmit-.
ted with exposed embryos. However, it has not been determined that the quantity of infectious virus associated with these embryos would constitute an infective dose for susceptible recipients via the intrauterine route.
Of course, risk assessment cannot be complete without establishing whether or not the amount of embryo-associated pathogen constitutes an infective dose. The question of infective dose has been addressed to some degree with bovine viral diarrhea virus. Exposed embryos were shown to be infective when they were washed, sonicated and
Ž .
injected intravenously into susceptible cattle Bielanski and Jordan, 1996 . However,
Ž .
under natural circumstances embryos would be intact not sonicated and would be delivered individually into recipients via the intrauterine route. Apparently contrasting
Ž .
results were presented in a report by Givens et al. 1999a . They discovered that the bovine viral diarrhea virus associated with single, washed, unsonicated, in vitro-derived embryos did not infect susceptible uterine tubal cells co-cultured with them for two days. In a subsequent study, the same investigators examined limited quantity and loss of infectivity of embryo-associated bovine viral diarrhea virus and antiviral influence of the embryo as possible explanations for failure of individual, developing, virus-exposed
Ž .
embryos to infect uterine tubal cells in co-cultures Givens et al., 1999b . Their results indicated that each of the three factors was partially responsible for preventing infection of the uterine tubal cells, but the relative importance of each factor could not be determined. These results are interesting and seem to indicate that an infective dose of bovine viral diarrhea virus might not be associated with exposed, transferred embryos. However, final resolution of the question can be accomplished only by transfer of
Table 3
Potential for transmission of pathogens through transfer of zona pellucida-intact, in vitro-derived, bovine embryos: Summarized results of embryo-pathogen research
Pathogen Potential for Pathogen associated with Recipientr
natural exposure washed embryos calf infected to pathogen After artificial After natural after transfer from infected exposure exposure
donor cows
Bluetongue virus Uncertain Yes ND ND
Bovine herpesvirus-1 Possible Yes Yes ND
Bovine viral diarrhea virus Possible Yes Yes ND
Foot-and-mouth disease virus Uncertain Yes ND ND
NDsNot determined. Washing procedures conformed to guidelines suggested by the International Embryo
Ž . Ž .
Transfer Society Stringfellow, 1998 . Information in this table summarized from Bielanski 1998 , Langston
Ž . Ž . Ž .
exposed embryos to susceptible recipients and monitoring recipients and offspring for signs of infection.
3. Embryo–pathogen research in small ruminants and swine
Information on embryo–pathogen interactions using in vitro-derived embryos of small ruminants and swine is insufficient to discuss here. Available information on research related to in vivo-derived embryo pathogen interactions in swine, sheep and
Ž
goats has been reviewed in detail elsewhere Stringfellow et al., 1991; Anonymous,
.
1998a; Thibier and Guerin, 1999 . A brief synopsis from these reviews and selected individual reports is provided in this paper.
3.1. Swine
Ž
Swine embryos were artificially exposed to seven different pathogens African swine fever virus, foot-and-mouth disease virus, hog cholera virus, porcine parvovirus,
pseu-.
dorabies virus, swine vesicular disease virus, and vesicular stomatitis virus , washed as
Ž .
recommended Stringfellow, 1998 and assayed for infective virus with the result that at
Ž
least some embryos were always positive for each of the pathogens reviewed in
.
Stringfellow et al., 1991; Anonymous, 1998a . Further, in one study, a nucleic acid probe was used to detect replicating porcine parvovirus, indicating that the virus had
Ž .
crossed the zona pellucida and infected embryonic cells Bane et al., 1990 .
Since all pathogens evaluated by artificial exposure remained associated with washed porcine embryos, it was especially important to evaluate the health status of recipients and offspring after transfer of washed or trypsin-treated, zona pellucida-intact embryos collected from infected or seropositive donors. In such experiments with embryo donors
Ž .
that were infected with hog cholera virus Anonymous, 1998a , porcine reproductive and
Ž .
respiratory syndrome virus Randall et al., 1999 and swine vesicular disease virus
ŽSingh et al., 1987 , recipients and offspring remained disease-free. In such studies with. Ž
donors that were pseudorabies virus-infectedrseropositive summarized in Stringfellow
.
et al., 1991 , when embryos were trypsin treated, there was no evidence of disease in recipients and offspring. Thus, it can be concluded that demonstrating association of a pathogen with swine embryos after artificial exposure and washing is not necessarily accompanied by a high risk that embryos collected from infected donors will infect recipients or offspring after transfer.
