www.elsevier.comrlocateranireprosci
Gonadal steroid receptors in the regulation of
GnRH secretion in farm animals
C.J. Scott
a,b,), A.J. Tilbrook
a, J.A. Rawson
a, I.J. Clarke
ba
Department of Physiology, Monash UniÕersity, Wellington Road, Clayton, Vic 3168, Australia
b
Prince Henry’s Institute of Medical Research, PO Box 5152, Clayton, Vic 3168, Australia
Abstract
The sites of action and mechanisms by which gonadal steroids regulate
gonadotrophin-releas-Ž .
ing hormone GnRH in domestic animals remain largely unknown. This review summarises information gained from sheep regarding the distribution of the gonadal steroid receptors in the brain, the neurochemical identity and the projections of these steroid receptor-containing neurones.
Ž
The cells in the hypothalamus that contain each of the gonadal steroid receptors oestrogen
Ž . Ž . Ž .
receptora ERa , oestrogen receptorb ERb, progesterone receptor PR and androgen receptor
ŽAR..show a remarkably similar distribution, although the PR and AR-containing cells are less
Ž .
widespread than oestrogen receptors ERs . There is considerable overlap in the distribution of ERa- and ERb-containing cells but also some unique sites for each subtype. This suggests differential regulation of the actions of oestrogen. There appears to be little sexual dimorphism in the distribution of the gonadal steroid receptors in the hypothalamus, with the notable exception of the ventromedial nucleus where females appear to have greater numbers of both ERa- and ERb-containing cells. Neuronal tracing studies have identified projections of some of the ERa-containing cells to sites that may allow interaction with the GnRH system. The receptor mapping, neuronal tracing and microimplantation studies suggest that the ventromedial nucleus is likely to be a key hypothalamic nucleus in the steroid regulation of GnRH secretion in sheep.
q2000 Elsevier Science B.V. All rights reserved.
Keywords: Gonadotrophin-releasing hormone; Oestrogen receptor; Progesterone receptor; Androgen receptor;
Sheep
)Corresponding author. Department of Physiology, Monash University, Wellington Road, Clayton, Vic 3168, Australia. Tel.:q61-3-99052552; fax:q61-3-99052547.
Ž .
E-mail address: [email protected] C.J. Scott .
0378-4320r00r$ - see front matterq2000 Elsevier Science B.V. All rights reserved. Ž .
1. Introduction
Oestrogens, androgens and progestogens, produced by the gonads, exert feedback
Ž . Ž
actions to regulate the secretion of luteinizing hormone LH in domestic animals e.g. sheep, Clarke, 1995; Tilbrook and Clarke, 1995; cattle, Burke et al., 1996; pig, Elsaesser
.
et al., 1992 . The major site of this action is within the brain to regulate the secretion of
Ž . Ž
gonadotrophin-releasing hormone GnRH sheep, Clarke, 1995; Tilbrook and Clarke,
.
1995; Skinner et al., 1998; cattle, Gazal et al., 1998 , although oestrogen has a direct
Ž
action on the gonadotrophs of the anterior pituitary gland also Clarke and Cummins,
.
1984 . The actions of these hormones are most likely through the classical steroid
Ž .
receptors Skinner et al., 1998 , which are ligand-activated transcription factors,
al-Ž
though evidence is accumulating for signalling via membrane-bound receptors Moss et
. Ž .
al., 1997 . Two forms of the oestrogen receptor ER have now been identified, the
Ž .
original ER, now designated ERa and a newly identified ERb Kuiper et al., 1996 . Despite reports that a small number of GnRH neurones in the rat brain may contain ERa
ŽButler et al., 1999 and progesterone receptors PRs. Ž . ŽKing et al., 1995 , studies in a.
variety of species consistently indicate that GnRH neurones do not contain ERa, ERb,
Ž . Ž
PR or androgen receptors ARs Shivers et al., 1983; Fox et al., 1990; Leranth et al.,
.
1992; Herbison et al., 1993; Herbison et al., 1996; Laflamme et al., 1998 . It is generally considered, therefore, that the actions of the gonadal steroid hormones must be via neurones that contain these receptors and that projectrrelay to the GnRH neurones.
The neural pathways involved in mediating the actions of the gonadal steroids on GnRH secretion in domestic species remain largely unknown. In recent years, work in our laboratory has focused on determining these pathways in female and male sheep. In this paper, we review some of the work undertaken by our group, and others, to determine the sites of action and pathways involved in the steroid regulation of GnRH secretion in domestic animals. It is worth noting that GnRH neurones from female sheep
Ž .
receive twice as many synaptic contacts as those from male sheep Kim et al., 1999 and, therefore, there are likely to be sex differences in the pathways mediating steroid feedback on GnRH secretion. Accordingly, this review will compare data obtained from both sexes.
