David Publishing Company www.davidpublishing.org
P u b l i s h i n g Dav i d
Journal of Life Sciences
Publication Information
Journal of Life Sciences is published monthly in hard copy (ISSN 1934-7391) and online (ISSN 1934-7405) by David Publishing Company located at 1840 Industrial Drive, Suite 160, Libertyville, Illinois 60048, USA.
Aims and Scope
Journal of Life Sciences, a monthly professional academic journal, covers all sorts of researches on molecular biology, microbiology, botany, zoology, genetics, bioengineering, ecology, cytology, biochemistry, and biophysics, as well as other issues related to life sciences.
Editorial Board Members
Dr. Stefan Hershberger (USA), Dr. Suiyun Chen (China), Dr. Farzana Perveen (Pakistan), Dr. Francisco Torrens (Spain), Dr. Filipa João (Portugal), Dr. Masahiro Yoshida (Japan), Dr. Reyhan Erdogan (Turkey), Dr. Grzegorz Żurek (Poland), Dr. Ali Izadpanah (Canada), Dr. Barbara Wiewióra (Poland), Dr. Valery Lyubimov (Russia), Dr. Amanda de Moraes Narcizo (Brasil), Dr. Marinus Frederik Willem te Pas (The Netherlands), Dr. Anthony Luke Byrne (Australia), Dr. Xingjun Li (China), Dr. Stefania Staibano (Italy), Dr. Wenle Xia (USA), Hamed Khalilvandi-Behroozyar (Iran).
Manuscripts and correspondence are invited for publication. You can submit your papers via Web Submission, or E-mail to [email protected] or [email protected]. Submission guidelines and Web Submission system are available at http://www.davidpublishing.org.
Editorial Office
1840 Industrial Drive, Suite 160, Libertyville, Illinois 60048 Tel: 1-847-281-9862, Fax: 1-847-281-9855
E-mail:[email protected], [email protected]
Copyright©2011 by David Publishing Company and individual contributors. All rights reserved. David Publishing Company holds the exclusive copyright of all the contents of this journal. In accordance with the international convention, no part of this journal may be reproduced or transmitted by any media or publishing organs (including various websites) without the written permission of the copyright holder. Otherwise, any conduct would be considered as the violation of the copyright. The contents of this journal are available for any citation. However, all the citations should be clearly indicated with the title of this journal, serial number and the name of the author.
Abstracted / Indexed in
Database of EBSCO, Massachusetts, USA Chemical Abstracts Service (CAS), USA Cambridge Scientific Abstracts (CSA), USA
Chinese Database of CEPS, American Federal Computer Library center (OCLC), USA Ulrich’s Periodicals Directory, USA
Chinese Scientific Journals Database, VIP Corporation, Chongqing, China
Subscription Information
Price (per year): Print $420, Online $300, Print and Online $560.
David Publishing Company
1840 Industrial Drive, Suite 160, Libertyville, Illinois 60048 Tel: 1-847-281-9862, Fax: 1-847-281-9855
J LS
Journal of Life Sciences
Volume 5, Number 11, November 2011 (Serial Number 43)
Contents
Molecular Biology and Biochemical Pharmacy
879 Newly Discovered Virus Associated Enzyme Capable of Alteration of Nucleic Acid Structures through Phosphotriester/-Diester Bonds
David Pan
884 Effects of Estradiol on 5-HT5A and 5-HT2C Receptor Immunolabeling in Rat Hippocampus
Laura Cristina Berumen, Marco Antonio Sánchez-Ramos, Martín García-Servín, Ataulfo Martínez-Torres, Angelina Rodríguez and Guadalupe García-Alcocer
890 Preliminary Functional Study on Wnt9a Cloning from Human Embryonic Stem Cells
Xueqin Zheng, Xiaonian Zhong, Chengneng Mi, Shuangmei Liu, Wenjing Meng, Yang Liu, Biao Xie, Yun Pan, Yuqing Gong, Shiying Yu, Chaobo Cai, Yanan Cui, Dongsong Nie and Yang Xiang
897 Effect of Therapeutic Ginger on Genotoxic of Taxol Drug (Anti-Cancer) in Bone Marrow Cell of Male Mice
Mona Mohammed Zaid AL-sharif
Genetics
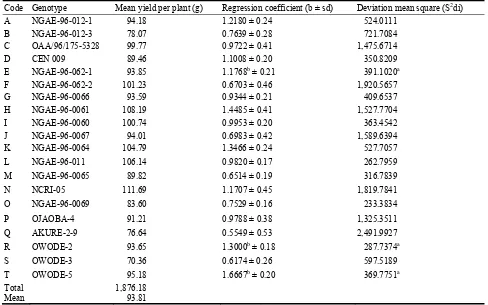
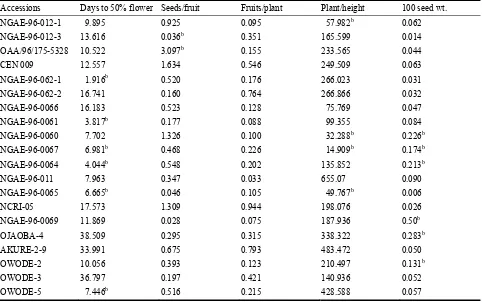
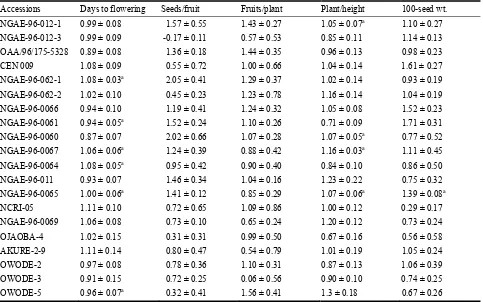
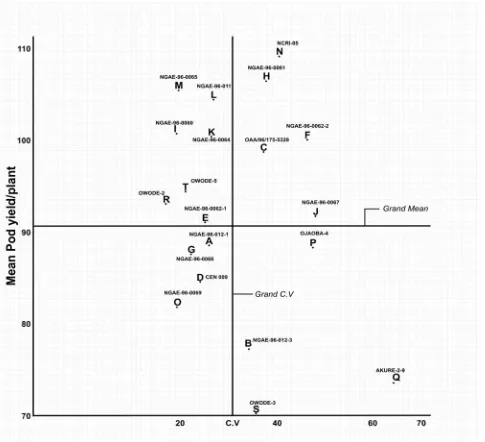
906 Stability Assessment of Some West African Okra (Abelmoschus caillei) Genotypes in Nigerian Genebank
Aladele Sunday Ezekiel, Omolayo Johnson Ariyo and Olusola Babatunde Kehinde
913 In Vitro Picloram-Induced Somatic Embryogenesis from Leaflets of Cherry (Prunus incisa Thunb.)
Ben Mahmoud Kaouther, Elloumi Nadhra Chakroun Ahlem, Ahmed Jemmali and Philippe Druart
921 Phenol Composition and Susceptibility to Fusarium oxysporum Dianthi in Carnation
Francesca Clematis, Joseph Tedeschini, Marcello Dolci, Virginia Lanzotti, Benedetta Cangelosi, Salvo Fascella and Paolo Curir
Physiology
926 Genotype and Environmental Effects on Cadmium Concentration in Maize
Spodoptera litura (F.) (Lepidoptera: Noctuidae)
Farzana Perveen
942 Shoot and Root Growth of Jatropha curcas Accessions Prospective for Rootstock on Rocky and Heavy Soil
Hamim Hamim, Andeng Sutrisna, Bambang Heliyanto, Mohammad Cholid and Miftahudin
954 Preliminary Studies and Antimicrobial Evaluation of the Aerial Parts of Genista numidica ssp. numidica
Oumessaad Toubal, Abdelghani Djahoudi and Amel Bouzabata
Biological Engineering
960 Yields of Polyhydroxyalkanoates (PHAs) during Batch Fermentation of Sugar Cane Juice by Alcaligenes latus and Alcaligenes eutrophus
Waranya Suwannasing, Samart Moonamart and Pakawadee Kaewkannetra
967 Bioremoval of Aquatic Environment Lead by Immobilized Cells of Enterobacter spp.
Harith Jabbar Fahad Al-Mathkhury, Adnan Hasan Afaj and Waad Emad Kasid
974 Differences in Browning Index and CIELAB Coordinates of the Two Grape Drying Processes, Traditional Sun-Drying and Chamber-Drying and during the Ageing of Pedro Ximenez Sweet Wine
Newly Discovered Virus Associated Enzyme Capable of
Alteration of Nucleic Acid Structures through
Phosphotriester/-Diester Bonds
David Pan
Laboratory of Animal Resources, Medical School, University of Wisconsin, Madison, Wisconsin 53706, USA
Received: June 24, 2011 / Accepted: July 25, 2011 / Published: November 30, 2011.
