www.elsevier.comrlocateranireprosci
Activation of ribosomal RNA genes in
preimplantation cattle and swine embryos
P. Hyttel
a,), J. Laurincik
b, D. Viuff
c, T. Fair
d,
V. Zakhartchenko
e, C. Rosenkranz
f, B. Avery
c, D. Rath
g,
H. Niemann
g, P.D. Thomsen
a, K. Schellander
h, H. Callesen
i,
E. Wolf
j, R.L. Ochs
k, T. Greve
ca
Department of Anatomy and Physiology, The Royal Veterinary and Agricultural UniÕersity,
GronnegaardsÕej 7, DK-1870 Frederiksberg C, Denmark
b
Konstantin the Philosopher UniÕersity, Nitra, SloÕak Republic
c
Department for Clinical Studies, Reproduction, The Royal Veterinary and Agricultural UniÕersity,
GronnegaardsÕej 7, DK-1870 Frederiksberg C, Denmark
d
Department of Animal Science and Production, UniÕersity College Dublin, Lyons Research Farm,
Co Dublin, Ireland e
Bayerisches Forschungszentrum fur Fortpflanzungsbiologie, Oberschleissheim, Germany¨
f
Veterinary UniÕersity, Vienna, Austria
g ( )
Institut fur Tiezucht und Tier¨ Õerhalten, Mariensee, FAL , Neustadt, Germany
h
UniÕersitat Bonn, Institut fur Tierzuchtwissenschaft, Bonn, Germany¨ ¨
i
Danish Institute of Agricultural Sciences, Tjele, Denmark j
Lehrstuhl fur Molekulare Tierzucht und Haustiergenetik, Lidwig-Maximilians-Uni¨ Õersitat,¨
Munchen, Germany¨
k
Precision Therapeutics, 3636 BouleÕard of the Allies, Pittsburgh, PA 15213, USA
Abstract
Ž .
Transcription of ribosomal RNA rRNA genes occurs in the nucleolus resulting in ribosome synthesis. In cattle and swine embryos, functional ribosome-synthesizing nucleoli become struc-turally recognizable towards the end of the fourth and third post-fertilization cell cycle, respec-tively. In cattle, a range of important nucleolar proteins become localized to the nucleolar anlage over several cell cycles and this localization is apparently completed towards the end of the fourth cell cycle. In swine, the localization of these proteins to the anlage is more synchronous and occurs towards the end of the third cell cycle and is apparently completed at the onset of the fourth.
)Corresponding author. Tel.:q45-3528-2541; fax:q45-3528-2547.
Ž .
E-mail address: [email protected] P. Hyttel .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
The rRNA gene activation and the associated nucleolus formation may be used as a marker for the activation of the embryonic genome in mammalian embryos and, thus, serve to evaluate the developmental potential of embryos originating from different embryo technological procedures. By this approach, we have demonstrated that in vitro produced porcine embryos display a lack of localization of nucleolar proteins to the nucleolar anlage as compared with in vivo developed counterparts. Similarly, bovine embryos produced by nuclear transfer from morulae display such deviations as compared with in vitro produced counterparts. Collectively, this information may help to explain the appearance of abnormalities seen in a certain proportion of offspring derived from in vitro produced embryos and after cloning. q2000 Elsevier Science B.V. All rights
reserved.
Keywords: Embryo; Cattle; Swine; rRNA genes; Nucleolus; Gene activation
1. Introduction
The initial development of mammalian pre-implantation embryos is governed by gene transcripts and polypeptides produced by, and stored in, the oocyte during its
develop-Ž .
ment Schultz, 1993 . However, following one to three cleavage divisions, control of development is taken over by the expression of portions of the embryonic genome as the
Ž
maternally derived transcripts and proteins are gradually degraded Telford et al., 1990; .
De Sousa et al., 1998; Watson et al., 1996, 1999 . This transition from maternal to embryonic control of development is a gradual phenomenon. In the mouse and rabbit, it has been clearly demonstrated that a minor transcriptional activation occurring at the G2 stage of the first post-fertilization cell cycle precedes a major activation taking place at the G2 stage of the second cell cycle in mouse and at the fourth to fifth cell cycle in
Ž .
rabbit for review see Thompson, 1996 . Accordingly, using long-term incubation with
3 Ž .