2. ERa
2.1. Location of cells containing ERa
The distribution of ERa-containing cells in the hypothalamus of the ewe has been
Ž
well described using immunocytochemistry Herbison et al., 1993; Lehman et al., 1993a;
. Ž
Blache et al., 1994 . The greatest concentration is in the preoptic area the region
.
hypothalamic area, with ERa-containing cells also found in the lateral septum, dorsome-dial hypothalamus and posterior hypothalamus. The distribution of ERa-containing cells in the hypothalamus of the female pig is similar, although numerous cells containing ERa were also found in the stigmoid nucleus and what the authors’ term the
dorsome-Ž .
dial extension of the supraoptic nucleus Van Leeuwen et al., 1995 . The distribution of ERa-containing cells in the hypothalamus of the male pigs appears to be the same as in
Ž .
females Van Leeuwen et al., 1995 . Notably, high numbers of ERa-containing cells are found in a region of the preoptic area of the female pig brain that corresponds to the sexually dimorphic nucleus of rodents. Female pigs also possess a greater number of
Ž
cells containing ERa in the arcuate and ventromedial nuclei than male pigs Van
.
Leeuwen et al., 1995 . The distribution of ERa-containing cells in the hypothalamus of
Ž
male sheep has not been delineated although Herbison unpublished observations cited
.
in Herbison, 1995 has described it as ‘‘approximately similar to that of AR-containing
Ž . Ž .
cells in the ram ’’ vide infra . Cells containing ERa cells have been described in a
Ž .
number of regions of the brainstem in the ewe Scott et al., 1998; Simonian et al., 1998 , including the ventrolateral medulla, nucleus of the solitary tract, and area postrema in the caudal brainstem, and parabrachial nucleus, subcoeruleus region and peri-aqueductal gray in the pons region.
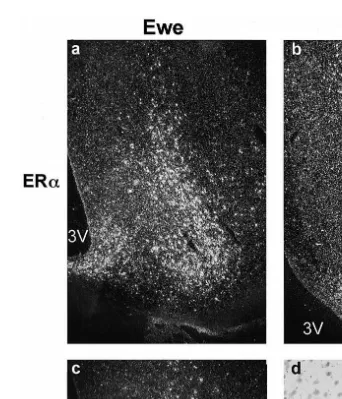
We examined the distribution of ERa mRNA-containing cells in the hypothalamus
Ž
and brainstem of ewes using in situ hybridisation Scott, C.J., Tilbrook, A.J., Simmons,
.
D.M., Rawson, J.A., Chu, S., Fuller, P.J., Ing, N.H., Clarke, I.J., unpublished data and found it to be essentially the same as reported for immunocytochemical studies. Two exceptions were the supraoptic and paraventricular nuclei, which contain only scattered ERa-containing cells as detected by immunocytochemistry, but a dense population of ERa mRNA-containing cells. It remains to be demonstrated if the difference in cell number reflects methodology or whether many ERa mRNA-containing cells in these regions do not produce the ERa protein. We compared the level of ERa mRNA expression in luteal phase ewes and intact rams and preliminary results indicate that the distribution is essentially the same in both sexes, although the amount of ERa mRNA
Ž
per cell is lower in the ventromedial nucleus in rams Scott, C.J., Tilbrook, A.J., Simmons, D.M., Rawson, J.A., Chu, S., Fuller, P.J., Ing, N.H., Clarke, I.J., unpublished
. Ž .
data Fig. 1 .
2.2. Projections of neurones containing ERa
For steroid receptor-containing neurones to regulate GnRH secretion, they must project to GnRH neurones or regions important in the regulation of GnRH secretion. Little is known about the neuronal inputs to GnRH neurones in domestic animals. Retrograde neuronal tracing has been performed in the ewe to characterise the cells that
Ž
project to the region of the GnRH cells, in the medial preoptic area e.g. Tillet et al.,
.
1993 . ERa-containing cells that project to the medial preoptic area of the ewe originate in the preoptic area, lateral septum, arcuate nucleus and caudal ventromedial nucleus
ŽGoubillon et al., 1999 , as well as the ventrolateral medulla of the brainstem Scott et. Ž .
Ž . Ž .
Fig. 1. Photomicrographs of ER mRNA in the ventromedial nucleus: a ERa in a ewe; b ERa mRNA in a
Ž . Ž . Ž
ram; c ERbmRNA in a ewe; d ERbmRNA in a ram bright field at higher power to show lack of silver
.
grain accumulation over Nissl stained cells . 3V: third ventricle. Scale bars100mm.
neurones, the ERa-containing cells in these hypothalamic and brainstem nuclei are well placed to regulate GnRH neurones.
It is also possible that oestrogen influences GnRH secretion through neurones that are activated by oestrogen and project to terminals within the neurosecretory zone of the
Ž .
Ž .
the lamina terminalis OVLT and arcuate nucleus project to the median eminence. It should be noted, however, that synaptic input is very difficult to find at this level and
Ž .
only one study Kuljis and Advis, 1989 has reported such interaction.
There is much evidence that retrochiasmatic area is involved in enhancing the
Ž .
negative feedback actions of oestrogen during anoestrus in the ewe Thiery et al., 1995 ,
Ž
although no ERa-containing cells have been detected in this nucleus Lehman et al.,
.