Abstract: A newly discovered enzyme, that can catalyze the formation of phosphotriester/-diester bonds between nucleic acids, was found to be associated with plant and animal viruses (i.e. southern bean mosaic virus, brome mosaic virus, influenza virus, avian virus and mouse retrovirus). A partially purified enzyme from maize developing endosperms was prepared through 15%-35% ammonium sulfate fractionation, DEAE-cellulose anion exchange column chromatography and Sephadex G150 gel filtration. The enzyme preparation was then used to demonstrate its main functional characteristics. The enzyme can use varieties of short and long chain length of nucleotides as substrates. However, the enzyme requires at least a minimum of 3 to 4 units of nucleotide chain length for the reaction to occur. The enzyme activity shows an optimum reaction in 50 mM sodium acetate buffer at pH 5.4 and is significantly inhibited by 6-azauridine as compared to other nucleotide analogs. By analyzing the data documented in literature and the results from the present study of the association of this enzyme with viruses and the distinctive inhibitory effect of 6-azauridine, it is speculated that this enzyme is likely associated with many other plant and animal viruses. The association of this enzyme on the surface of virus particles can be explored as a common antigen for developing a versatile antiviral vaccine.
Key words: Virus associated enzyme, “phosphotriester/-diester bond linkase”, sugar phosphate backbone of DNA, 6-azauridine.
1. Introduction
Viruses are important because many cause serious illness in humans, animals and can damage crop plants. During the last century, progress in the control of infectious diseases through new vaccines, drugs and chemicals has reduced the threat to humans and to the production of agricultural products. The advance of new knowledge and technology relevant to viruses provides a better way to control viral diseases. We report here a newly discovered virus associated enzyme capable of altering nucleic acid structure through the formation of phosphotriester/-diester bond. The unique phenomenon of the association of host cell proteins and enzymes with infectious viruses has been
Corresponding author: David Pan, Ph.D., scientist, research field: genetics. E-mail: [email protected].
2. Materials and Methods
2.1 Materials
Virus preparations were gifts from following laboratories, University of Wisconsin-Madison, USA: Poliovirus and influenza virus were obtained from Prof. Roland Rueckert’s Lab; Brome mosaic virus was obtained from Prof. Paul Ahlquist’s Lab, Institute of Molecular Virology; Southern Bean mosaic virus was obtained from Prof. Thormas German’s Lab, Department of Entomology; Avian virus was obtained from Prof. Virginia Hindshaw’s Lab, Veterinary school; Mouse retroviruses were obtained from the late Prof. Howard Temin’s Lab, McArdle Cancer Research Laboratory–UW and the University of Laval, Quebec, Canada. All chemicals were obtained from Sigma Biochem Company, St Louis, Missouri, USA.
2.2 Partial Enzyme Purification
A partial purification of this enzyme was prepared from maize developing endosperm of W64A line after 22 days of post pollination according to the procedures of 15%-35% ammonium sulfate fractionation, DEAE-cellulose anion exchange column chromatography and Sephadex G150 gel filtration as described by Pan [10]. The 50 mM NaCl fraction eluted from DEAE column in 50 mM Tris-HCl, pH 7.0 was used for all enzyme assays to characterize the enzyme activities. The enzyme reactions were assayed in 50 mM sodium acetate buffer at pH 5.4.
2.3 Enzyme Assays
All enzyme essays were performed in Beckman DU-6 spectrophotometer at room temperature. The scale and speed of recorder were adjusted each time to provide optimum conditions for conducting the essays. The reactions and the spectrum of reactions were automatically recorded on chart paper by following the change of OD at 260 nm after either the addition of enzyme or the virus preparations to the reaction mixture. Routinely, the reaction mixture contained
5 µL of virus preparations. 5 µL of nucleic acids and 5 µL of other tested chemical compounds in a final volume of 0.6 mL of 50 mM of sodium acetic acid buffer, pH 5.4 [10].
3. Results and Discussion
3.1 Enzyme Associated with Viruses
Fig. 1 shows the enzyme activities were found to be associated with several of the tested viruses including influenza virus, avian virus, brome mosaic virus, southern bean mosaic virus and mouse retrovirus. The data indicate the nature and reality of the association of this enzyme with the listed viruses, but not as a comparative study on enzyme activities. The association of host cell enzymes/proteins with viruses and their possible functions has been documented in literature [1-4]. This is the first time we are able to report a new enzyme associated with the viruses that can carry out the unique function of linking either short or long chain lengths of nucleic acids together through phosphotriester/-diester bonds with or without the intercalation of virus particles.
3.2 The Formation of Complex through
Phosphotriester/-Diester Bonds
Fig. 2 demonstrates that the end product complex was hydrolyzed by phosphodiesterase, but not by trypsin
Fig. 1 Enzyme activities with yeast RNA as substrate catalyzed by virus associated enzyme for the formation of phosphotriester/-disester bonds as complex.
through Phosphotriester/-Diester Bonds
and pepsin. Phosphodiesterase can hydrolyze both phosphotriester and -diester bonds [11], although the endonucleolytic is much slower than the exonucleolytic action [12, 13]. The results support the contention that the increase of OD at 260 nm during enzyme reaction is a result of the formation of phosphotriester/-diester bonding complex but not related to peptide bonds. Furthermore, the end product complexes, that were derived from the reactions using alkalated short chain length poly(A) or poly(dT) with
Fig. 2 Formation of RNA polymer complex which is sensitive to phosphodiesterase I (Crotalus atrox venom), but insensitive to pepsin or trypsin digestions.
The arrows indicate when the digestion enzymes were added: 1: Pepsin; 2: Trypsin; 3: Phosphodiesterase. The 50 mM NaCl eluted enzyme fractions from DEAE cellulose anion exchange column were used for enzyme assays with yeast RNA as substrate.
N-methyl-N-nitrosourea reagent as substrates, were reduced to about 50% due to the alkylation of the sugar phosphate backbone of nucleotides. In addition, the complexes were consistently stayed at the origin of loading well of 1% agarose electrophoresis gel without migration due to its larger molecular weight regardless of whether short or long chain length of nucleotides used as substrate (data not shown).
3.3 6-Azauridine Is Specific Inhibitor
Table 1 shows that 6-azauridine is specific inhibitor on the enzyme activity. It has been known that 6-azauridine is the most important inhibitor on many viral infections (Table 2). Also, 6-azaurdine has been commonly used for the treatment of many viral infections [14-18]. However, the detailed mechanism of the inhibitory effect of 6-azauridine on viral infectivity is not completely understood. It has been suggested that 6-azauridine may act on some other site(s) of action in the inhibition of virus multiplication besides blocking orotidylic acid decarboxylase activity. The finding of this new enzyme to be associated with viruses and its activity is selectively inhibited by 6-azauridine can lead to further study for understanding the mechanism of its inhibitory effect. By analyzing the results of this study together with the Table 1 Effect of nucleotide analogs on enzyme activity.
Nucleotide analogs % of activity Nucleotide analogs % of activity
Control 100.0 Control 100.0
UMP 52.3 6-Methyluracil 98.1
UDP 50.1 5-Bromo-2-deoxyuridine 97.2
UTP 48.3 Sulferuridine 86.4
UDPG 74.8 Propanyl thiouracil 71.6
Adenosine 68.4 Amino uracil 62.5
Guanosine 50.5 Pseudouridin 62.1
Cytidine 48.6 1,3-Dimethyl uracil 60.5
Thiamine 95.2 Uracil carboxylic acid 56.7
Xanthosine 47.8 2-Azido-2-deoxyuridine 47.3
Uric acid 97.2 6-Aza-2,3,5-acetouridine 46.7
Uracil 60.2 Uracil 20.6
8-Azahypoxanthin 82.3 6-Azauridine 0.02 µMol 16.4
8-Azaguanidin 102.2 6-Azauridine 0.04 µMol 4.5
Table 2 Virus infectivity inhibited by 6-azauridine [16, 17].
Names of viral infectivity inhibited by 6-azauridine Dengue 2 Lymphotic chorimeningitis
Adeno 5 Reo 3
Parainfluenza 3 Polyoma
Fowl plague Venezuelanequine encephalitis
Vaccinia Japanese encephalitis
Rous sarcoma Encephalmyocarditis Herpes hominis Cytomegalo Herpes Simplex virus Influenza virus
Measles Rhino virus
Simian rotovirus Simian foamy Vesicular stomatitis
Fig. 3 Effect of nucleotide chain length on enzyme reaction.
The 50 mM NaCl eluted enzyme fraction from DEAE-cellulose anion exchange column was used for enzyme assays with d(T)2→d(T)8 as substrates.
data documented in literature about the unique inhibitory effect of 6-azauridine on the viral infectivity of a variety of viruses, this new enzyme could be considered likely to be associated with most, if not all, plant and animal viral particles.