Ž .
The report by Bane et al. 1990 that porcine parvovirus penetrated the intact zona pellucida and replicated in embryonic cells after artificial exposure remains a concern. Since this is the only report of a viral pathogen penetrating the zona pellucida of an embryo from any livestock species, it seems appropriate that the experiment be confirmed by duplication.
3.2. Goats
Specific information on epidemiology of embryo production in goats is very limited
Ž .
study testing the efficacy of washing caprine embryos Thibier, 1990 . In this study,
Ž 3.
embryos collected from disease-free does were exposed in vitro to low 10 , medium
Ž 5. Ž 7.
10 and high 10 concentrations of M. mycoides mycoides, washed using
recom-Ž .
mended procedures Stringfellow, 1998 and assayed for the presence of the pathogen. Mycoplasmas were isolated from all washed embryos that had been exposed to medium or high concentrations of the organism.
There have been three reports of studies in which caprine embryos were collected from donors that were naturally or artificially exposed to pathogen and transferred to
Ž .
disease-free recipients. Chemineau et al. 1986 collected embryos from does in a flock in which approximately half of the animals were seropositive to bluetongue virus. The embryos were washed 10 times, cryopreserved and later transferred to disease-free
Ž . Ž .
recipients. None of the recipients ns19 or offspring ns19 developed antibody to
Ž .
bluetongue virus. Wolfe et al. 1987 collected embryos from does that were seroposi-tive to caprine arthritis-encephalitis virus. The embryos were washed only three times
Ž .
before transfer to negative recipients. However, the recipients ns8 and offspring
Žns1 remained seronegative. Finally, Foster et al 1999 collected embryos from does. Ž . Ž .
that had been artificially infected with bovine spongiform encephalopathy BSE . Embryos were washed 10 times and transferred to BSE-free recipients. Again, recipients
Žns22 and offspring n. Ž s37 remained free of signs of disease and were negative by.
all analyses. Presumably, the latter study represented the use of a caprine model to study possible transmission of BSE via embryo transfer, since BSE is generally considered a disease of cattle and not of goats.
Although the work with caprine embryos has been limited, one trend is similar to that found with both bovine and porcine embryos. When properly washed embryos from pathogen-exposed donors are transferred to disease-free recipients, both offspring and recipients remained free of disease.
3.3. Sheep
Ž
Zona pellucida-intact ovine embryos were exposed in vitro to one viral bovine viral
. Ž .
diarrhea virus and three bacterial pathogens B. abortus, B. oÕis, Campylobacter fetus . Then embryos were washed and examined in vitro for infectious agent. The combination of washing and treatment with anti-bovine viral diarrhea virus antibody was effective for
Ž .
removalrinactivation of bovine viral diarrhea virus Evermann et al., 1981 . Further,
Ž .
washing was effective for removal of Campylobacter fetus Guerin et al., 1988 , but in the absence of antibiotics, washing was unreliable for the removal of B. abortus
ŽRiddell et al., 1989 . Also, neither washing nor treatment with antibiotics was effective.
Ž .
for the removal of B. oÕis Wolfe et al., 1988; Guerin et al., 1992 , and when embryos
Ž .
exposed in vitro to B. oÕis were washed no antibiotics and transferred, four of seven
Ž .
recipients seroconverted Riddell et al., 1990 .
Possible transmission of bluetongue virus, C. psittaci, sheep pulmonary adenomatosis and scrapie under natural circumstances were evaluated by the collection of embryos from affected ewes and transferred to recipients.
Ž . Ž .
using procedures that have since been adopted by the International Embryo Transfer
Ž . Ž .
Society Stringfellow, 1998 and transferred to bluetongue virus-free recipients ns27 .
Ž .