1993a . Nevertheless, the influence of estrogen could be via cells from other brain regions that project to the retrochiasmatic area. Using retrograde tracing coupled with immunocytochemistry, ERa-containing cells that input to the retrochiasmatic area of the ewe were identified as projecting from the rostral medial preoptic area, anterior
Ž
hypothalamic area, ventromedial nucleus and arcuate nucleus Jansen, Heiko T.,
per-.
sonal communication . In a study comparing anoestrous and cycling ewes, Lehman et al.
Ž1996 used immunocytochemistry for the Fos-related antigens as markers of long-term.
cellular activation by oestrogen. They found that only cells in the rostral preoptic area and the retrochiasmatic area showed activation specifically during anoestrus and not during the breeding season. Thus, there appears to be a population of cells in these nuclei that are oestrogen responsive only during anoestrus. Taken together, these two studies would suggest that the negative feedback action of oestrogen during anoestrus may be mediated in part via cells in the preoptic area that contain ERa and project to the retrochiasmatic area. An alternative hypothesis for the actions of oestrogen on this nucleus is outlined in Section 3.2.
In an effort to determine which sites in the hypothalamus are relevant to the regulation of GnRH secretion by oestrogen, microimplants of oestrogen were placed into various brain sites of gonadectomised ewes and rams and the effect on LH was
Ž .
evaluated. Implants in the ventromedial nucleus but not the preoptic area induced an
Ž .
LH surge in ovariectomized ewes Blache et al., 1991 and implants into the arcuate nucleus–ventromedial region of castrated rams were effective in reducing LH secretion
ŽBlache et al., 1997; Scott et al., 1997 . These data, combined with those of retrograde.
tracing studies, suggest that one important pathway for oestrogen feedback in ewes and rams may involve ERa-containing cells in the ventromedial nucleus that project to the preoptic area. Notably, the nature of this feedback differs between sexes, with oestrogen having a positive feedback action at this site in ewes and a negative feedback action on GnRH secretion in males. The origin of ERa-containing cells that project to the preoptic area has not been described in males.
2.3. Neurochemical identity of neurones containing ERa
A key to understanding the feedback actions of gonadal steroids is a determination of the neurochemical identity of the neurones containing their receptors. The neurochemi-cal identity of the majority of ERa-ir cells remain to be elucidated, even in the arcuate
Ž .
Table 1
Neuronal cells that contain ERa-ir in the sheep brain
Neurotransmitter Nucleus Percentage of Reference
or marker labelled cells that
contain ERa-ir
Ž . Ž
Tyrosine hydroxylase Arcuate A12 -10% of Batailler et al.
ŽDopamine. ER-ir cells. Ž1992.
Preoptic area A14 0% Lehman and
Ž .
Glutamic acid decarboxylase Preoptic area 44% Herbison et al.
ŽGABA. Ž1993.
b-Endorphin Arcuate 15–20% Lehman and
Ž .
Karsch 1993b
Neuropeptide Y Arcuate 3% Skinner and
Ž .
Herbison 1997
Ž Ž .
Somatostatin Ventromedial Nucleus ;35% ;70% Herbison 1995
.
of ER-ir cells
Ž .
Dopaminebhydroxylase Ventrolateral medulla A1 20–70% Simonian et al.
Žnoradrenaline. Ž1998.
Ž .
4–20% Scott et al. 1999
Nucleus of the solitary 16–60% Simonian et al.
Ž . Ž .
tract A2 1998
Ž .
8–25% Scott et al. 1999
GnRH Preoptic area 0% Herbison et al.
Ž1993.
Lehman and Karsch
Ž1993b.
produce neurotransmitters not yet identified. The neurochemical identity of ERa -con-taining cells in the brain of male sheep has not been described.
3. ERb
3.1. Location of cells containing ERb
Ž .
ERa mRNA-containing cells Hileman et al., 1999 . The highest levels of ERb
expression were found in the preoptic area, bed nucleus of the stria terminalis, paraventricular nucleus and supraoptic nucleus. In contrast to ERa mRNA, ERb
mRNA was observed in the retrochiasmatic area, but very few ERb mRNA-containing cells were observed in the ventromedial nucleus and arcuate nucleus.
Ž .
We have cloned a portion of ovine ERb Genebank accession number applied for and compared the distribution and abundance of ERb mRNA in the hypothalamus of ewes in the luteal phase of the oestrous cycle and intact rams using in situ hybridisation
ŽScott, C.J., Tilbrook, A.J., Simmons, D.M. Rawson, J.A., Chu, S., Fuller, P.J., Ing,
.
N.H., Clarke, I.J., unpublished data . Preliminary results indicate that the distribution and level of expression of ERbmRNA is similar in rams and ewes with the exception of the ventromedial and arcuate nuclei where moderate levels were detected in the ewe;
Ž .
there was almost no ERb mRNA in this region of the ram brain Fig. 1 . We compared
Ž
the distribution of ERb mRNA with ERa mRNA in ewes and rams as outlined in
. Ž
Section 2.1 and a number of differences were observed Scott, C.J., Tilbrook, A.J., Simmons, D.M., Rawson, J.A., Chu, S., Fuller, P.J., Ing, N.H., Clarke, I.J., unpublished
.
data . In both sexes, ERa-mRNA was widely distributed in the preoptic area, whereas in the rostral preoptic area, ERb mRNA was localised to a thin strip down the midline of the brain. In addition, the preoptic area, lateral septum, ventromedial and arcuate nuclei all contained higher numbers of ERa than ERb mRNA-containing cells. By contrast, the median preoptic nucleus, and the zona incerta contained higher numbers of ERb
than ERa mRNA-containing cells, with the retrochiasmatic nucleus containing only ERb mRNA.