3.4 Requirement of Minimum Chain Length of
Nucleotides for Reaction
Fig. 3 shows that the enzyme requires at least a minimum of 3 to 4 units of nucleotide chain length for initiating reaction. Both short and longer chain length of nucleotides can be used as substrates for enzyme reaction. Calf thymus DNA and yeast RNA, including polyC, polyG, polyCG, polyGU, polydA, polydT and polydAdT etc. had been used for all enzyme assays
during investigation of this enzyme over the past 20 plus years.
4. Conclusion
This study demonstrates the presence of a new virus associated enzyme that can catalyze the formation of polymeric form of nucleic acid through linking of phosphotriester/-diester bond between the phosphate backbones of nucleic acid. The fact of the enzyme can use variety of short and long chain length of nucleotides as substrates suggests that the reaction products could be a result of different polymeric forms of nucleic acid depending on the type of substrates being used. The enzyme is selectively inhibited by 6-azauridine. Based on the result of the enzyme is not only associated with plant and animal viruses, but is also widely distributed in nature ranging from microbial, plant to animal tissues (data not shown) which indicates this enzyme is an evolutionary significant protein.
Acknowledgments
This investigation was carried out in the laboratory of the late Prof. Oliver E. Nelson (member of National Academy of Science) in the Department of Genetics, University of Wisconsin-Madison since 1982. The author would like to dedicate this finding to remember him for his continued support and encouragement. The author is grateful to the laboratories for providing the viral preparations as gifts.
References
[1] L.O. Arthur, J.W. Bess jr., R.C. Sowder(II), R.E. Benveniste, D.L. Mann, J.C Chermann, et al., Cellular proteins bound to immunodeficiency viruses: Implications for pathogenesis and vaccines, Science 258 (1992) 1935-1937.
[2] M.L. Shaw, K.L. Stone, C.M. Colangelo, E.E. Gulcicek, P. Palese, Cellulars proteins in influenza virus particles, PLoS Pathog 4 (6) (2008) e1000085. doi:10.1371/Journal.ppat.1000085.
through Phosphotriester/-Diester Bonds
phosphodiester bonding complex: An event leading to a new frontier of research and development for viral diseases, in: Abstract in 14th International Congress for Infectious Diseases, Miami, USA, March, 2010.
[4] M. Hammarstedt, J. Ahlqvist, S. Jacobson, H. Garoff, A. Fogdell-Hahn, Purification of infectious human herpesvirus 6A virions and association of host cell proteins, Virology J. 4 (2007) 101-112.
[5] S. Valadkhan, J.L. Manley, Characterization of the catalytic activity of U2 and U6 snRNAs, RNA (Cold Spring Harbor Lab. Press) 9 (2003) 892-904.
[6] K.V. Shooter, DNA phosphotriesters as indicators of cumulative carcinogen-induced damages, Nature 274 (1978) 612-614.
[7] J. Haglund, W.V. Dongen, F. Lemiere, E.L. Esmans, Analysis of DNA-phosphate adducts in vitro using miniaturized LC-ESI-MS/MS and column switching: Phosphotriesters and alkyl cobalamins, J. Am. Soc. Mass Spectrum 15 (2004) 593-606.
[8] E. Wimmer, Genome-linked proteins of viruses, Cell 28 (1982) 199-201.
[9] Y.F. Drygin, Natural covalent complexes of nucleic acids and proteins: Some comments on practice and theory on the path from well-known complexes to new ones, Nucleic Acids Res. 26 (21) (1988) 4791-4796.
[10] D. Pan, An endosperm enzyme catalyzes the formation of phosphotriester and phospdiester bonding complex between nucleic acids with altering their structure, in: 53rd Annual Maize Genetic Meeting, Abstract 22, 2011.
[11] P.J. O’Connor, G.P. Margison, A.W. Craig,
Phosphotriesters in rat liver deoxyribonucleic acid after the administration of the carcinogen NN-dimethylnitrosamine in vivo, Biochem. J. 145 (1975) 475-482.
[12] W.E. Razzel, H.G. Khorana, Studies on polynucleotides. IV. Enzymic degradation; the stepwise action of venom phosphodiesterase on deoxyribo-oligonucleotides, J. Biol. Chem. 234 (8) (1959) 2114-2117.
[13] P. Bannon, W. Verly, Alkylation of phosphates and stability of phosphate triesters in DNA, Eur. J. Biochem. 31 (1972) 193-111.
[14] R.J. Klein, A.E. Friedman-Kien, E. Brady, Herpes simplex virus skin infection in hairless mice: Treatment with antiviral compounds, Antimicrobial, Agents & Chemother 5 (3) (1974) 318-322
[15] B. Rada, M. Dragun, Antiviral action and selectivity of 6-azauridine, Annals. N.Y. Sci. 284 (1977) 410-417. [16] D.F. Smee, P.A. MeKernan, L.D. Nord, R.C. Willis, C.R.
Petrie, T.M. Riley, et al., Novel pyrazolo [3,4-d] pyridimidine nucleoside analog with broad-spectrum antiviral activity, Antimicrob. Agents Chemother. 31 (10) (1987) 1535-1541.
[17] J.D. Morrey, D.F. Smee, R.W. Sidwell, C. Tseng, Identification of active antiviral compounds against a New York isolate of West Nile virus, Antiviral Res. 55 (2002) 107-116.
Effects of Estradiol on 5-HT
5A
and 5-HT
2C
Receptor
Immunolabeling in Rat Hippocampus
Laura Cristina Berumen1, Marco Antonio Sánchez-Ramos2, Martín García-Servín3, Ataulfo Martínez-Torres3, Angelina Rodríguez1 and Guadalupe García-Alcocer1
1. Facultad de Química, Universidad Autónoma de Querétaro, Centro Universitario, Querétaro 76010, México
2. Facultad de Ciencias Naturales, Universidad Autónoma de Querétaro, Campus Juriquilla, Juriquilla Querétaro 76230, México
3. Department of Cellular and Molecular Neurobiology, Instituto de Neurobiología, Universidad Nacional Autónoma de México,
Campus Juriquilla, Juriquilla Querétaro 76230, México
Received: April 05, 2011 / Accepted: June 07, 2011 / Published: November 30, 2011.
Abstract: Steroid hormones participate in the modulation of serotonergic transmission, including the regulation of synthetic and metabolic enzyme production, as well as receptor and transporter activity. The changes of 5-HT5A and 5-HT2C immunolabeling induced
by steroids in the hippocampus of ovariectomized rats were studied in this work. Densitometric analysis in rat hippocampi were carried out for adjacent brain coronal immunolabeled sections after treatment with subcutaneous injections of vehicle, estradiol, progesterone or the combination of both steroids in ovariectomized rats. Exposure to estradiol and the combination of estradiol and progesterone significantly reduced the 5-HT5A–like immunosignal in the CA1 region while progesterone did not induce changes. On the other hand,
exposure to the combination of estradiol and progesterone or estradiol alone increased the 5-HT2C immunosignal in the same region.
These results indicate that estradiol is involved in the discrete regulation of serotonin receptors 5-HT5A and 5-HT2C in rat hippocampus.
Key words: Serotonin receptor, 5-HT5A, 5-HT2C, hippocampus, estradiol, progesterone.
1. Introduction
Steroid hormones, which include estrogens, progestins, androgens, glucocorticoids and mineralocorticoids, have ligand-inducible transcription factors-mediated actions; they also have several non-genomic effects that modify the trascriptional activity of different genes. These effects include essential roles played by steroid hormones in: brain differentiation, neural plasticity and neurotransmission [1-3].
Steroid hormones have modulatory effects on the synthesis and release of several neurotransmitters such as serotonin [4, 5] and affect serotonergic transmission in some extent by regulating the density of receptors in neuronal plasma membranes [6-8]. The 5-HT5A
Corresponding author: Laura Cristina Berumen, Ph.D., professor, research fields: neurobiology, neuroendocrinology. E-mail: [email protected].
receptor, a G protein-coupled metabotropic receptor, is involved in the modulation of exploratory behavior and in certain LSD-psychoactive effects; it is expressed in the brain, with the highest concentrations found in the hippocampus [9-11]. Activation of 5-HT5A receptors inhibits the production of cAMP but its precise metabolic cascade has not been fully elucidated [12-14].
On the other hand, 5-HT2C receptors are heterogeneously distributed in the brain with the highest levels in the choroid plexus [15, 16]; they are involved in the control of appetite and tonic inhibition of neuronal network excitability [17, 18]. The 5-HT2C receptor regulates a number of effectors through PLC-, Ca2+-, or PKC-dependent mechanisms [14].
hippocampus and the modulation of a number of serotonin receptors by steroids, tests were conducted to ascertain if estradiol and progesterone alter the expression and location of 5-HT5A receptors in the rat hippocampus compared to 5-HT2C.