H-uridine we have observed transcription during the first Hay-Schmidt et al., 1997
Ž .
and second cell cycle Plante et al., 1994; Hyttel et al., 1996; Viuff et al., 1996 in cattle, a stage which is well before the major transcriptional activation which takes place
Ž .
during the fourth cell cycle Camous et al., 1986 . In swine, the major transcriptional
Ž .
activation is seen during the fourth cell cycle Tomanek et al., 1989 , but whether this activation is preceded by an earlier minor one has not been investigated.
It is a common phenomenon that the major transcriptional activation includes
Ž . Ž
activation of the ribosomal RNA rRNA genes King et al., 1988; Kopecny et al., 1989; .
From the presented data, it is evident that the activation of the rRNA genes can be visualized ultrastructurally through the formation of the nucleolus in the blastomeres of the embryo. However, the application of different cell biological techniques in embryo technology during the past few years has allowed for a more molecular understanding of this process by the use of immunocytochemistry for the localization of specific proteins
Ž .
of importance for nucleolar function, and fluorescence in situ hybridization FISH for the localization of the rRNA genes and transcripts. On the following pages, the ultrastructure and the molecular composition of the developing nucleolus in pre-implan-tation cattle and swine embryos will be discussed.
2. Ultrastructure of rRNA gene activation
The active ribosome-synthesizing nucleolus contains three main ultrastructural
com-Ž .
ponents: the fibrillar components consisting of the fibrillar centres FCs , and the dense
Ž . Ž . Ž
fibrillar component DFC , and the granular component GC for review see Wachtler .
and Stahl, 1993 . These components of the so-called fibrillo-granular nucleolus reflect the steps in the biosynthesis of ribosomes according to the following model: the FCs house the enzymatic apparatus for the transcriptional process, the DFC carries the primary unprocessed transcripts, while the GC represents processed transcripts associ-ated with proteins in the form of pre-ribosomal particles.
2.1. Cattle
It is known that active fibrillo-granular nucleoli are established in cattle embryos Ž
during the fourth cell cycle under in vivo conditions Camous et al., 1986; King et al., .
1988; Kopecny et al., 1989 . In order to elucidate the chronology of this event in greater detail, we analyzed in vitro produced cattle zygotes and embryos fixed at different
Ž . Ž
intervals during the one-cell stage first cell cycle , the two-cell stage second cell
. Ž . Ž .
cycle , the four-cell stage third cell cycle , the eight-cell stage fourth cell cycle and
Ž .
the 16-cell stage fifth cell cycle; Laurincik et al., 2000 by transmission electron
Ž .
microscopy TEM . The embryos were produced by in vitro oocyte maturation, in vitro fertilization and subsequent culture.
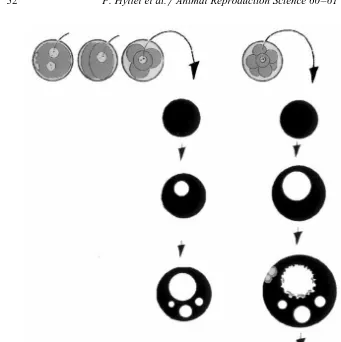
The structurally most prominent nuclear entities observed during the initial three cell
Ž .
cycles were large electron-dense fibrillar spheres Fig. 1 . These spheres typically developed one or several vacuoles with the progression of the cell cycles. A central primary vacuole developed first, followed by the formation of several peripheral secondary vacuoles.
Fig. 1. Schematic illustration of the ultrastructural aspects of nucleolus development in cattle embryos. During the first, second and third cell cycles, the nuclei display spherical fibrillar spheres that undergo vacuolization
Ž .
during the cell cycle. During the fourth cell cycle, the formation of dense fibrillar component DFC and
Ž .
fibrillar centres FCs occurs in the periphery of the fibrillar spheres. DFC also forms at the rim of the central vacuole. Later during the cell cycle, the substance of the sphere is gradually displaced by the granular
Ž .
component GC . During the fifth cell cycle, a fibrillo-granular nucleolus emerges from the start.
Subsequently, the DFC and the FCs developed and a GC consisting of pre-ribosomal particles emerged and occupied the remaining portion of the former fibrillar sphere. Thus, through this process a functional fibrillo-granular nucleolus had emerged. At the fifth cell cycle, fibrillo-granular nucleoli were formed already from the start of the cycle indicating that the meiotically induced inactivation of the rRNA genes had ceased. In addition to the precise chronological mapping, this study established that the DFC and the FCs were formed initially at the periphery of the fibrillar sphere, which is a new finding. Previously, this event was thought to occur at the rim of the primary vacuole
Ž .