3.2. A role for ERb — the control of LH secretion?
Ž .
Male mice lacking a functional ERb appear to be fully fertile Krege et al., 1998 . This, combined with a paucity of ERb-containing cells in hypothalamic nuclei known to
Ž .
be important in the regulation of GnRH secretion vide supra , suggests that ERb may not be involved in the feedback regulation of GnRH secretion. Nevertheless, the
Ž
presence of ERb mRNA-containing cells in the retrochiasmatic nucleus A15
dopamin-.
ergic cell group of the sheep, raises an intriguing possibility of involvement in
Ž .
oestrogen negative feedback during anoestrus Thiery et al., 1995 . Implantation of oestrogen directly into the retrochiasmatic nucleus suppressed plasma LH levels in
Ž .
ovariectomised ewes during an inhibitory photoperiod Gallegos-Sanchez et al., 1997 ,
Ž . Ž .
yet ERa has not been detected in this nucleus Lehman et al., 1993a vide supra . It remains possible, therefore, that oestrogen acts via ERb within the retrochiasmatic nucleus to inhibit LH secretion during the non-breeding season in the sheep.
4. Progesterone receptor
4.1. Location of cells containing PR
Recent evidence suggests that the negative feedback actions of progesterone are
Ž .
immunocytochem-Ž .
istry to map the distribution of PR in the hypothalamus of the ewe Scott et al., 2000 . The largest population of PR-containing cells was found in the preoptic area, especially in the medial region, around the OVLT. The arcuate and ventromedial nuclei also contained large numbers of cells containing PR, with moderate numbers in the periven-tricular and supraoptic nuclei, and scattered cells in the diagonal band of Broca. We did not detect any PR-containing cells in the brainstem.
We were readily able to detect PR cells in ovariectomised ewes, which was surprising considering that, in other species, significant numbers have been detected following
Ž .
oestrogen treatment e.g. Blaustein and Turcotte, 1989; Bethea et al., 1992 . To confirm
Ž .
this, we used in situ hybridisation with a riboprobe for ovine PR Ing et al., 1996 . PR mRNA-containing cells were clearly observed in the hypothalamus of ovariectomised
Ž .
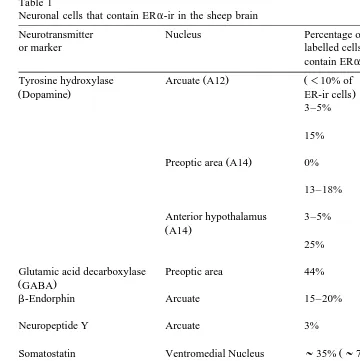
ewes Scott et al., 2000 , with a similar distribution to the PR protein as detected with immunocytochemistry. Treatment of ovariectomised ewes with a subcutaneous oestro-gen implant for 14 days increased the number of PR-containing cells in the ventromedial
Ž . Ž .
and arcuate nuclei Fig. 2 , but not in other hypothalamic regions Scott et al., 2000 . This observation highlights the importance of these nuclei in the regulation of steroid feedback in the sheep. In the ewe, LH secretion is altered by implantation of
proges-Ž . Ž .
terone Blache et al., 1996 or oestrogen Blache et al., 1991 into the ventromedial nucleus. These steroids are thought to act in synergy to regulate LH secretion in the
Ž .
sheep Goodman and Karsch, 1980 , with oestrogen enhancing the efficacy of
proges-Fig. 2. Low power photomicrographs showing the effect of oestrogen treatment on PR-immunoreactive cells in
Ž . Ž .
the mediobasal hypothalamus: a ventromedial nucleus in an oestrogen treated ewe; b ventromedial nucleus
Ž . Ž .