2. Materials and Methods
Adult female Sprague-Dawley rats (200-250 g) were maintained with food and water ad libitum in 12 h/12 h light-dark cycles. Each experimental group consisted of five rats ovariectomized under anesthesia (80 mg/kg ketamine + 6 mg/kg xylazine) between 9-12 h in the morning of diestrus. Administration of all treatments was carried out between 9-10 h in the morning of days 1 through 5 after surgery using a single daily subcutaneous injection. The control group of rats was administered only vehicle (100 μL corn oil subcutaneous injection). A group of ovariectomized rats was treated with 50 μg/kg body weight of estradiol benzoate (EB). Another group was treated with 7.5 mg/kg of progesterone (P) and a last group was injected with the combination of EB and P. After treatments rats were anesthesized with sodium pentobarbital (40 mg/kg) and decapitated between 9-12 h the next day following the treatment. Brains were fixed in 4% paraformaldehyde-PBS and coronal sections of 12 μm were made in a Leica CM 1850 cryostat, then thaw mounted on superfrost slides [20].
Immunohistochemistry was performed with specific antibodies used to determine the distribution of the receptors: 1) an anti 5-HT5A receptor (Sigma; 1:500); and 2) an anti 5-HT2C (Santa Cruz Biotechnologies; 1:500). The slices were incubated overnight at room temperature with the primary antibody; after rinsing, they were incubated with the biotin-conjugated goat anti-rabbit antibody (Chemicon) for two hours at room temperature and then with Vector ABC system. Finally, 3,3’-diaminobenzidine (DAB) and hydrogen peroxide were used for color development. Digitized images of nine sections for each rat were analyzed using densitometry (Axiovision, Zeiss). One-way ANOVA and Tukey tests were used for statistical analysis of differences between group means. All experiments were conducted according to the international guidelines of the care and use of experimental animals.
3. Results
Immunodetection of 5-HT5A receptor in the hippocampus of untreated rats revealed that it is distributed in all regions. Rats treated with EB or EB + P showed an overall reduced staining for 5-HT5A (Fig. 1) in CA1 region. In contrast, P did not elicit significant changes compared to controls.
Densitometric analysis of the samples (Fig. 2) confirmed the observations made under the microscope, with a 11.4% reduction of intensity for 5-HT5A receptor-like
Fig. 1 Coronal sections of rat brains stained by immunohistochemistry (DAB, H2O2) with an antibody against the 5-HT5A or 5-HT2C serotonin receptors.
immunosignal for EB treatment and 10.2% for EB + P treatment (P < 0.01). No-statistical difference for the 3.9% reduction of intensity in P treatment compared to control, and no-statistical differences between EB and EB + P were noted.
The effects of EB and EB + P on 5-HT2C receptors (Fig. 3) were the opposite to those on 5-HT5A receptors; consistently, both treatments gave rise to higher levels of 5-HT2C immunolabeling in CA1 (Fig. 1) compared to controls that showed 2.5% and 2% respectively, P < 0.01 for EB + P. In contrast, rats treated with P alone did not differ from the control samples in that less than 1% reduction with no significant difference noted.
The densitometry values are arbitrary optical density
Fig. 2 Densitometry of 5-HT5A serotonin receptors in CA1 of rat hippocampus after treatment with steroids.
Fig. 3 Densitometry of 5-HT2C (arbitrary optic density units) serotonin receptors in CA1 of rat hippocampus after treatment with steroids.
Estradiol (EB) and Estradiol + Progresterone (EB + P) increased receptor expression with statistical significance (¶) compared to controls (P < 0.01).
units normalized against negative controls (no primary antibody). Estradiol (EB) and Estradiol + Progresterone (EB + P) decreased receptor expression with statistical significance (¶) compared to controls (P < 0.01).
4. Discussion
The activity of different neurotransmitter systems can be modulated by steroid hormones. The effect of steroids over the serotonergic pathway has been widely studied by different means, although the partial agonism of classical drugs and ligands for the various serotonin receptors sometimes overlapping, makes it complex to analyze [21, 22]. In this work we found that estradiol changed the expression of 5-HT5A and 5-HT2C serotonin receptor subtypes. It has been reported that the administration of estrogen sharply reduced [3H]5-HT binding-sites in the hypothalamus and preoptic nucleus [7, 8, 23]; however the response is different for subtypes of serotonin receptor and brain areas. The effects of steroids have been studied in different areas of rat brain for several receptor subtypes, especially 5-HT1A and 5-HT2A, but 5-HT5A has received less attention. For example, estradiol reduced the 5-HT1A receptor mRNA in the piriform cortex and the anterodorsal medial amygdale whereas it remained unaltered in the hippocampus and the prefrontal and cingulate cortex as well as in the dorsal nucleus of raphe [24, 25]. Furthermore, estradiol increased 5-HT2A mRNA in several limbic regions of gonadectomized rats treated with estradiol or testosterone [26, 27] although no changes or reduced levels for 5-HT2C mRNA were found for discrete areas of hippocampus [28].
It has been previously reported that the 5-HT5A receptor undergoes developmental modifications and is a suitable candidate for fine-tuning regulation of the serotonergic system [20]. Changing conditions such as steroid levels may also exert different modulatory effects on the number and class of serotonin receptors present in the plasma membrane. In this project it was
1.4
1.3
1.2
1.1
1
1.1
1.08
1.06
1.04
1.02
found that estradiol down-regulated 5-HT5A receptors. The results herein regarding this receptor were consistent with the down-regulation expected for the negative cAMP G-protein coupled receptor 5-HT1A although differences were found particularly in hippocampus [25]. On the other hand, progesterone action requires estrogen-priming in order to be effective [29]. No-changes were found with the subcutaneous high dosis administration of progesterone alone in these experiments. However, administering both estradiol and progesterone showed no statistical differences versus estradiol alone therefore it can be inferred that progesterone does not have a differential effect at this high acute dosis.
For the 5-HT2C receptor-like immunodetection there were found significant increases of signal noted after treatment with estradiol and estradiol plus progesterone which support the idea for specific cellular modifications in the serotonergic transmission. These results also correspond with the changes in PLC G-protein coupled serotonin receptor 5-HT2A [26] and 5-HT2C [30]. Nonetheless, it was found found that there were differences in receptor expression in hippocampus using subcoutaneously implanted pellets [28] that might reflect differences in the dosages used and way of administration.
The different effects of estrogen and progesterone on the expression of serotonin receptors need to be further investigated in order to determine whether the effects are directly on the transcriptional regulation of the receptor protein, or reflect the contribution of the serotonin itself and its pathways. Although there are no recognizable canonical ERE in 5-HT5A sequence (ratCHR4:2707261-2716944) [31] and its 5′ and 3′ flanking regions (5 kbp), there are ERE-like motifs with different spacing regions between palindromic and direct repeats [32, 33], particularly one pentameric modified palindrome sequence with a four-bp spacer (CHR4:2717587-2717574) and the glucocorticoid receptor recognized sequence 3-bp spaced from an ERE-like motif (CHR4:2702313-2702299). This direct
interaction needs to be tested, although it has been reported the estradiol induced expression for this receptor in rat anterior pituitary cell aggregates [34].
Moreover, the steroids may regulate receptor expression via other transcription factors, including cyclic AMP response element binding proteins. Estradiol causes phosphorilation of CREB in the CA1 region as well as in the CA3, which might be involved in the tissue-specific modification of serotonin receptors and differences found in in vitro experiments [1, 28, 35]; furthermore, pCREB immunolabeling is increased in hippocampus after estradiol administration as well as BDNF expression [36], which are involved in the expression of some serotonin receptors.
5. Conclusion
Administration of acute dosis of estradiol in ovariectomized rats down-regulates 5-HT5A and up-regulates 5-HT2C receptors in CA1 region of hippocampus, whereas progesterone alone does not induce significant changes in the expression of these receptors.
Acknowledgments
The authors appreciate the efficient work and help of Aaron Tecozautla, Jesica Escobar, Berenice Flores and Karina Hernández. They would also like to acknowledge Silvia C. Stroet of Universidad Autónoma de Querétaro and Nancy Arent for editing the English content of this document. This work was supported (funds) by ProMeP for Berumen, CONACYT and DGAPA-PAPIIT for Martínez-Torres.
References
[1] J.L. Spencer, E.M. Waters, T.A. Milner, B.S. McEwen, Estrous cycle regulates activation of hippocampal Akt, LIM kinase, and neurotrophin receptors in C57BL/6 mice, Neuroscience 155 (4) (2008) 1106-1119.
