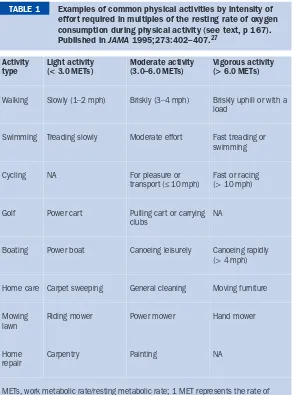
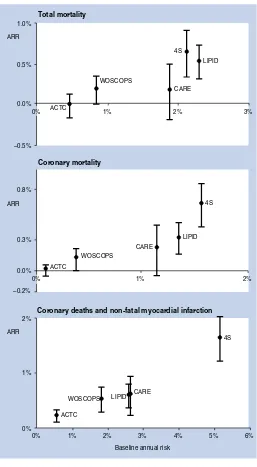
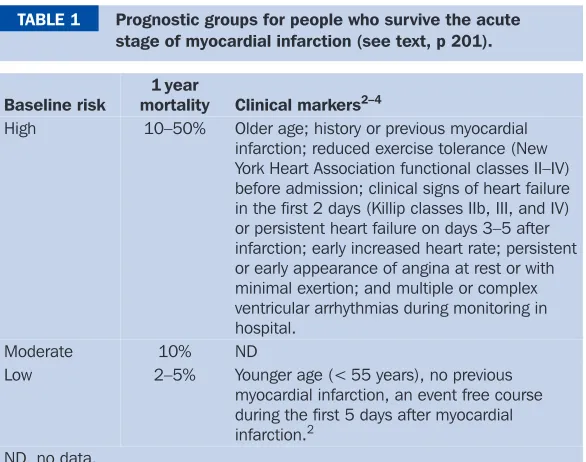
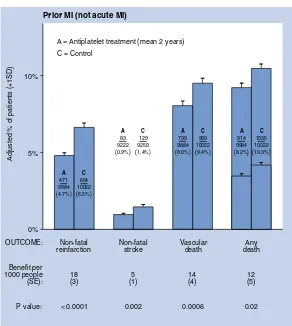
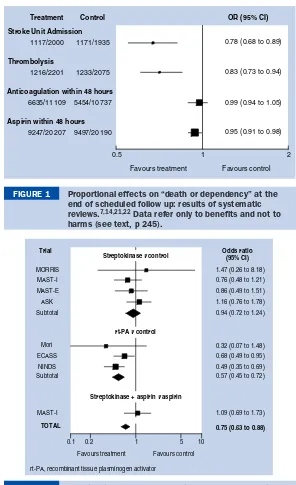
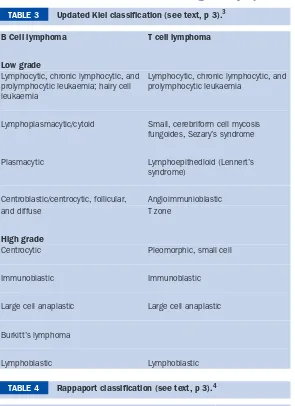
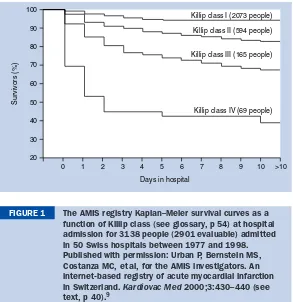
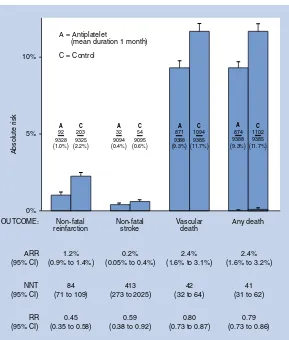
BMJ Clinical Evidence and Clinical Evidence Concise EBM2004 pdf
Teks penuh
Gambar
Dokumen terkait
Kekuatan yang dimiliki UPK antara lain prose- dur dan syarat pengajuan kredit mudah dan ringan, ada pendampingan kelompok, pelaksa- naan tanggung renteng berjalan
Secara khusus untuk mengetahui pengaruh motivasi terhadap kinerja pegawai di kantor kecamatan Siantar Utara kota Pematangsiantar. Metode yang digunakan dalam penelitian ini
Anda harapkan termasuk imbalan-imbalan tambahan yang akan diterima jika mereka dibutuhkan untuk kerja lembur, pekerjaan di luar tugas, waktu libur dipanggil, dan sebagainya,
Film Talak 3 adalah sebuah film ber genre romantic comedy yang disutradarai oleh Hanung Bramantyo dan Ismail Basbeth pada tahun 2016. Ismail Basbeth merupakan sutradara
King James ataupun Alkitab berbahasa Indonesia versi Terjemahan Baru. b) Terdapat fitur Go To , yang dimana user dapat menampilkan ayat yang diinginkannya. Namun
6.2.1 Alat uji ketahanan sobek metode Elmendorf dengan perlengkapan sebagai berikut. a) Alat penjepit yang terdiri dari sebuah penjepit statis dan sebuah penjepit yang dapat
Japan was industrialized countries having a culture is very fast in many ways .This usually measured by work of one of his people that can cut through a market in every country in
Jumlah populasi pengusaha jahit yang ada di Pasar Sunan Giri berdasarkan penghitungan yang telah dilakukan dalam pra riset, yaitu berjumlah 84 orang, yang terdiri dari