2.2. Swine
It has been documented that swine embryos display the first signs of development of FCs towards the end of the third cell cycle, i.e. one cell cycle earlier than in cattle ŽTomanek et al., 1989 . In order to elucidate the chronology of this event in greater. detail, we analyzed in vivo developed swine zygotes and embryos by TEM. Following flushing from the oviducts, the zygotes and embryos were cultured into the subsequent
Ž
cell cycle in vitro to be fixed at different intervals during the one-cell stage first cell
. Ž .
cycle; the zygotes were not cultured in vitro , the two-cell stage second cell cycle , the
Ž . Ž .
four-cell stage third cell cycle , the eight-cell stage fourth cell cycle and the 16-cell
Ž .
stage fifth cell cycle; Laurincik et al., 1996; Hyttel et al., unpublished data .
The structurally most prominent nuclear entities observed during the initial two cell cycles were electron-dense fibrillar spheres that were markedly larger than in cattle
Ž .
embryos Fig. 2 . In contrast to the observations in cattle, the spheres did not undergo vacuolization.
Fig. 2. Schematic illustration of the ultrastructural aspects of nucleolus development in swine embryos. During the first and second cell cycle, the nuclei display spherical fibrillar spheres. During the third cell cycle, dense
Ž . Ž . Ž .
At the onset of the third cell cycle, the fibrillar spheres were again observed. Around the midpoint of the third cell cycle, the nuclei of the blastomeres displayed different stages of nucleolus formation, ranging from the inactive fibrillar spheres to fibrillo-gran-ular nucleoli presenting a DFC, FCs and a GC. Different stages of nucleolus develop-ment were observed within the same nucleus, i.e. fibrillar spheres and fibrillo-granular nucleoli were found side by side. Apparently, nucleolus formation was initiated by the formation of the DFC and GC and later the FCs on the surface of, but not in, the fibrillar spheres. Throughout this process, semilunar fibrillo-granular nucleoli more or less encapsulated the fibrillar sphere. At the fifth cell cycle, fibrillo-granular nucleoli, as described for the previous cycle were already formed at the start of the cycle, indicating that the meiotically induced inactivation of the rRNA genes had ceased. Again, the fibrillo-granular nucleoli were found together with inactive fibrillar spheres.
2.3. ComparatiÕe aspects
In cattle and swine embryos, fibrillo-granular ribosome-synthesizing nucleoli become structurally recognizable towards the end of the fourth and third cell cycle, respectively. In cattle, the formation of DFC and FCs occurs in the periphery of pre-existing nucleolar anlages appearing as fibrillar spheres, whereas in the swine the DFC and the FCs appear on the surface of these anlages instead.
3. Molecular aspects of rRNA gene activation
From a molecular perspective, the nucleolus consists of the rRNA genes and their transcripts associated with a long list of proteins that play different roles in rRNA transcription and processing to the final pre-ribosomal particles. The location of several important nucleolar proteins is well established and their function is to some degree
Ž .
known for review see Wachtler and Stahl, 1993 . Likewise, the localization of the rRNA genes within the nucleolus has been established although the actual site of rRNA transcription is still a controversial issue: some reports suggest that transcription occurs in the DFC, others that it takes place in the FCs, while a third and tempting model
Ž
suggests that the process occurs at the interface between the DFC and the FCs Hozak et .
al., 1994 .
In order to elucidate the molecular composition of the nucleolus before and during Ž the activation of the rRNA genes, we have analyzed in vitro produced cattle embryos as
. Ž .
for TEM, see above and in vivo developed swine embryos as for TEM, see above by immunocytochemistry and FISH. For immunocytochemistry, antibodies for six
impor-Ž .
tant nucleolar proteins see later were applied on whole mounts of embryos that were Ž
subsequently analyzed by confocal laser scanning microscopy Laurincik et al., 2000; .
localizes certain nucleolar proteins characterized by silver affinity. The resultant speci-Ž
mens were analyzed by both bright field and fluorescence microscopy Viuff et al., .
1998 .
The nucleolar proteins against which antibodies were applied were: Topoisomerase I,
Ž .
RNA polymerase I, upstream binding factor UBF , fibrillarin, nucleolin and nucle-ophosmin. The nucleolar proteins investigated in the present study may be divided into two categories: proteins such as topoisomerase I, RNA polymerase I and UBF that are characterized by a well-defined action during transcription and processing, while others, such as fibrillarin, nucleolin and nucleophosmin, are characterized by their spatial localization within the nucleolus. The putative role of each of these proteins in relation to rRNA transcription and processing is addressed in the following paragraph.