[2] M. Beato, J. Klug, Steroid hormone receptors: an update, Hum Reprod. Update 6 (2000) 225-236.
Clin. Exp. Pharmacol. Physiol. 25 (1998) 764-775. [4] T. Inagaki, C. Gautreaux, V. Luine, Acute estrogen
treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas, Horm. Behav. 58 (3) (2010) 415-426.
[5] P.J. Schmidt, D.R. Rubinow, Sex hormones and mood in the perimenopause, Ann N Y Acad. Sci. 1179 (2009) 70-85.
[6] P. Zheng, Neuroactive steroid regulation of neurotransmitter release in the CNS: action, mechanism and possible significance, Prog. Neurobiol. 89 (2) (2009) 134-152.
[7] A. Biegon, B.S. McEwen, Modulation by estradiol of serotonin receptors in brain, J. Neurosci. 2 (1982) 199-205.
[8] A. Biegon, H. Bercovitz, D. Samuel, Serotonin receptor concentration during the estrous cycle of the rat, Brain Res 187 (1980) 221-225.
[9] R.A. Glennon, Higher-end serotonin receptors: 5-HT(5), 5-HT(6), and 5-HT(7), J. Med. Chem. 46 (14) (2003) 2795-2812.
[10] R. Grailhe, C. Waeber, S.C. Dulawa, J.P. Hornung, X. Zhuang, D. Brunner, et al., Increased exploratory activity and altered response to LSD in mice lacking the 5-HT(5A) receptor, Neuron. 22 (1999) 581-591.
[11] M.G. Erlander, T.W. Lovenberg, B.M. Baron, L. Lecea, P.E. Danielson, M. Racke, et al., Two members of a distinct subfamily of 5-hydroxytryptamine receptors differentially expressed in rat brain, Proc. Nat. Acad. Sci. USA 90 (1993) 3452-3456.
[12] J. Bockaert, S. Claeysen, C. Bécamel, A. Dumuis, P. Marin, Neuronal 5-HT metabotropic receptors: fine-tuning of their structure, signaling, and roles in synaptic modulation, Cell Tissue Res. 326 (2006) 553-572. [13] W.K. Kroeze, B.L. Roth, Molecular biology and genomic
organization of G protein-coupled serotonin receptors, in: B.L. Roth (Ed.), The Serotonin Receptors: from Molecular Pharmacology to Human Therapeutics, Humana Press, New Jersey, 2006, pp. 1-38.
[14] J.R. Raymond, J.H. Turner, A.K. Gelasco, H.B. Ayiku, S.D. Coaxum, J.M. Arthur, et al., 5-HT receptor signal transduction pathways, in: B.L. Roth (Ed.), The Serotonin Receptors: From Molecular Pharmacology to Human Therapeutics, Humana Press, New Jersey, 2006, pp. 143-206.
[15] D. Hoyer, J.P. Hannon, G.R. Martin, Molecular, pharmacological and functional diversity of 5-HT receptors, Pharm. Biochem. Beh. 71 (2002) 533-554. [16] G. Mengod, H. Nguyen, H. Le, C. Waeber, H. Lübbert,
J.M. Palacios, The distribution and cellular localization of the serotonin 1C receptor mRNA in the rodent brain examined by in situ hybridization histochemistry.
Comparison with receptor binding distribution, Neuroscience 35 (1990) 577-591.
[17] A. Kimura, P.L. Stevenson, R.N. Carter, G. Maccoll, K.L. French, J. Paul Simons, et al., Overexpression of 5-HT2C receptors in forebrain leads to elevated anxiety and hypoactivity, Eur. J. Neurosci. 30 (2) (2009) 299-306. [18] L.H. Tecott, L.M. Sun, S.F. Akana, A.M. Strack, D.H.
Lowenstein, M.F. Dallman, et al., Eating disorder and epilepsy in mice lacking 5-HT2c serotonin receptors, Nature 374 (1995) 542-546.
[19] S. Campbell, G. Macqueen, The role of the hippocampus in the pathophysiology of major depression, J. Psychiatry Neurosci. 29 (2004) 417-426.
[20] G. García-Alcocer, L.C. Berumen Segura, M. García-Peña, A. Martinez Torres, R. Miledi, Ontogenic distribution of 5-HT2C, 5-HT5A, and 5-HT7 receptors in the rat hippocampus, Gene Expression 13 (2006) 53-57. [21] L.C. Berumen, A. Tecozautla, M.A. Sánchez-Ramos, M.
García-Servín, G. García-Alcocer, Steroid hormone effects on 5-HT5A serotonin receptor-like immunolabelling in the rat hippocampus, 4th International Meeting Steroids and Nervous System Abstract Book, 2007, p. 163.
[22] S. Mouillet-Richard, M. Pietri, B. Schneider, C. Vidal, V. Mutel, J.M. Launay, et al., Modulation of serotonergic receptor signaling and cross-talk by prion protein, J. Biol. Chem. 280 (6) (2005) 4592-4601.
[23] A. Biegon, A. Reches, L. Snyder, B.S. McEwen, Serotonergic and noradrenergic receptors in the rat brain: modulation by chronic exposure to ovarian hormones, Life Sci. 32 (1983) 2015-2021.
[24] M. Landry, T. Di Paolo, Effect of chronic estradiol, tamoxifen or raloxifene treatment on serotonin 5-HT1A receptor, Mol. Brain Res. 12 (2003) 82-89.
[25] M.K. Osterlund, D.H. Overstreet, Y.L. Hurd, The flinders sensitive line rats, a genetic model of depression, show abnormal serotonin receptor mRNA expression in the brain that is reversed by 17beta-estradiol, Brain Res Mol Brain Res. 74 (1999) 158-166.
[26] B.E. Sumner, G. Fink, Testosterone as well as estrogen increases serotonin2A receptor mRNA and binding site densities in the male rat brain, Mol. Brain Res. 59 (1998) 205-214.
[27] B.E. Sumner, G. Fink, Estrogen increases the density of 5-hydroxytryptamine(2A) receptors in cerebral cortex and nucleus accumbens in the female rat, J. Steroid Biochem. Mol. Biol. 54 (1995) 15-20.
progesterone on serotonin turnover in rats primed with estrogen implants into the ventromedial hypothalamus, Brain Res. Bull. 32 (3) (1993) 293-300.
[30] W. Zhou, K.A. Cunningham, M.L. Thomas, Estrogen regulation of gene expression in the brain: a possible mechanism altering the response to psychostimulants in female rats, Mol. Brain Res. 100 (2002) 75-83.
[31] Ensembl, 2010, http://www.ensembl.org/Rattus_novergicus. [32] F.J. Shu, N. Sidell, D. Yang, C.B. Kallen, The
tri-nucleotide spacer sequence between estrogen response element half-sites is conserved and modulates ER-mediated transcriptional responses, J. Steroid Biochem Mol Biol 20 (2010) 172-179.
[33] K. Pettersson, K. Grandien, G.G. Kuiper, J.A. Gustafsson, Mouse estrogen receptor β forms estrogen response element-binding heterodimers with estrogen receptor α,
Mol. Endocrinol. 11 (10) (1997) 1486-1496.
[34] A. Papageorgiou, C. Denef, Estradiol induces expression of 5-hydroxytryptamine (5-HT) 4,5-HT5, and 5-HT6 receptor messenger ribonucleic acid in rat anterior pituitary cell aggregates and allows prolactin release via the 5-HT4 receptor, Endocrinology 148 (3) (2007) 1384-1395
Preliminary Functional Study on
Wnt9a
Cloning from
Human Embryonic Stem Cells
Xueqin Zheng, Xiaonian Zhong, Chengneng Mi, Shuangmei Liu, Wenjing Meng, Yang Liu, Biao Xie, Yun Pan, Yuqing Gong, Shiying Yu, Chaobo Cai, Yanan Cui, Dongsong Nie and Yang Xiang
Department of Chemistry and Chemical Engineering, Hunan Institute of Science and Technology, YueYang 414000, China
Received: March 08, 2011 / Accepted: May 04, 2011 / Published: November 30, 2011.
Abstract: Wnts are secreted lipid-modified signaling proteins that influence multiple processes ranging from cell proliferation and differentiation, fate decisions, apoptosis, axial polarity and axonal guidance to stem cell loss, kidney and reproductive tract defects. Activation of Wnt signalling in many tissues has also been associated with cancer. In many eukaryotes, expression of nuclear-encoded mRNA can be strongly inhibited by the presence of a small double-stranded RNA corresponding to exon sequences in the mRNA. In this study, human Wnt9a was cloned from undifferentiated hES cells. The results of immunohistochemistry showed that Wnt9a protein was expressed in undifferentiated hES cells. pAVU6+27 vectors were used to construct the siRNA expression vectors for human Wnt9a. One kind of small interfering RNA inserts was designed, synthesized and tested for human Wnt9a. The results of in situ hybridization demonstrated that Wnt9a signal was dramatically reduced in the cells transfected with U6+27/siWnt9a compared to the untransfected cells. The results of flow cytometry analysis showed that the human breast cancer MCF-7 cells proliferation was promoted after lowering the expression of human Wnt9a by RNAi, but inhibited after over-expression of human Wnt9a. All those suggest the expression level of human Wnt9a may play a role in adjusting the rate of cell proliferation of hES and MCF-7 cells.