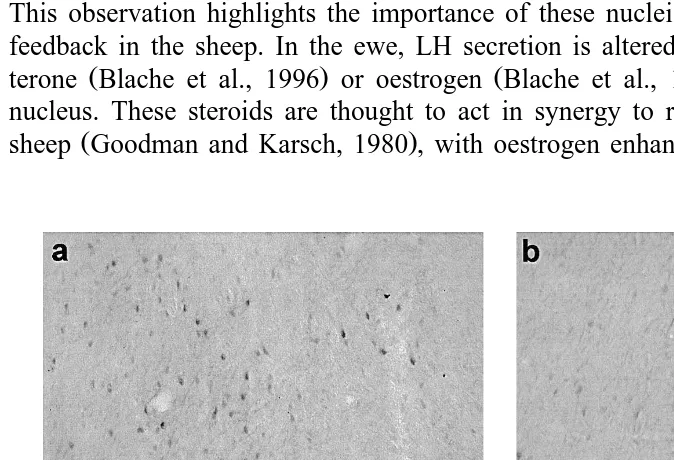
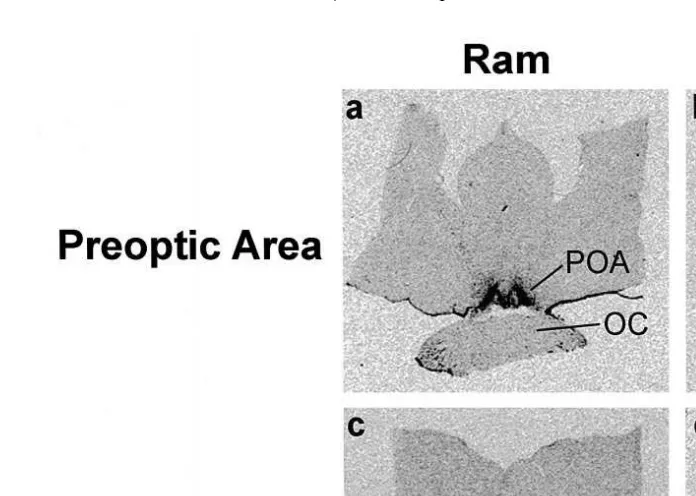
Fig. 3. Film autoradiograms showing the distribution of PR mRNA-containing cells in the hypothalamus of a
Ž . Ž . Ž . Ž .
ram and ewe: a preoptic area of a ram; b preoptic area of a ewe; c mediobasal hypothalamus of a ram; d mediobasal hypothalamus of a ewe. 3V: third ventricle; ARC: arcuate nucleus; OC: optic chiasm; POA: preoptic area; VMN: ventromedial nucleus. Scale bars2 mm.
terone negative feedback. The results above suggest that this effect of oestrogen may be achieved via an increase in PR-containing cells. This reinforces the notion that, in the ewe, the ventromedial and arcuate nuclei are major sites for these steroids to exert dual regulation of LH secretion.
Progesterone, although a precursor for testosterone synthesis, is not normally associ-ated with feedback regulation of gonadotropin secretion in males. Nonetheless, synthetic
Ž
progestogens are showing great promise as a reversible contraceptive for men WHO,
.
1999 by virtue of their ability to suppress gonadotropin secretion. Accordingly, we used in situ hybridisation to determine if PR mRNA is produced in the brain of male sheep
ŽScott, C.J., Tilbrook, A.J., Clarke, I.J., unpublished observations and found expression.
Ž .
similar to that in females Fig. 3 . Whether these receptors have a functional role in the feedback regulation of GnRH secretion in male sheep is currently being determined.
4.2. Neurochemical identity of neurones containing PR
Ž .
the preoptic area Robinson and Kendrick, 1992 , dopamine levels in the mediobasal
Ž . Ž
hypothalamus Fabre-Nys et al., 1994 , andb-endorphin in the arcuate nucleus Yang et
.
al., 1988; Whisnant et al., 1991 . Progesterone treatment altered levels of
pre-proen-Ž
kephalin mRNA in the ventromedial nucleus of ovariectomised ewes Broad et al.,
. Ž
1993 , although we did not detect any changes across the estrous cycle Walsh, J.P.,
.
Clarke, I.J., unpublished . There is also evidence that endogenous opioids mediate
Ž . Ž .
progesterone feedback in cattle Stumpf et al., 1993 and pigs Okrasa et al., 1992 . Thus, in these species, the cells producing these neurotransmitters are good candidates to also co-express PR.
5. AR
5.1. Location of cells containing AR
The distribution of AR-containing cells in the ram hypothalamus has been described using immunocytochemistry and large populations were found in the preoptic area,
Ž .
ventromedial, arcuate and premamillary nuclei Herbison et al., 1996 . Numerous cells containing AR were also detected in the bed nucleus of the stria terminalis and anterior
Ž
hypothalamic area. We have used in situ hybridisation for AR in the ram Scott, C.J.,
.
Tilbrook, A.J., Rao, A., Chu, S., Fuller, P.J., Clarke, I.J., unpublished and found a similar distribution. We also found moderate labelling of cells in the median preoptic nucleus and the stria terminalis. This distribution of ARs is similar to that in the rat
ŽSimerly et al., 1990 . It is notable, however, that the bed nucleus of the stria terminalis.
contains very high levels of AR expression in the rat brain, but only moderate numbers of AR-containing cells are found at this site in the ram brain.
5.2. Neurochemical identity of neurones containing AR
Ž .
Herbison et al. 1996 have shown that GnRH neurones do not contain AR, nor do the majority of dopaminergic neurones. Only a small number of tyrosine hydroxylase
ŽTH positive cells in the lateral hypothalamus contained AR, while none of the TH.
positive cells in the major hypothalamic dopamine cell groups contained AR. It therefore appears unlikely that testosterone feedback is mediated via dopaminergic cells in the ram. Around 40% of somatostatin cells in the ventromedial nucleus contain AR, which accounted for nearly half of the AR-ir cells in that nucleus. It is possible that these somatostatin cells may be involved in the feedback regulation of GnRH secretion in the ram, but the role of somatostatin in the control of GnRH secretion has not been examined. On the other hand, dihydrotestosterone implants into the ventromedial
Ž .