As a prerequisite for transcription of the rRNA genes, the supercoiled DNA must be
Ž .
uncoiled. This process is mediated by topoisomerase I Muller et al., 1985 which has
Ž .
also been localized to the DFC and FCs Wachtler and Stahl, 1993 . The actual transcription of the rRNA genes is dependent on the activity of RNA polymerase I
Ž which also has been localized mainly to the FCs and to some degree to the DFC Raska
.
et al., 1989 . The binding of RNA polymerase I to the DNA requires several
transcrip-Ž .
tion factors, one of which is UBF Schnapp et al., 1994 . UBF is thought to bind to the Ž promoter and to recruit another transcription factor, the promotor selectivity factor SL1,
.
Maeda et al., 1992 , thus forming a pre-initiation complex to which RNA polymerase I
Ž .
can bind and initiate transcription Jordan et al., 1996 . The roles of the remaining three nucleolar proteins investigated is more putative and they are primarily characterized by their nucleolar localization. Fibrillarin was first identified by human autoimmune sera
Ž
from patients with scleroderma and was localized to the DFC and the FCs Ochs et al.,
. Ž .
1985; Raska et al., 1989 . Fibrillarin is a small nucleolar ribonucleoprotein snoRNP Ž
associated with U3 small nucleolar RNA snoRNA; Lischwe et al., 1985; Lapeyere et
. Ž .
al., 1990 and with U8 and U13 snoRNAs Tyc and Steitz, 1989; Baserga et al., 1991 . SnoRNAs are believed to be involved in the processing of the primary rRNA transcript ŽJansen et al., 1991; Aris and Bloberg, 1991; Tollervey et al., 1991, 1993 . Nucleolin is. a phosphorylated protein present in large amounts in nucleoli with active ribosomal
Ž . Ž
biogenesis Lapeyere et al., 1987 . It has RNA-binding properties Ghisolfi-Nieto et al., .
1996 and, furthermore, it is identical to the human DNA helicase IV, which unwinds
Ž .
RNA–RNA, DNA–DNA and DNA–RNA duplexes Teteja et al., 1995 . The protein is associated with the primary rRNA transcripts. Nucleolin may act in promoting the functional secondary structure of the rRNA that is necessary for the assembly of the
Ž .
pre-ribosomal particles Ghisolfi et al., 1990 . However, it is not part of the final product ŽHernandez-Verdun, 1991 . Nucleolin has been localized to the DFC and the GC. ŽBiggiogera et al., 1990 . Nucleophosmin may be involved in shuttling other proteins,.
Ž . Ž .
such as nucleolin Li et al., 1996 and the nucleolar protein p120 Valdez et al., 1994 , into the nucleolus. However, nucleophosmin also has DNA and RNA binding properties ŽWang et al., 1994 , ribonuclease activity Herrera et al., 1995 and associates with the. Ž .
Ž .
In the following, the immunocytochemical labelling of structural nuclear entities by antibodies directed against the above mentioned proteins is presented together with observations on the localization of the rRNA genes and their transcripts in order to elucidate the gradual assembly of the nucleolus from a molecular point of view.
3.1. Cattle
During the first cell cycle, topoisomerase I, RNA polymerase I, UBF, fibrillarin, nucleolin and nucleophosmin may be localized to discrete foci in the pronuclei. We believe that the foci are identical with the fibrillar spheres observed by TEM. During the second cell cycle, only fibrillarin and UBF could be localized to such foci, suggesting that the proteins observed during the first cycle were of maternal origin. During the third cell cycle, fibrillarin and UBF displayed the same labelling pattern as during the previous cycle and, in addition, nucleolin and nucleophosmin were localized to foci or spherical bodies presenting a central vacuole, i.e. appearing shell-like in the confocal sections.