Key words: Wnt9a, cloning, hES cells (human embryonic stem cells), function.
1. Introduction
Human embryonic stem cells (hES cells) will be the unlimited cell source for future cell therapy, contributing to their characteristics of pluripotency, capability of differentiation to almost any type of cells [1]. At present a lot of inactive mouse embryonic fibroblasts (mEFs) are required to maintain and support the hES cell growth in an undifferentiated state [2-4]. The Wnt family of signaling molecules regulates numerous processes in animal development and has increasingly been implicated in tissue homeostasis in adult organisms [5]. Wnts are secreted lipid-modified signaling proteins [6] and powerful regulators of cell proliferation and differentiation, and
Corresponding author: Yang Xiang, Ph.D., professor, research fields: molecular and cell biology. E-mail: [email protected].
Dongsong Nie, Ph.D., research fields: molecular and cell biology. E-mail: [email protected].
many tissues, activation of Wnt signalling has also been associated with cancer [10]. Unchecked Wnt signaling [11] and/or the loss of cell-cell adhesion [12] are involved in cancer induction and progression. Loss of cadherin expression can also promote tumorigenesis [13]. RNA interference (RNAi) is a process of posttranscriptional gene silencing, by which double-stranded RNA (dsRNA) induces sequence-specific degradation of homologous gene transcripts [14, 15]. Efficient RNA interference technologies are used to antagonize gene function [16]. Wnt9a predominantly expressed in the mural trophoblast and inner cell mass cells surrounding [17]. Wnt9a has been implicated as being a player in joint induction, based on gain-of function experiments in chicken and mouse. Wnt9a is a temporal and spatial regulator of Indian hedgehog (Ihh), a central player of skeletogenesis. Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis [18].
In this study, human Wnt9a was cloned from hES cells. The preliminary function of human Wnt9a was studied by RT-PCR, immunohistochemistry, in situ hybridization, RNAi, over expression Wnt9a and analyses of flow cytometry. The results showed that the expression level of human Wnt9a adjust the rate of cell proliferation.
2. Materials and Methods
2.1 hES Cells Culture
The hES cell line chESC-3, one of the several human ES cell lines was established as described [1] and cultured on mEFs in a culture medium consisting of 85% Knock-out Dulbecco’s modified Eagle’s medium (KO-DMEM), 15% Knock-out serum replacement, 1 mmol/L L-glutamine, 0.1 mmol/L β-mercaptoethanol, 1% non-essential amino acids, and 4 ng/mL human basic fibroblast growth factor (hbFGF) (all Gibco Invitrogen products, USA). Undifferentiated hES cells were passaged every 5 days with fresh medium and mEFs by mechanical dissociation using a “Stem Cell Tool” (Swemed Lab International AB, Billdal, Sweden, http://www.swemed.com). Medium
change was performed every 2-3 days. For subsequent analyses, the hES cells were either fixed for immunohistochemical evaluation or rapidly harvested by mechanical dissociation and frozen at −80°C for RNA extraction.
2.2 RNA Extraction and RT-PCR Analysis
Extraction of total RNA from undifferentiated hES cells maintained on mEFs; human three month abortion fetus testis, cerebra, cerebellum, kidney, adrenal gland, skeletal muscle, heart, liver, small intestine, thymus, stomach and lung; and human breast cancer MCF-7 cells (was given friendly by cancer research institute of central south university) were performed using the Gentra system RNA isolation (Gentra reagents, USA) according to the protocol provided. DNase treatment was performed on-column using RNase-free DNase Kit (Qiagen). Reverse transcription was performed using 1-2 μg of total RNA in a final volume of 20 μL, using iScript First Strand Synthesis Kit (Bio-Rad Laboratories, Hercules, CA,). PCR primer pair for human Wnt9a was: F: 5′-tcaaggagactgccttcctctat-3′, R: 5′-actccacatagcagc accaac-3′. Semiquantitative RT-PCR was performed by the following procedure: denaturation (94°C for 90 s); 35 cycles of amplification (94°C for 40 s, 62°C for 40 s, 72°C for 90 s), and extension (72°C for 5 min) for human Wnt9a.
2.3 Construction pEGFP-C3/Wnt9a Recombinant
Plasmid
checked by using 2.0% agarose gel electrophoresis, and then the pEGFP-C3 vector and the gene fragments digested using HindⅢ and BamHⅠ were purified. Then, a ligation reaction was done according to the manufacturer’s instructions (TaKaRa Co., Japan). DH5α cells were transformed with 10 μL of the ligation reaction products and spread on an LB agar plate containing 100 μg/mL ampicillin. Finally, individual clones were checked using DNA-sequencing to ensure that the fusion plasmids were correctly constructed.
2.4 Immunohistochemistry
Human embryonic stem cells were cultured by our stem cell centre. Undifferentiated hES cells maintained on mouse embryonic fibroblasts were fixed in 4% paraformaldehyde. Immunohistochemistry was performed according to the procedure described by themanufacturer (SABC kit, Boster Co. Wuhan, China). Briefly, after permeabilization and blocking, the cells were incubated for 20 hours at 4°C with primary antibodies directed against the human Wnt9a antigens (final antibody concentration 1 μg/mL), Negative controls were included in which the primary antibodies were omitted. Fluorescein is Cy-3-conjugated secondary antibodies (invitrogen products, USA) and 4,6-diamidino-2-phenylindole were used for detection of the primary antibodies and for nuclei staining, respectively. The stained hES cells cultures were mounted and visually inspected in an inverted fluorescence microscope.
2.5 Construction of siRNA Expression Vectors
pAVU6+27, which contains human U6 promoter and the first 27-bp of U6 RNA coding sequence, has been described by Paul et al [19]. A series of shRNA expression vectors were generated by inserting annealed oligos sequence into pAVU6+27 vector between SalⅠand XbaⅠ sites [20]. The pAVU6+27 vector was digested with SalⅠ and XbaⅠ to generate compatible ends for cloning. The sequence of one
siRNA for human Wnt9a was designed by using the Ambion web-based criteria. Their primer pairs for RT-PCR were synthesized by Invitrogen Co. as follows: siWnt9a F1: 5′-tcgaaaccttaagtacagcagcaagcttgcttgctg ctgtacttaaggtttttt-3′ (SalⅠ), siWnt9a R1: 5′-ctagaaaa aaccttaagtacagcagcaagcaagcttgctgctgtacttaaggtt-3′ (XbaⅠ); The pairs of primers were incubated at 95°C for 5 min with 1×annealing buffer (10 mmol/L Tris-HCL and 100 mmol/L NaCl), and then gradually cooled to room temperature. The annealed shDNAs were inserted into the digested pAVU6+27 vectors with SalⅠ and XbaⅠ clone sites. The shRNA expression vectors, pAVU6+27/siWnt9a was transformed into E. coli DH5α and identified by RT-PCR using sense primer, 5′-ctaactgacacacattccac-3′ and antisense primer, 5′-gcaataaacaagttactagtcc-3′. The resulting products were analyzed by electrophoresis on a 2.0% agarose gel and sequenced.
2.6 Cell Culture and Transfection
A human MCF-7 breast cancer cells were maintained in RPMI-1640 medium supplemented with heat-inactivated 10% fetal calf serum (FCS) and 1% antibiotic/antimycotic solution at 37°C in a humidified incubator with 5% CO2. The cell line was routinely split three to four times a week after trypsinization. Twenty-four hours before transfection, MCF-7 cells were splitted and seeded in 6-well culture plates at 1 × 105 cells per well. The cells were transiently transfected with 2.0 μg of the empty vectors pAVU6+27 and 2.0 μg of the RNA expression vector pAVU6+27/siWnt9a using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instructions. The cells were splitted after one day transiently transfected and then the cells were collected at three days post-tansfection for flow cytometry analysis, or were reserveted for in situ hybridization.