6. Conclusions
Receptors for the gonadal steroids are widespread in the hypothalamus and in other brain regions, but the distribution is remarkably consistent between sexes and across receptor types. It seems likely, therefore, that there are common pathways for the actions of gonadal steroids on GnRH secretion. Much work is required, however, to establish the different neurochemical identities of these cells. Since they are potentially involved in a number of different brain functions, it becomes important to ‘dissect’ those that subserve neuroendocrine systems. The largely anatomical studies of steroid receptor-containing cells now provide a useful framework that will allow more discrete mapping of the neural pathways to the GnRH neurones.
Acknowledgements
Original work performed in this laboratory was supported by grants from the National
Ž .
Health and Medical Research Council of Australia NH & MRC , the Australian Re-search Council and Monash University. C.J.S. was the recipient of an Australian Postdoctoral Fellowship of the NH & MRC.
References
Batailler, M., Blache, D., Thibault, J., Tillet, Y., 1992. Immunohistochemical colocalization of tyrosine hydroxylase and estradiol receptors in the sheep arcuate nucleus. Neurosci. Lett. 146, 125–130. Bethea, C.L., Fahrenbach, W.H., Sprangers, S.A., Freesh, F., 1992. Immunocytochemical localization of
progestin receptors in monkey hypothalamus: effect of oestrogen and progestin. Endocrinology 130, 895–905.
Blache, D., Batailler, M., Fabre-Nys, C., 1994. Oestrogen receptors in the preoptico-hypothalamic continuum: immunohistochemical study of the distribution and cell density during induced oestrous cycle in ovariec-tomized ewe. J. Neuroendocrinol. 6, 329–339.
Blache, D., Fabre-Nys, C.J., Venier, G., 1991. Ventromedial hypothalamus as a target for oestradiol action on proceptivity, receptivity and luteinizing hormone surge of the ewe. Brain Res. 546, 241–249.
Blache, D., Fabre-Nys, C., Venier, G., 1996. Inhibition of sexual behaviour and the luteinizing hormone surge by intracerebral progesterone implants in the female sheep. Brain Res. 741, 117–122.
Blache, D., Tjondronegoro, S., Blackberry, M.A., Anderson, S.T., Curlewis, J.D., Martin, G.B., 1997. Gonadotrophin and prolactin secretion in castrated male sheep following subcutaneous or intracranial treatment with testicular hormones. Endocrine 7, 235–243.
Blaustein, J.D., Turcotte, J.C., 1989. Estradiol-induced progestin receptor immunoreactivity is found only in oestrogen receptor-immunoreactive cells in guinea pig brain. Neuroendocrinology 49, 454–461. Broad, K.D., Kendrick, K.M., Sirinathsinghji, D.J., Keverne, E.B., 1993. Changes in pro-opiomelanocortin
and pre-proenkephalin mRNA levels in the ovine brain during pregnancy, parturition and lactation and in response to oestrogen and progesterone. J. Neuroendocrinol. 5, 711–719.
Burke, C.R., Macmillan, K.L., Boland, M.P., 1996. Oestradiol potentiates a prolonged progesterone-induced suppression of LH release in ovariectomised cows. Anim. Reprod. Sci. 45, 13–28.
Butler, J.A., Sjoberg, M., Coen, C.W., 1999. Evidence for oestrogen receptor alpha-immunoreactivity in gonadotrophin-releasing hormone-expressing neurones. J. Neuroendocrinol. 11, 331–335.
Clarke, I.J., Cummins, J.T., 1984. Direct pituitary effects of oestrogen and progesterone on gonadotropin secretion in the ovariectomized ewe. Neuroendocrinology 39, 267–274.
Elsaesser, F., Parvizi, N., Foxcroft, G.R., 1992. Control of the LH surge mechanism in the female pig. J. Physiol. Pharmacol. 43, 69–78.
Fabre-Nys, C., Blache, D., Hinton, M.R., Goode, J.A., Kendrick, K.M., 1994. Microdialysis measurement of neurochemical changes in the mediobasal hypothalamus of ovariectomized ewes during oestrus. Brain Res. 649, 282–296.
Fox, S.R., Harlan, R.E., Shivers, B.D., Pfaff, D.W., 1990. Chemical characterization of neuroendocrine targets for progesterone in the female rat brain and pituitary. Neuroendocrinology 51, 276–283.
Gallegos-Sanchez, J., Delaleu, B., Caraty, A., Malpaux, B., Thiery, J.C., 1997. Estradiol acts locally within the retrochiasmatic area to inhibit pulsatile luteinizing-hormone release in the female sheep during anestrus. Biol. Reprod. 56, 1544–1549.