None of the recipients or offspring ns16 seroconverted. However, in a similar study
Ž . Ž .
by Gilbert et al. 1987 , 20 embryos were collected from viremic donors ns8 and
Ž .
transferred to susceptible recipients ns15 . Viremia and seroconversion occurred in 2 of the 15 recipients, although one of these recipients gave birth to a lamb that was seronegative and virus-negative. Unfortunately, there was no mention of an attempt to wash the embryos, raising concern that embryos were not properly washed and making accurate interpretation of the results difficult.
The feasibility of using embryo transfer to break the cycles of infection for C. psittaci
Ž .
and sheep pulmonary adenomatosis SPA was demonstrated in two other studies.
Ž . Ž .
Williams et al. 1998 reported that properly washed embryos ns12 from ewes
Žns3 artificially infected with C. psittaci did not result in infection of recipients. Žns7 or their offspring. Also, Parker et al. 1998 collected 215 embryos from 76. Ž .
Ž .
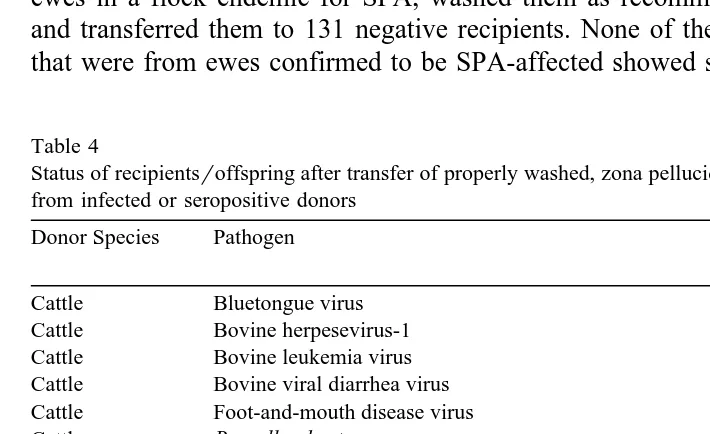
ewes in a flock endemic for SPA, washed them as recommended Stringfellow, 1998 and transferred them to 131 negative recipients. None of the recipients or 38 offspring that were from ewes confirmed to be SPA-affected showed signs of disease.
Table 4
Status of recipientsroffspring after transfer of properly washed, zona pellucida-intact, in vivo-derived embryos from infected or seropositive donors
Cattle Bovine viral diarrhea virus No
Cattle Foot-and-mouth disease virus No
Swine Porcine reproductive and respiratory syndrome virus No
a
Swine Pseudorabies virus No
Swine Swine vesicular disease virus No
Sheep Bluetongue virus No
Results reported as unpublished. Washing procedures conformed to guidelines suggested by the
Interna-Ž .
The potential for transmission of scrapie via embryos has been the topic of
investiga-Ž .
tion by two research teams. Foote et al. 1993 collected embryos from scrapie-inoc-ulated ewes, washed them three times without regard to condition of the zona pellucida and transferred them fresh to recipients in a scrapie-free flock. None of the recipients, including 129 that lambed, or offspring, including 56 that survived to 5 years of age, developed signs or lesions indicative of scrapie. However, conflicting results were
Ž .
presented by Foster et al. 1992 . In this study, 37 embryos were transferred from donor ewes artificially infected with scrapie into 16 recipients with birth of 26 lambs resulting. They described this study as a worst-case circumstance in which embryos were not washed. Six of the lambs developed scrapie at 2 to 2.5 years of age. Then, in a
Ž .
subsequent study Foster et al., 1996 , the same investigators reported that properly washed embryos from infected ewes resulted in lambs that later developed scrapie. However, validity of their results has to be questioned, since the flock of origin of recipients had a low incidence of natural scrapie, and they reported that the offspring
Žtwo of four from transfer of susceptible sAsA , negative-control, washed embryos. Ž .
also developed scrapie.