2.7 Exam the Efficiency of RNAi by in situ Hybridization Analyses
purchased from Boster Bio-engineering Co. (Wuhan, China). Being longer probes were difficult to penetrate the cell, short oligonucleotide probes were used in this study. To strengthen the signals, three probes were used. The sequences of oligonucleotide probes were as follows: (1) 5′-gagcg gaagc agcgg cgcat gtgcc gccgg gaccc-3′; (2) 5′-gactt ccaca acaac ctcgt gggtg tgaag gtgat-3′; (3) 5′-cataa cacac agagc cgggt ggtga caagg ccctg-3′. The probes were labeled with digoxigenin. To get specific signals, in situ hybridization was done as follows: Cells were washed in 0.1 M PBS (pH 7.2-7.6) twice, each for 5 min, treated with H2O2 mix (H2O2:methanol = 1:50) for 30 min and washed in distilled water for three times, each for 5 min. Then the cells were digested by 3% pepsin (diluted by citric acid) for 30 s, washed in 0.5 M PBS (pH 7.2-7.6) three times, each for 5 min. Washed cells in distilled water for 5 min and then post-fixed in buffered 1% paraformaldehyde in 0.1 M PBS (pH 7.2-7.6) containing 0.1% DEPC and washed cells in distilled water three times, each for 5 min. Pre-hybridization was performed for 3 h. Subsequently, hybridization was performed in a solution containing digoxigenin-labeled human Wnt9a probes. The control cells were treated with the solution without Wnt9a probes. Slides were incubated at 38°C for 16 h in a humidified chamber. Post-hybridization washes were performed at 37°C by incubation in 2×SSC twice, each for 5min; in 0.5×SSC for 15 min and in 0.2×SSC twice, each for15 min. The cells were incubated in blocking buffer for 30min at 37°C and then incubated with biotined antidigoxigenin antibody at 37°C for 2 h. The slides were washed in 0.5 M PBS (pH 7.2-7.6) four times, each for 5 min. Cells were incubated in SABC at 37°C for 20 min. Slides were washed in 0.5 M PBS (pH 7.2-7.6) three times, each for 5 min. After that, cells were incubated in biotined peroxidase at 37°C for 20 min. Slides were washed in 0.5 M PBS (pH 7.2-7.6) four times, each for 5 min. The specific signals were visualized by incubation in DAB buffer. Water was used to end the reactions. Cells re-dyed with haematoxylon and photographs were taken.
2.8 Cell Cycle Analysis by Flow Cytometry
Cell-cycle phase distribution was analyzed by flow cytometry using PI staining. Briefly, control for treating MCF-7 cells were harvested after trypsinization and washing with 1×PBS, then fixed in 70 % ethanol at a cell concentration of 1 × 106/mL with samples not exceeding 3 × 106 cells, and then stained with 50 μg/mL propidium iodide (containing 10 mg/mL RNase A) for 30 min at 4°C. Resulting DNA distributions were analyzed on a FACSort (Becton Dickinson, San Jose, CA) with Cell Quest software (version 313) for the proportions of cells in G1, S and G2 phases of the cell cycle.
3. Results
3.1 Cloning and Sequencing of the Full-Length cDNA
of Human Wnt9a
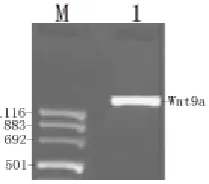
The full-length cDNA of human Wnt9a was successfully cloned using PCR from hES cells (Fig. 1). The PCR product (1,621 bps) was sequenced and corrected.
3.2 Immunohistochemistry
The results of immunohistochemistry (Fig. 2) showed that Wnt9a was expressed in undifferentiated hES cells.
3.3 Construction of Expression Vector for Silence Human Wnt9a
Fig. 1 PCR amplification of Human Wnt9a ORF from hES cells. M: Marker (pUCmix 8); 1: Human Wnt9a gene.
Fig. 2 Immunhistochemical staining for human Wnt9 protein in hES cells.
A: undifferentiated hES cells under optics microscope; B: undifferentiated hES cells dyed by DAPI under fluorescence microscope; C: undifferentiated hES cells addition Wnt9a antibody under fluorescence microscope; D: undifferentiated hES cells without addition Wnt9a antibody under fluorescence microscope.
Fig. 3 Insert of anti-human Wnt9a RNA and sequenced. A: one kind of tested insert of anti-human Wnt9a RNA is shown that it begins immediately after the SalⅠ sequence, and termination occur after the UUUU at 3' terminus of the insert; B: sequenced the cDNA insert of anti-human Wnt9a.
Fig. 4 5′ partial sequence of pEGFP-C3/human Wnt9a
recombinant plasmid.
3.4 Construction pEGFP-C3/Human Wnt9a Recombinant Plasmid
To allow a directional insertion of the whole ORF of human Wnt9a into the pEGFP-C3 vector, primers 5'-gaagcttatgctggatgggtccccg-3′ (HindⅢ) and 5'-aggatccgcaatgcctgcaccctgt-3′ (BamHⅠ) were used to amplify the target gene. After PCR amplification, the Wnt9a encoding sequences were subcloned in the pMD18-T vector. Subsequently, there combinant pMD18-T/human Wnt9a and pEGFP-C3 vectors were digested with the restriction endonucleases HindⅢ
and BamHⅠ (MBI, Lithuanian). Both digestion reactions were checked by using 1.5% agarose gel electrophoresis, and then the pEGFP-C3 vector and the gene fragments digested using HindⅢ and BamHⅠ were purified. Then, a ligation reaction was done according to the manufacturer’s instructions (TaKaRa Co., Japan). DH5α cells were transformed with 10 μL of the ligation reaction products and spread on an LB agar plate containing 100 μg/mL ampicillin. Finally, individual clones were checked using DNA-sequencing to ensure that the fusion plasmids were correctly constructed (Fig. 4).
3.5 Examined the Expression Level of Wnt9a after RNAi by in Situ Hybridization
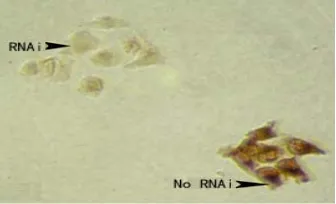
The results demonstrated that the cells showed, when U6+27 cassettes were used with anti-Wnt9a hairpin siRNA inserts, dramatic reduction of Wnt9a signals (Fig. 5 RNAi) whereas as compared to the untransfected cells (Fig. 5 No RNAi) in the same fields under microscope, shown the hybridization probes having specific for human Wnt9a.
3.6 Flow Cytometry Analyses
Fig. 5 The effect of U6+27/siWnt9a transcripts.
Cells were stained lightly in cells (RNAi), whereas cytoplasms are deep brown in untransfected cells (No RNAi).
Fig. 6 The influence of cell cycle after RNAi and over expression of Wnt9a.
A: MCF-7 cells transfected with pAVU6+27 as control, MCF-7 cells transfected with pAVU6+27/siWnt9a, for down-expression of Wnt9a; B: MCF-7 cells transfected with pEGFP-C3 as a control and MCF-7 cells transfected with pEGFP-C3/Wnt9a for over-expression of Wnt9a.
cytometry analyses showed that the number of the cells transfected with pEGFP-C3/Wnt9a were decreased in S, G2/M phase and increased in G1 phase (Fig. 6B carmine) as compared with control cells transfected pEGFP-C3 (Fig. 6B blue).
4. Disscussion
In this study, we cloned human Wnt9a from hES cells (Fig. 1). The immunohistochemistry result shows that human Wnt9a protein was expressed in human embryonic stem cell (Fig. 2). In order to study on the preliminary function of human Wnt9a, we sued pAVU6+27 vector, which contains the SalⅠ/XbaⅠ cloning sites [19], to construct the RNAi expression vector for the human Wnt9a. One kind of tested insert of anti-human Wnt9a RNA is shown that it begins
Wnt9a predominantly expressed in the mural trophoblast and inner cell mass cells surrounding, so Wnt9a may be play an important role of regulating the rate of hES cells proliferation.
5. Conclusion
In summary, our results demonstrated that human Wnt9a was cloned from hES cells and Wnt9a protein was expressed in hES cells. The pAVU6+27/siWnt9a expression vector can effectively induce RNAi-mediated gene silencing in mammalian cells. The cells proliferation was promoted after lowering expression of human Wnt9a in MCF-7 cells by RNAi, but reversely after over-expression of Wnt9a. All suggest the expression level of human Wnt9a may play a role in adjusting the rate of cell proliferation.
Acknowledgment
This work was supported by grants from the National Nature Science Foundation of China (No.31071091; No.30971570; No.31171196) and Department of Education Key Project of HuNan Province, China (NO:09A035 and NO:06C370).
References
[1] C.Q. Xie, G. Lin, K.L. Luo,Newly expressed proteins of mouse embryonic fibroblasts irradiated to be inactive, Biochemical and Biophysical Research Communications 315 (2004) 581-588.
[2] J.A. Thomson, J. Itskovitz-Eldor, S.S. Shapiro, Embryonic stem cell lines derived from human blastocysts, Science 282 (1998) 1145-1147.