Gazal, O.S., Leshin, L.S., Stanko, R.L., Thomas, M.G., Keisler, D.H., Anderson, L.L., Williams, G.L., 1998. Gonadotropin-releasing hormone secretion into third-ventricle cerebrospinal fluid of cattle: correspondence with the tonic and surge release of luteinizing hormone and its tonic inhibition by suckling and neuropeptide Y. Biol. Reprod. 59, 676–683.
Goodman, R.L., Karsch, F.J., 1980. Pulsatile secretion of luteinizing hormone: differential suppression by ovarian steroids. Endocrinology 107, 1286–1290.
Goubillon, M.-L., Delaleu, B., Tillet, Y., Caraty, A., Herbison, A.E., 1999. Localization of estrogen-receptive neurons projecting to the GnRH neuron-containing rostral preoptic area of the ewe. Neuroendocrinology 70, 228–236.
Herbison, A.E., 1995. Neurochemical identity of neurones expressing oestrogen and androgen receptors in sheep hypothalamus. J. Reprod. Fertil., Suppl. 49, 271–283.
Herbison, A.E., Robinson, J.E., Skinner, D.C., 1993. Distribution of oestrogen receptor-immunoreactive cells in the preoptic area of the ewe: co-localization with glutamic acid decarboxylase but not luteinizing hormone-releasing hormone. Neuroendocrinology 57, 751–759.
Herbison, A.E., Skinner, D.C., Robinson, J.E., King, I.S., 1996. Androgen receptor-immunoreactive cells in ram hypothalamus: distribution and co-localization patterns with gonadotropin-releasing hormone, somato-statin and tyrosine hydroxylase. Neuroendocrinology 63, 120–131.
Hileman, S.M., Handa, R.J., Jackson, G.L., 1999. Distribution of oestrogen receptor-beta messenger ribonu-cleic acid in the male sheep hypothalamus. Biol. Reprod. 60, 1279–1284.
Ing, N.H., Spencer, T.E., Bazer, F.W., 1996. Oestrogen enhances endometrial oestrogen receptor gene expression by a posttranscriptional mechanism in the ovariectomized ewe. Biol. Reprod. 54, 591–599. Jansen, H.T., Hileman, S.M., Lubbers, L.S., Jackson, G.L., Lehman, M.N., 1996. A subset of oestrogen
receptor-containing neurons project to the median eminence in the ewe. J. Neuroendocrinol. 8, 921–927. Kim, S.-J., Foster, D.L., Wood, R.I., 1999. Prenatal testosterone masculinizes synaptic input to
gonadotropin-releasing hormone neurons in sheep. Biol. Reprod. 61, 599–605.
King, J.C., Tai, D.W., Hanna, I.K., Pfeiffer, A., Haas, P., Ronsheim, P.M., Mitchell, S.C., Turcotte, J.C., Blaustein, J.D., 1995. A subgroup of LHRH neurons in guinea pigs with progestin receptors is centrally positioned within the total population of LHRH neurons. Neuroendocrinology 61, 265–275.
Krege, J.H., Hodgin, J.B., Couse, J.F., Enmark, E., Warner, M., Mahler, J.F., Sar, M., Korach, K.S., Gustafsson, J.A., Smithies, O., 1998. Generation and reproductive phenotypes of mice lacking oestrogen receptor beta. Proc. Natl. Acad. Sci. U. S. A. 95, 15677–15682.
Kuiper, G.G.J.M., Enmark, E., Pelto-Huikko, M., Nilsson, S., Gustafsson, J.-A., 1996. Cloning of a novel estrogen receptor expressed in rat prostate and ovary. Proc. Natl. Acad. Sci. U. S. A. 93, 5925–5930. Kuljis, R.O., Advis, J.P., 1989. Immunocytochemical evidence of a synapse between dopamine-and luteinizing
hormone releasing hormone-containing neurons in the ewe median eminence. Endocrinology 124, 1579– 1581.
Laflamme, N., Nappi, R.E., Drolet, G., Labrie, C., Rivest, S., 1998. Expression and neuropeptidergic
Ž .
characterization of oestrogen receptors ERalpha and ERbeta throughout the rat brain: anatomical evidence of distinct roles of each subtype. J. Neurobiol. 36, 357–378.
Lehman, M.N., Ebling, F.J., Moenter, S.M., Karsch, F.J., 1993a. Distribution of oestrogen receptor-im-munoreactive cells in the sheep brain. Endocrinology 133, 876–886.
Lehman, M.N., Karsch, F.J., 1993b. Do gonadotropin-releasing hormone, tyrosine hydroxylase-, and beta-en-dorphin-immunoreactive neurons contain oestrogen receptors? A double-label immunocytochemical study in the Suffolk ewe. Endocrinology 133, 887–895.
Lehman, M.N., Robinson, J.E., Karsch, F.J., Silverman, A.J., 1986. Immunocytochemical localization of
Ž .
luteinizing hormone-releasing hormone LHRH pathways in the sheep brain during anestrus and the mid-luteal phase of the estrous cycle. J. Comp. Neurol. 244, 19–35.