It appears that the trend established through research in cattle, swine and goats has continued in research with sheep. When embryos from pathogen exposed donors were
Ž .
properly treated Stringfellow, 1998 before transfer to disease-free recipients, both
Ž .
offspring and recipients remained free of disease Table 4 . The one possible exception
Ž .
is found in the report by Foster et al. 1996 . However, in their study, recipients came from a flock with a low incidence of natural scrapie, and some negative control animals developed scrapie. Thus, there was a realistic possibility that scrapie was not actually transmitted with washed embryos.
4. Discussion about safe embryo handling procedures
As stated, a conservative approach to health certification of embryos is to test donor
Ž
animals. Serological tests are generally conducted in pairs one around the time of
.
embryo collection and another a few weeks later to allow adequate time for incubation of any newly acquired pathogens. The rationale for donor testing is that donor females serve as isolation units for embryos prior to their recovery, and these embryos cannot be exposed to pathogens if donors are not infected. If flocks or herds of origin of donors are free of specific pathogens, the security of the isolation is higher. If the region or country of origin is disease-free, the security is still higher. In theory, this method could be used effectively for any donor species, but to be effective, reliable tests for the pathogens of concern in the donor species must be available.
Ž
The justification for the use of embryo processing i.e., washing or washing with
. Ž . Ž .
trypsin has been that 1 it is relatively inexpensive, 2 it is easily incorporated into
Ž .
embryo production schemes, 3 it has been shown in cattle to be effective after in vitro
Ž .
The standardized methods for treatment of embryos are described in detail and
Ž
illustrated in the Manual of the International Embryo Transfer Society Stringfellow,
. Ž .
1998 . Briefly, essential requirements of washing include: 1 only 10 or fewer, zona
Ž .
pellucida-intact embryos from one donor are washed as a group. 2 At least 10 washes
Ž .
are applied, using separate sterile pipets between each two washes. 3 Each wash is at
Ž .
least a one-hundred-fold dilution of the previous wash. 4 After washing, embryos are observed over all surfaces at a minimum of 50=magnification to ensure that the zona pellucida is free of defects and attached debris. Washing with trypsin has the same general requirements except that there are 12 washes. Embryos are washed five times, then exposed to fresh active trypsin in a sixth and seventh wash for a total time of 60 to 90 s and, finally, washed five more times without trypsin.
Whether donor testing andror embryo treatments are used to certify the health of embryos will depend on circumstances. However, of major importance is the ethical and technical excellence of those responsible for the procedures. It is their obligation not only to conduct procedures according to recommendations, but to insure that media, reagents and equipment as well as the working environment are free of pathogens and contaminating microorganisms.
References
Anonymous, 1998a. Appendix B. Data on embryo–pathogen studies in cattle, sheep, goats and swine from
Ž . Ž .
tables in the IETS Manual, 2nd edn. 1990 . In: Stringfellow, D.A., Seidel, S.M. Eds. , Manual of the International Embryo Transfer Society. 3rd edn. IETS, Savoy, IL, pp. 151–163.
Anonymous, 1998b. Conclusions of the research subcommittee of the International Embryo Transfer Society
ŽIETS ImportrExport Committee. Rev. Sci. Tech. Off. Int. Epiz. 17, 839..
Baker, J.C., 1995. The clinical manifestations of bovine viral diarrhea infection. Vet. Clin. N. Am: Food Anim. Pract. 11, 425–445.
Bane, D.P., James, J.E., Gradil, C.M., Molitor, T.W., 1990. In vitro exposure of porcine embryos to porcine parvovirus. Theriogenology 33, 553–561.
Bielanski, A, 1998. Potential for disease control or transmission by embryos produced in vitro: a review of
Ž .
current literature. In: Stringfellow, D.A., Seidel, S.M. Eds. , Manual of the International Embryo Transfer Society. 3rd edn. IETS, Savoy, IL, pp. 45–53.
Bielanski, A., Dubuc, C., 1993. In vitro fertilization of bovine oocytes exposed to bovine herpesvirus-1
ŽBHV-1 . Reprod. Dom. Anim. 28, 285–288..
Bielanski, A., Dubuc, C., 1994. In vitro fertilization and culture of ova from heifers infected with bovine
Ž .
herpesvirus-1 BHV-1 . Theriogenology 41, 1211–1217.