During the fourth cell cycle, fibrillarin was initially localized to spherical bodies that later during the cell cycle developed a central vacuole. Towards the end of the cell cycle, this protein was localized to more complex bodies showing several distinct intensely labelled foci embedded in a less intensely labelled matrix. During this cell cycle, UBF was initially localized to small discrete foci which later during the cycle appeared to cluster. Towards the end of the cell cycle, UBF was localized to complexes similar to those described for fibrillarin. Nucleolin and nucleophosmin were initially localized to small discrete foci that during the cell cycle developed a central vacuole. Towards the end of the cell cycle, these entities developed into large shell-like bodies as the vacuole expanded in size. Topoisomerase I was detected for the first time around the midpoint of the cell cycle. It was localized to discrete foci which appeared to cluster towards the end of the cycle. RNA polymerase I was detected for the first time towards the end of the cycle in clusters of discrete foci. The gradual localization of this palette of proteins to more complex entities is compatible with the formation of fibrillo-granular nucleoli within the fibrillar spheres as observed by TEM towards the end of the fourth cell cycle. In this context, topoisomerase I, UBF, RNA polymerase I and fibrillarin are thought mainly to be confined to the fibrillar components and nucleolin and nucleophos-min to the GC. During the fifth cell cycle, the same labelling pattern as observed at the end of the fourth was observed throughout the cell cycle.
The FISH analyses demonstrated that the rRNA genes were already associated with silver staining nucleolar proteins from at least the second cell cycle. During the fourth cell cycle, however, they became co-localized with large silver deposits, substantiating their localization within the nucleoli developing during this cell cycle. Interestingly, the non-RNase-treated embryos processed during the third cell cycle displayed a granular labelling pattern of the probe associated with the stronger labelling of the presumptive rRNA genes. This pattern was not identified in the RNase-treated specimens. This feature signals that rRNA may be transcribed already during the third cell cycle before nucleoli are structurally assembled, which is in accordance with results demonstrating a small but repeatable increase in the rRNA hybridization signal on Northern blots of
Ž
.
Schultz, 1997 . On the other hand, it is also possible that the phenomenon is caused by the association of rRNA transcripts of maternal origin with the rRNA genes as has been
Ž .
observed in the Xenopus Verheggen et al., 1998 .
In conclusion, the labelling patterns observed by immunocytochemistry and FISH are compatible with the formation of fibrillo-granular nucleoli towards the end of the fourth cell cycle in which topoisomerase I, UBF, RNA polymerase I and fibrillarin are mainly confined to the fibrillar components and nucleolin and nucleophosmin to the GC, and in which the rRNA genes are located.
3.2. Swine
During the first cell cycle, labelling of nucleophosmin was localized to large shell-like bodies. It is tempting to suggest that these bodies are identical with the electron-dense spheres observed by TEM. The remaining proteins were not localized to nuclear entities. During the second and early third cell cycles, none of the proteins were localized to nuclear entities. This feature indicates that the nucleophosmin observed during the first cell cycle is probably of maternal origin. However, towards the end of the third cell cycle, RNA polymerase I was localized to discrete foci arranged in a shell-like pattern. None of the remaining proteins were localized to nuclear entities. This observation is in accordance with the observation by TEM of the first fibrillo-granular nucleoli established as lentiform structures on the surface of the fibrillar spheres towards the end of this cell cycle. Early during the fourth cell cycle, labelling of topoisomerase I, RNA polymerase I, UBF and fibrillarin were localized to small foci arranged in a shell-like pattern while labelling for nucleolin and nucleophosmin was localized to more or less shell-like bodies. This labelling pattern is compatible with the presence of fibrillo-granular nucleoli as lentiform structures on the surface of the fibrillar sphere with topoisomerase I, UBF, RNA polymerase I and fibrillarin mainly being confined to the fibrillar components and nucleolin and nucleophosmin to the GC. Similar observa-tions were made during the fifth cell cycle.
In conclusion, the labelling patterns observed by immunocytochemistry are compati-ble with the formation of fibrillo-granular nucleoli towards the end of the third cell cycle in which topoisomerase I, UBF, RNA polymerase I and fibrillarin are mainly confined to the fibrillar components and nucleolin and nucleophosmin to the GC.
3.3. ComparatiÕe aspects
In both cattle and swine, nucleolar proteins become gradually localized to the nucleolar anlage. In cattle, the process occurs over several cell cycles with a full compliment of the investigated nucleolar proteins being established towards the end of the fourth cell cycle. In swine, the localization of the proteins to the anlage is more synchronous and occurs towards the end of the third and from the onset of the fourth cell cycle when the full compliment of the investigated proteins is established.