Leranth, C., MacLusky, N.J., Brown, T.J., Chen, E.C., Redmond, D.E.J., Naftolin, F., 1992. Transmitter content and afferent connections of oestrogen-sensitive progestin receptor-containing neurons in the primate hypothalamus. Neuroendocrinology 55, 667–682.
Moss, R.L., Gu, Q., Wong, M., 1997. Oestrogen: nontranscriptional signaling pathway. Recent Prog. Horm. Res. 52, 33–68.
Okrasa, S., Kalamarz, H., Tilton, J.E., Ziecik, A.J., 1992. Influence of opioids on LH secretion in gilts during the estrous cycle. J. Physiol. Pharmacol. 43, 105–116.
Robinson, J.E., Kendrick, K.M., 1992. Inhibition of luteinizing hormone secretion in the ewe by progesterone: associated changes in the release of gamma-aminobutyric acid and noradrenaline in the preoptic area as measured by intracranial microdialysis. J. Neuroendocrinol. 4, 231–236.
Scott, C.J., Kuehl, D.E., Ferreira, S.A., Jackson, G.L., 1997. Hypothalamic sites of action for testosterone, dihydrotestosterone, and oestrogen in the regulation of luteinizing hormone secretion in male sheep. Endocrinology 138, 3686–3694.
Scott, C.J., Pereira, A.M., Rawson, J.A., Simmons, D.M., Rossmanith, W.G., Ing, N.H., Clarke, I.J., 2000.
Ž . Ž .
The distribution of progesterone receptor PR immunoreactivity -ir and PR mRNA in the preoptic area and hypothalamus of the ewe; up regulation of PR mRNA in the mediobasal hypothalamus by estrogen. J. Neuroendocrinol. 12, 565–575.
Scott, C.J., Rawson, J.A., Pereira, A.M., Clarke, I.J., 1998. The distribution of oestrogen receptors in the brainstem of female sheep. Neurosci. Lett. 241, 29–32.
Scott, C.J., Rawson, J.A., Pereira, A.M., Clarke, I.J., 1999. Oestrogen receptors in the brainstem of the female sheep: relationship to noradrenergic cells and cells projecting to the medial preoptic area. J. Neuroen-docrinol. 11, 745–755.
Shivers, B.D., Harlan, R.E., Morrell, J.I., Pfaff, D.W., 1983. Absence of oestradiol concentration in cell nuclei of LHRH-immunoreactive neurones. Nature 304, 345–347.
Simerly, R.B., Chang, C., Muramatsu, M., Swanson, L.W., 1990. Distribution of androgen and oestrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J. Comp. Neurol. 294, 76–95.
Simonian, S.X., Delaleu, B., Caraty, A., Herbison, A.E., 1998. Oestrogen receptor expression in brainstem noradrenergic neurons of the sheep. Neuroendocrinology 67, 392–402.
Skinner, D.C., Evans, N.P., Delaleu, B., Goodman, R.L., Bouchard, P., Caraty, A., 1998. The negative feedback actions of progesterone on gonadotropin-releasing hormone secretion are transduced by the classical progesterone receptor. Proc. Natl. Acad. Sci. U. S. A. 95, 10978–10983.
Skinner, D.C., Herbison, A.E., 1997. Effects of photoperiod on oestrogen receptor, tyrosine hydroxylase, neuropeptide Y, and beta-endorphin immunoreactivity in the ewe hypothalamus. Endocrinology 138, 2585–2595.
Stumpf, T.T., Roberson, M.S., Wolfe, M.W., Hamernik, D.L., Kittok, R.J., Kinder, J.E., 1993. Progesterone, 17 beta-estradiol, and opioid neuropeptides modulate pattern of luteinizing hormone in circulation of the cow. Biol. Reprod. 49, 1096–1101.
Thiery, J.C., Gayrard, V., Le, C.S., Viguie, C., Martin, G.B., Chemineau, P., Malpaux, B., 1995. Dopaminer-gic control of LH secretion by the A15 nucleus in anoestrous ewes. J. Reprod. Fertil., Suppl. 49, 285–296. Tilbrook, A.J., Clarke, I.J., 1995. Negative feedback regulation of the secretion and actions of GnRH in male
ruminants. J. Reprod. Fertil., Suppl. 49, 297–306.
Tillet, Y., Batailler, M., Thibault, J., 1993. Neuronal projections to the medial preoptic area of the sheep, with special reference to monoaminergic afferents: immunohistochemical and retrograde tract tracing studies. J. Comp. Neurol. 330, 195–220.
Ž
the distribution of oestrogen receptors in the septal area and hypothalamus of the domestic pig Sus .
scrofa . Neuroscience 64, 261–275.
Whisnant, S.C., Havern, R.L., Goodman, R.L., 1991. Endogenous opioid suppression of luteinizing hormone pulse frequency and amplitude in the ewe: hypothalamic sites of action. Neuroendocrinology 54, 587–593. WHO, 1999. In: Annual Technical Report 1998. World Health Organization, Geneva, pp. 105–109. Yang, K., Haynes, N.B., Lamming, G.E., Brooks, A.N., 1988. Ovarian steroid hormone involvement in