Bielanski, A., Dubuc, C., 1995. In vitro fertilization of ova from cows experimentally infected with a noncytopathic strain of bovine viral diarrhea virus. Anim. Reprod. Sci. 38, 215–221.
Bielanski, A., Jordan, L., 1996. Washing or washing with trypsin treatment is ineffective for removal of noncytopathic bovine viral diarrhea virus from bovine oocytes or embryos after viral contamination of an in vitro fertilization system. Theriogenology 46, 1467–1476.
Bielanski, A., Loewen, K., 1994. In vitro fertilization of bovine oocytes with semen from bulls persistently infected with bovine viral diarrhea virus. Anim. Reprod. Sci. 35, 183–189.
Bielanski, A., Lutz-Wallace, C., Sapp, T., Jordan, L., 1997. The efficacy of trypsin for disinfection of in vitro fertilized bovine embryos exposed to bovine herpesvirus-1. Anim. Reprod. Sci. 47, 1–8.
Bielanski, A., Sapp, T., Lutz-Wallace, C., 1998b. Association of bovine embryos produced by in vitro fertilization with a noncytopathic strain of BVDV type II. Theriogenology 49, 1231–1238.
Bielanski, A., Surujballi, O., 1996. Association of Leptospira borgpetersenii serovar hardjo type hardjoboÕis
with bovine ova and embryos produced by in vitro fertilization. Theriogenology 46, 45–55.
Bielanski, A., Surujballi, O., 1998. Leptospira borgpetersenii serovar hardjo type hardjoboÕis in embryos
fertilized in vitro. Can. J. Vet. Res. 62, 234–236.
Bielanski, A., Surujballi, O., Golsteyn, T.E., Tanaka, E., 1998c. Sanitary status of oocytes and embryos collected from heifers experimentally exposed to Leptospira borgpetersenii serovar hardjo type
hardjobo-Õis. Anim. Reprod. Sci. 54, 65–73.
Booth, P.J., Stevens, D.A., Collins, M.E., Brownlie, J., 1995. Detection of bovine viral diarrhea virus antigen and RNA in oviduct and granulosa cells of persistently infected cattle. J. Reprod. Fertil. 105, 17–24. Brock, K.V., 1998. Quality control for materials of animal origin used in embryo production and transfer. In:
Ž .
Stringfellow, D.A., Seidel, S.M. Eds. , Manual of the International Embryo Transfer Society. 3rd edn. IETS, Savoy, IL, pp. 151–163.
Brownlie, J., Booth, P.J., Stevens, D.A., Collins, M.E., 1997. Expression of non-cytopathic bovine viral
Ž .
diarrhea virus BVDV in oocytes and follicles of persistently infected cattle. Vet. Rec. 141, 335–337. Chemineau, P., Procureur, R., Cognie, Y., Lefevre, P.C., Locatelli, A., Chupin, D., 1986. Production, freezing
and transfer of bluetongue virus-free goat embryos. Theriogenology 26, 279–290.
Evermann, J.F., Faris, M.A., Niemi, S.M., Wright, R.W., 1981. Pestivirus persistence and pathogenesis: comparative diagnostic aspects of Border disease virus of sheep and bovine viral diarrhea virus. In: 24th Ann. Proc. Am. Assoc. Vet. Lab. pp. 407–426.
Foote, W.C., Clark, W., Maciulis, A., Call, J.W., Hourrigan, J., Evans, R.C., Marshall, M.R., deCamp, M., 1993. Prevention of scrapie transmission in sheep using embryo transfer. Am. J. Vet. Res. 54, 1863–1868. Foster, J.D., McKelvey, W.A.C., Mylne, M.J.A., Williams, A., Hunter, N., Hope, J., Fraser, H., 1992. Studies
on maternal transmission of scrapie using embryo transfer. Vet. Rec. 132, 341–343.
Foster, J.D., Hunter, N., Williams, A., Mylne, M.J.A., McKelvey, W.A.C., Hope, J., Fraser, H., Bostock, C., 1996. Observations on the transmission of scrapie in experiments using embryo transfer. Vet. Rec. 138, 559–562.