4. Conclusions and perspectives
cycle, respectively. In cattle, the formation of DFC and FCs occurs in the periphery of pre-existing nucleolar anlages appearing as fibrillar spheres, whereas in the swine the DFC and the FCs appear on the surface of these anlages instead. Nucleolar proteins become gradually localized to the nucleolar anlage. In cattle, the process occurs over several cell cycles with a full compliment of the investigated nucleolar proteins being established towards the end of the fourth cell cycle. In swine, the localization of the proteins to the anlage is more synchronous and occurs towards the end of the third cell cycle and from the onset of the fourth, where the full compliment of the investigated proteins is established.
The cell biology of embryonic rRNA gene activation may have some practical implications as the nucleolus formation may serve as a marker for the activation of the embryonic genome. Data obtained in our laboratories indicate that differences exist in nucleolus formation between in vivo developed and in vitro produced swine embryos as well as between in vitro produced cattle embryos and cloned cattle embryos produced by nuclear transfer.
In swine embryos produced by oocyte maturation and fertilization in vitro followed by embryo culture, only fibrillarin and nucleolin displayed a localization to nucleolus-like
Ž .
structures towards the end of the third cell cycle Hyttel et al., unpublished data . The remaining proteins were not localized to nucleolus-like structures except for RNA polymerase I which was localized to foci arranged in shell-like patterns during the fifth cell cycle. In conclusion, in vitro produced swine embryos display a lack of allocation of essential proteins to the nucleolus signalling aberrations in the activation of the rRNA genes.
In cattle embryos produced by nuclear transfer from in vitro produced cattle morulae, all the investigated proteins except for topoisomerase I were localized to nuclear entities
Ž .
during the first and second cell cycle Laurincik et al., unpublished data . The same applied for the third cycle, except that localization of RNA polymerase I was lacking in half of the blastomeres in most embryos. During the fourth cell cycle, only a proportion of the nuclear transfer embryos displayed localization of the investigated proteins to nuclear entities in all blastomeres. In conclusion, as compared with in vitro produced cattle embryos, a substantial proportion of cattle nuclear transfer embryos display aberrations in rRNA gene activation in one or more blastomeres as evaluated by the localization of key nucleolar proteins to nuclear entities.
It is our hope that the increasing basic cell biological understanding of the deviations in embryonic development superimposed by embryo technological procedures will be of value in the continued development of these technologies.
Acknowledgements
The work was supported by the Danish Agricultural and Veterinary Research
¨
Council, Bundesministerium fur Wissenschaft und Verkehr der Republik Osterreich GZ
¨
References
Aris, J.P., Bloberg, G., 1991. cDNA cloning and sequencing of human fibrillarin, a conserved nucleolar protein recognized by autoimmune antisera. Proc. Natl. Acad. Sci. U.S.A. 88, 931–935.
Baserga, S.J., Yang, X.D., Steitz, J.A., 1991. An intact Box C sequence in the U3 snRNA is required for binding of fibrillarin, the protein common to the major family of nucleolar snRNPs. EMBO J. 10, 2645–2651.
Biggiogera, M., Buerki, K., Kaufmann, S.H., Shaper, J.H., Gas, N., Amalric, F., Fakan, S., 1990. Nucleolar distribution of proteins B23 and nucleolin in mouse preimplantation embryos as visualized by immunoelec-tron microscopy. Development 110, 1263–1270.
Bilodeau-Goeseels, S., Schultz, G.A., 1997. Changes in ribosomal ribonucleic acid content within in vitro produced bovine embryos. Biol. Reprod. 56, 1323–1329.
w3 x
Camous, S., Kopecny, V., Flechon, J.E., 1986. Autoradiographic detection of the earliest stage of´ H -uridine incorporation into the cow embryo. Biol. Cell 58, 195–200.
De Sousa, P.A., Watson, A.J., Schultz, G.A., Bilodeau-Goeseels, S., 1998. Oogenetic and zygotic gene expression directing early bovine embryogenesis: a review. Mol. Reprod. Dev. 51, 112–121.
Dumbar, T.S., Gentry, G.A., Olson, M.O.J., 1989. Interaction of nucleolar phosphoprotein B23 with nucleic acid. Biochemistry 28, 9495–9501.
Ghisolfi, L., Joseph, G., Erard, M., Escoubas, J.-M., Mathieu, C., Amalric, F., 1990. Nucleolin-pre-rRNA interactions and preribosome assembly. Mol. Biol. Rep. 14, 113–114.
Ghisolfi-Nieto, L., Joseph, G., Puvion-Dutilleul, F., Amalric, F., Bouvet, P., 1996. Nucleolin is a sequence-specific RNA-binding protein: characterization of targets on pre-ribosomal RNA. J. Mol. Biol. 260, 34–53. Hay-Schmidt, A., Viuff, D., Hyttel, P., 1997. Transcription in in vivo produced bovine zygotes and embryos.
Theriogenology 47, 215.
Hernandez-Verdun, D., 1991. The nucleolus today. J. Cell Sci. 99, 465–471.
Herrera, J.E., Savkur, R., Olson, M.O., 1995. The ribonuclease activity of nucleolar protein B23. Nucleic Acids Res. 23, 3974–3979.
Hozak, P., Cook, P.R., Schofer, C., Mosgoller, W., Wachtler, F., 1994. Site of transcription of ribosomal RNA and intranucleolar structure in HeLa cells. J. Cell Sci. 107, 639–648.
Hyttel, P., Viuff, D., Avery, B., Laurincik, J., Greve, T., 1996. Transcription and cell cycle-dependent development of intranuclear bodies and granules in two-cell bovine embryos. J. Reprod. Fert. 108, 263–270.
Jansen, R.P., Hurt, E.C., Kern, H., Lehtonen, H., Carmo-Fonseca, M., Lapeyre, B., Tollervey, D., 1991. Evolutionary conservation of the human nucleolar protein fibrillarin and its functional expression in yeast. J. Cell Biol. 113, 715–729.
Jordan, P., Mannervik, M., Tora, L., Carmo-Fonseca, M., 1996. In vivo evidence that TATA-binding proteinrSL1 colocalizes with UBF and RNA polymerase I when rRNA synthesis is either active or inactive. J. Cell Biol. 133, 225–234.
King, W.A., Niar, A., Chartrain, I., Betteridge, K.J., Guay, P., 1988. Nucleolus organizer regions and nucleoli in preattachment bovine embryos. J. Reprod. Fert. 82, 87–95.
Kopecny, V., Flechon, J.E., Camous, S., Fulka, J. Jr., 1989. Nucleologenesis and the onset of transcription in the eight-cell bovine embryo: fine-structural autoradiographic study. Mol. Reprod. Dev. 1, 79–90. Lapeyere, B., Bourbon, H., Amalric, F., 1987. Nucleolin, the major nucleolar protein of growing eucaryotic
cells: an unusual protein structure revealed by the nucleotide sequence. Proc. Nat. Acad. Sci. U.S.A. 84, 1472–1476.
Lapeyere, B., Mariottini, P., Mathieu, C., Ferrer, P., Maldi, F., Amalric, F., Caizergues-Ferrer, M., 1990. Molecular cloning of Xenopus fibrillarin, a conserved U3 small nuclear ribonucleoprotein recognized by antisera from humans with autoimmune disease. Mol. Cell. Biol. 10, 430–434.
Laurincik, J., Kopecny, V., Hyttel, P., 1996. Detailed analysis of pronucleus development in bovine zygotex in vivo: ultrastructure and cell cycle chronology. Mol. Reprod. Dev. 43, 62–69.
Laurincik, J., Thomsen, P.D., Hay-Schmidt, A., Avery, B., Greve, T., Ochs, L.R., Hyttel, P., 2000. Nucleolar proteins and nuclear ultrastructure in bovine embryos obtained in vitro. Biol. Reprod. 62, 1024–1032. Li, Y.P., Busch, R.K., Valdez, B.C., Busch, H., 1996. C23 interacts with B23, a putative
Lischwe, M.A., Ochs, R.L., Reddy, R., Cook, R.G., Yeoman, L.C., Tan, E.M., Reichlin, M., Busch, H., 1985.
Ž .
Purification and partial characterization of a nucleolar scleroderma antigen Mrs34,000; pI, 8.5 rich in
NG, NG-dimethylarginine. J. Biol. Chem. 260, 14304–14310.
Maeda, Y., Hisatake, K., Kondo, T., Hanada, K., Song, C.Z., Nishimura, T., Muramatsu, M., 1992. Mouse rRNA gene transcription factor mUBF requires both HMG-box1 and an acidic tail for nucleolar accumulation: molecular analysis of the nucleolar targeting mechanism. EMBO J. 11, 3695–3704. Muller, M.T., Pfund, W.P., Mehta, V.B., Trask, D.K., 1985. Eukaryotic type I topoisomerase is enriched in the
nucleolus and catalytically active on ribosomal DNA. EMBO J. 4, 1237–1243.