Foster, J., McKelvey, W., Fraser, H., Chong, A., Ross, A., Parnham, D., Goldman, W., Hunter, N., 1999. Experimentally induced bovine spongiform encephalopathy did not transmit via goat embryos. J. Gen. Virol. 80, 517–524.
Fray, M.D., Prentice, H., Clarke, M.C., Charleston, B., 1998. Immunohistochemical evidence for the localization of bovine virus diarrhea virus, a single-stranded RNA virus, in ovarian oocytes in the cow. Vet. Path. 35, 253–259.
Gilbert, R.O., Coubrough, R.I., Weiss, K.E., 1987. The transmission of bluetongue virus by embryos in sheep. Theriogenology 27, 527–540.
Givens, M.D., Galik, P.K., Riddell, K.P., Stringfellow, D.A., 1999a. Uterine tubal cells remain uninfected after culture with in vitro produced embryos exposed to bovine viral diarrhea virus. Vet. Micro. 70, 7–20. Givens, M.D., Galik, P.K., Riddell, K.P., Brock, K.V., Stringfellow, D.A., 1999b. Quantity and infectivity of embryo-associated bovine viral diarrhea virus and antiviral influence of a blastocyst impede in vitro infection of uterine tubal cells. Theriogenology 52, 887–900.
Givens, M.D., Stringfellow, D.A., 1999. Potential of embryo transfer for infectious disease control. In:
Ž .
Howard, J.L., Smith, R.A. Eds. , Current Veterinary Therapy: 4. Food Animal Practice. Saunders, Philadelphia, PA, pp. 592–595.
Guerin, B., Builly, J.P., Humblot, P., Nibart, M., Thibier, M., 1988. Effets de la contamination experimentale in vitro des embryones de souris et de brebis par Campylobacter fetus. Bull. Acad. Vet. France 61, 63–78. Guerin, B., LeGuienne, B., Chaffaux, St., Harlay, T., Allietta, M., Thibier, M., 1989. Contamination des ovocytes et des embryons fecondes in fitro apres infection experimentale de vaches donneuses par le virus
Ž .
bovine de type 1 BHV-1 . Rec. Med. Vet. 165, 827–833.
Guerin, B., Nibart, M., Marquant-LeGuienne, B., Humblot, P., 1997. Sanitary risks related to embryo transfer in domestic species. Theriogenology 47, 33–42.
Guerin, B., Quirin, R., Diemert, S., Thibier, M., 1992. In vitro contamination of ovine embryos with Brucella
oÕis and attempt of treating the contaminated embryos with gentamycin. In: Proc. 12th I.C.A.R., The
Hare, W.C.D., Luedke, A.J., Thomas, F.C., Bowen, R.A., Singh, E.L., Eaglesome, M.D., Randall, G.L.B., Bielanski, A., 1988. Non-transmission of bluetongue virus by embryos from bluetongue virus-infected sheep. Am. J. Vet. Res. 49, 468–472.
Langston, N.L., Stringfellow, D.A., Galik, P.K., Garrett, G.E., 1999. Failure to wash bluetongue virus from
Ž .
bovine IVF embryos. Theriogenology 51, 273 abstract .
Marquant-LeGuienne, B., Remond, M., Cosquer, R., Humblot, P., Kaiser, C., Lebreton, F., Cruciere, C., Guerin, B., LaPorte, J., Thibier, M., 1998. Exposure of in vitro produced bovine embryos to foot-and-mouth disease virus. Theriogenology 50, 109–116.
Parker, B.N.J., Wrathall, A.E., Saunders, R.W., Dawson, M., Done, S.H., Francis, P.G., Dexter, I., Bradley, R., 1998. Prevention of transmission of sheep pulmonary adenomatosis by embryo transfer. Vet. Rec. 142, 687–689.
Randall, A.E., Pettitt, M.J., Plante, C., Buckrell, B.C., Randall, G.C.B., Henderson, J.M., Larochelle, R., Magar, R., Pollard, J., 1999. Elimination of porcine reproductive and respiratory syndrome virus through embryo transfer. Theriogenology 51, 274, abstract.