Ochs, R.L., Lischwe, M.A., Spohn, W.H., Busch, H., 1985. Fibrillarin: a new protein of the nucleolus identified by autoimmune sera. Biol. Cell 54, 123–133.
Plante, L., Plante, C., Shepherd, D.L., King, W.A., 1994. Cleavage and3H-uridine incorporation in bovine embryos of high in vitro developmental potential. Mol. Reprod. Dev. 39, 375–383.
Raska, I., Reimer, G., Jarnik, M., Kostrouch, Z., Raska, K., 1989. Does the synthesis of ribosomal RNA take place within nucleolar fibrillar centers or dense fibrillar component? Biol. Cell 65, 79–82.
Schnapp, G., Santori, F., Carles, C., Riva, M., Grumm, I., 1994. The HMG box-containing nucleolar transcription factor UBF interacts with a specific subunit of RNA polymerase I. EMBO J. 13, 190–199. Schultz, R.M., 1993. Regulation of zygotic gene activation in the mouse. Bioessays 15, 531–538.
Telford, N.A., Watson, A.J., Schultz, G.A., 1990. Transition form maternal to embryonic control in early mammalian development: a comparison of several species. Mol. Reprod. Dev. 26, 90–100.
Teteja, N., Huang, N.W., Skopac, D., Tuteja, R., Hrvatic, S., Zhang, J., Pongor, S., Joseph, G., Faucher, C., Amalric, F. et al., 1995. Human DNA helicase IV is nucleolin, an RNA helicase modulated by phosphorylation. Gene 160, 143–148.
Thompson, E.M., 1996. Chromatin structure and gene expression in the preimplantation mammalian embryo. Reprod. Nutr. Dev. 36, 619–635.
Tollervey, D., Lehtonen, H., Carmo-Fonseca, M., Hurt, E.C., 1991. The small nucleolar RNP protein NOP1
Žfibrillarin is required for pre-rRNA processing in yeast. EMBO J. 10, 573–583..
Tollervey, D., Lehtonen, H., Jansen, R., Kern, H., Hurt, E.C., 1993. Temperature-sensitive mutations demonstrate roles for yeast fibrillarin in pre-rRNA processing, pre-rRNA methylation, and ribosome assembly. Cell 72, 443–457.
Tomanek, M., Kopecny, V., Kanka, J., 1989. Genome reactivation in developing early pig embryos: an ultrastructural and autoradiographic analysis. Anat. Embryol. 180, 309–316.
Tyc, K., Steitz, J.A., 1989. U3, U8 and U13 comprise a new class of mammalian snRNPs localized in the cell nucleolus. EMBO J. 8, 3113–3119.
Valdez, B.C., Perlaky, L., Henning, D., Saijo, Y., Chan, P.K., Busch, H., 1994. Identification of the nuclear and nucleolar localization signals of the protein p 120. Interaction with translocation protein B23. J. Biol. Chem. 269, 23776–23783.
Verheggen, C., Le Panse, S., Almouzni, G., Hernandez-Verdun, D., 1998. Presence of pre-rRNAs before activation of polymerase I transcription in the building process of nucleoli during early development of
Xenopus laeÕis. J. Cell Biol. 142, 1167–1180.
Viuff, D., Avery, B., Greve, T., King, W.A., Hyttel, P., 1996. Transcriptional activity in in vitro produced bovine two- and four-cell embryos. Mol. Reprod. Dev. 43, 171–179.
Viuff, D., Hyttel, P., Avery, B., Vajta, G., Greve, T., Callesen, H., Thomsen, P.D., 1998. Ribosomal ribonucleic acid is transcribed at the 4-cell stage in in vitro produced bovine embryos. Biol. Reprod. 59, 626–631.
Wachtler, F., Stahl, A., 1993. The nucleolus: a structural and functional interpretation. Micron 24, 473–505. Wang, D., Baumann, A., Szebeni, A., Olson, M.O., 1994. The nucleic acid binding activity of nucleolar
protein B23.1 resides in its carboxyl-terminal end. J. Biol. Chem. 269, 30994–30998.
Watson, A.J., Barcroft, L.C., Betts, D.H., De Sousa, P.A., Gilfoyle, E., Looye, J., Pierre-Louis, J., Winger, Q.A., 1996. Maternal and embryonic control of bovine pre-attachment development: expression of oviductal and embryonic genes. Arch. Tierz. 49, 55–69.