Riddell, M.G., Stringfellow, D.A., Wolfe, D.F., Galik, P.K., 1989. In vitro exposure of ovine ova to Brucella
abortus. Theriogenology 31, 895–901.
Riddell, M.G., Stringfellow, D.A., Wolfe, D.F., Galik, P.K., Lauerman, L.H., 1990. Seroconversion of recipient ewes after transfer of ova exposed to Brucella oÕis in vitro. Theriogenology 34, 965–973.
Rossi, C.R., Bridgman, C.R., Kiesel, G.K., 1980. Viral contamination of fetal bovine serum. Am. J. Vet. Res. 41, 1680–1681.
Singh, E.L., 1987. The disease control potential of embryos. Theriogenology 27, 9–20.
Singh, E.L., Thomas, F.C., Hare, W.C.D., Eaglesome, M.D., 1987. Embryo transfer as a means of controlling viral infections: X. The in vivo exposure of zona pellucida-intact porcine embryos to swine vesicular disease virus. Theriogenology 27, 451–457.
Stringfellow, D.A., 1985. The potential of bovine embryo transfer for infectious disease control. Rev. Sci. Tech. Off. Int. Epiz. 4, 859–866.
Stringfellow, D.A., 1998. Recommendations for the sanitary handling of in-vivo-derived embryos. In:
Ž .
Stringfellow, D.A., Seidel, S.M. Eds. , Manual of the International Embryo Transfer Society. 3rd edn. IETS, Savoy, IL, pp. 79–84.
Stringfellow, D.A., Givens, M.D., 2000. Preventing disease transmission through the transfer of in-vivo-de-rived bovine embryos. Lives. Prod. Sci. 62, 237–251.
Stringfellow, D.A., Riddell, K.P., Zurovac, O., 1991. The potential of embryo transfer for infectious disease control in livestock. N. Z. Vet. J. 39, 8–17.
Thibier, M., 1990. Le transfert embryonnaire: le mogen le plus sur, au plan sanitaire, d’exchanges de genes. In: Proc. 6e Reunione A.E.T.E. Lyon, Sept., 1990. pp. 67–81.
Thibier, M., Guerin, B., 2000. Embryo transfer in small ruminants: the method of choice for health control in germ plasm exchanges. Lives. Prod. Sci. 62, 253–270.
Trachte, E.A., Stringfellow, D.A., Riddell, K.P., Galik, P.K., Riddell, M.G., Wright, J.C., 1998. Washing and trypsin treatment of in vitro derived bovine embryos exposed to bovine viral diarrhea virus. Theriogenol-ogy 50, 717–726.
Tsuboi, T., Imada, T., 1998. Bovine viral diarrhea virus replication in bovine follicular epithelial cells derived from persistently infected heifers. J. Vet. Med. Sci. 60, 569–572.
Vanroose, G., Nauwynck, H., Van Soom, A., Ysebaert, M., Charlier, G., Van Oostueldt, P., deKruif, A., 1999. Why is the zona pellucida of in vitro-produced embryos an efficient barrier for viral infection? Theri-ogenology 51, 276, abstract.
Williams, A.F.J., Beck, N.F.G., Williams, S.P., 1998. The production of EAE-free lambs from infected dams using multiple ovulation and embryo transfer. Vet. J. 155, 79–84.
Wolfe, D.F., Nusbaum, K.E., Lauerman, L.H., Mysinger, P.W., Riddell, M.G., Putnam, M.R., Shumway, L.S., Powe, T.A., 1987. Embryo transfer from goats seropositive for caprine arthritis-encephalitis virus. Theriogenology 28, 307–316.
Wolfe, D.F., Stringfellow, D.A., Riddell, M.G., Lauerman, L.H., Galik, P.K., 1988. Adherence of Brucella
oÕis to preimplantation ovine ova. Theriogenology 30, 387–393.
Ž .
Ž .
method for investigating the transmission of Maedi-Visna virus MVV by pre-implantation embryos. In: Proc. 3rd International Sheep Vet. Conf., Edinburgh. p. 126.
Wrathall, A.E., Sutmoller, P., 1998. Potential of embryo transfer to control transmission of disease. In:
Ž .