[3] B.E. Reubinoff, M.F. Pera, C.Y. Fong, Embryonic stem cell lines from human blastocysts: somatic differentiation
in vitro, Nat. Biotechnol. 18 (2000) 399-404.
[4] C.H. Xu, M.S. Inokuma, J. Denham, M.K. Carpenter, Freeder-free growth of undifferentiated human embryonic
stem cells, Nat. Biotechnol 19 (2001) 971-974.
[5] N. Roel, Wnt signaling in disease and in development, Cell Research 15 (2005) 28-32.
[6] K. Willert, J.D. Brown, E. Danenberg, Wnt proteins are lipid-modified and can act as stem cell growth factors, Nature 423 (2003) 448-452.
[7] W.J. Nelson, R. Nusse, Convergence of Wnt, β-catenin, and cadherin pathways, Science 303 (2004) 1483-1487. [8] K.M. Cadigan, R. Nusse, Wnt signaling: A common
theme in animal development, Genes Dev. 11 (1997) 3286-3305.
[9] C. Jamora, E. Fuchs, Intercellular adhesion, signaling and the cytoskeleton,Nature Cell Biol. 4 (2002) 101-108. [10] T. Reya1, H. Clevers, Wnt signaling in stem cells and
cancer, Nature 434 (2005) 843-850.
[11] P. Polakis, Wnt signaling and cancer, Genes Dev. 14 (2000) 1837-1851.
[12] J.P. Thiery, Epithelial-mesenchymal transitions in tumor progression, Nature Rev. Cancer 2 (2002) 442-454. [13] R.A. Pagliarini, T. Xu, A genetic screen in Drosophila
for metastatic behavior, Science 302 (2003) 1227-1231. [14] A. Fire, S. Xu, M.K. Montgomery, S.A. Kostas, Potent
and specific genetic interference by double-stranded RNA in Caenorhabditis elegans, Nature 391 (1998) 806-811. [15] M.K. Montgomery, S. Xu, A. Fire, RNA as a target of
double-stranded RNA-mediated genetic interference in
Caenorhabditis elegans, Proc Nati. Acad. Sci. USA 95 (1998) 15502-15507.
[16] E.R. Fearon, K.M. Cadigan, Wnt signaling glows with RNAi, Science 308 (2005) 801-808.
[17] G. Kemp, E. Willems, S. Abdo, Expression of all Wnt genes and their secreted antagonists during mouse blastocystand postimplantation development, Development Dynamics 233 (2005) 1064-1075.
[18] D. Spater, T.P. Hill, R.J. Osullivan, Wnt9a signaling is required for joint integrity and regulation of Ihh during chondrogenesis, Development 133 (2006) 3039-3049. [19] R. Agami, RNAi and related mechanisms and their
potential use for therapy, Curr. Opin. Chem. Biol. 6 (2002) 829-834.
Effect of Therapeutic Ginger on Genotoxic of Taxol Drug
(Anti-Cancer) in Bone Marrow Cell of Male Mice
Mona Mohammed Zaid AL-Sharif
Biology Department, Faculty of Science for Girls, King Abdul-Aziz University, Jeddah 21493, Kingdom of Saudi Arabia
Received: June 13, 2011 / Accepted: July 22, 2011 / Published: November 30, 2011.
Abstract: Medicinal use of spices/herbs has been gradually increased in the developed countries, Zingiber officinale (Ginger) is known to possess potent antioxidant and anti inflammatory properties. Therefore, the aim of this study is to determine the possible anti-mutagenic effect of ginger against the genotoxic effect of anti-cancer drug Taxol 0.6 mg/kg. This study is conducted by using two types of cytogenetic studies in bone marrow cell of mal albino mice Mus musculus (average weight 25-30 g). The animals were randomly distributed into six groups, each of 14 mice, (G1) was given the solvent, (G2) treatment of the medical dose of Taxol drug, (G3) treatment of ginger, (G4) a pre-treatment of ginger prior to treatment of drug, (G5) a simultaneous treatment of ginger and treatment of drug, (G6) a post-treatment of ginger after treatment drug. The study results show that significant increase in total chromosomal aberrations and significant increase in the number of micronuclei were observed after treatment drug. The significant structural aberrations were in the form of end-to-end associations. The numerical chromosomal aberrations were endomitosis and polyploid. The results showed that the frequencies of chromosomal aberrations and micronuclei in ginger treated group were not significantly different from control. Simultaneous treatment of ginger was found to be effective in reducing the genotoxic effects induced by drug Taxol especially in the total number of the chromosomal aberrations and the number of micronuclei.
Key words: Cytogenetic, genotoxic, taxol, ginger, bone marrow, mice, chromosomal aberrations, micronuclei.
1. Introduction
The majority of anticancer (antieoplastic) drugs are especially designed to interfere with DNA synthesis, cellular metabolism and cell division [1]. Due to this mode of action, these drugs are expected to cause mutations and cytogenetic abnormalities. Therefore, anticancer drugs are used as mutagens in most antimutagenic tests [2]. During recent years, considerable efforts have been focused on using antimutagens to modulate the genotoxic effects of the mutagenic antineoplastic drugs.
In the recent past, many natural compounds derived from plants crude extracts proved to have a protective effect against the toxic effects of many chemicals [3-5]. Medicinal use of spices/herbs has been gradually
Corresponding author: Mona Mohammed Zaid AL-Sharif, Ph.D., assistant professor, research field: genetics. E-mail: [email protected].
gastrointestinal, prostate, breast, and other cancers than their Western counterparts [8], and it is thought that constituents of their diet may play significant role in protection. Indeed, phenolic substances present in fruit and vegetables, and in medicinal plants, have cancer chemo preventive activities, both in vitro as well as in vivo animal models [9-12].
Therefore, the aim of this study is to determine the possible antimutagenic effect of ginger against the genotoxic effect of Taxol anticancer drug using of studies Chromosomal Aberration (CA) and Micronucleus (MN) in bone marrow cells of male mice.
2. Materials and Methods
2.1 Animals
Male Swiss albino mice (Mus musculus) MFI strain, 8-9 weeks old, weighed 25-30 g, were obtained from the animal house of King Fahad Medical Research Centre, KAU. Animals were housed in plastic cages with steel wire tops in an air conditioned room (22 ± 1°C,45-75% relative humidity) maintained in a controlled atmosphere of 12 h light/12 h dark cycle. The mice were maintained basal diet (20% crude protein, 4% crude fat, 3.5% crude fiber, and energy 2,850 kcal/kg diet), and water were provided ad labium. 2.2 Test Compounds
Taxol (Paclitaxel) which has been recently introduced into Kingdom of Saudi Arabia as a natural anticancer drug was obtained from Dr. Soliman Fakeeh Hospital Pharmacy. The dose used in the present study was calculated based on human therapeutic dose (0.6 mg/kg).
Ginger (Zingiber officinale Rosc.) was prepared by heating 400 mL of distilled water at 80°C; then soaked 20 g of dry ginger for 60 min and then permitted to interfere with his candidacy and get rid of the sludge and then put in dark bottles. Each animal was given a dose of about 0.2 mL/kg of ginger using the Tube infectious which is placed directly in the mouth.
2.3 Treatments and Route of Administration
The control animals received an equal volume of the solvent 9% NaCl by intra peritoneal injection (i.p.) for one day. The route of administration for ginger was oral intubation (o.i.) for one day, Taxol was acutely by a single intra peritoneal injection (i.p.) for one day. 2.4 Experimental Design
The animals were randomly distributed into six groups, each of 14 mice. Animals in group one (G1) were given the solvent (i.p.); in group two (G2): treatment of therapeutic dose of Taxol drug (i.p.); G3: treatment of ginger (o.i.); G4: a pre -treatment of ginger 2 h prior to treatment of drug; G5: a simultaneous treatment of ginger and treatment of drug; G6: a post-treatment of ginger 2 h after treatment drug. 2.5 Cytogenetic Methods
Chromosomal aberrations (CA): Bone marrow cells of male albino mice were examined for chromosomal aberrations according to the method described by Adler [13]. Mice were intraperitoneal injected with 0.05% colchicines dissolved in distilled water for 2 h before killing to arrest dividing cells. At least 50 metaphases were examined using research microscope with oil immersion lens.
2.6 Protective Effect
The protective index of ginger against the clastogenic and cytotoxic effects of Taxol on the induction of CA and MN was calculated according to the equations in Ref. [17] as following:
100 – % CA (G + Taxol DDP) groups × 100 % CA (Taxol DDP) groups
100 – % MN (G + Taxol DDP) groups × 100 % MN (Taxol DDP) groups
2.7 Statistical Analysis
SPSS program was used for analyzing chromosomal aberrations and micronuclei T-test and analysis of variance (ANOVA) followed by the least significant differences (LSD).
3. Results
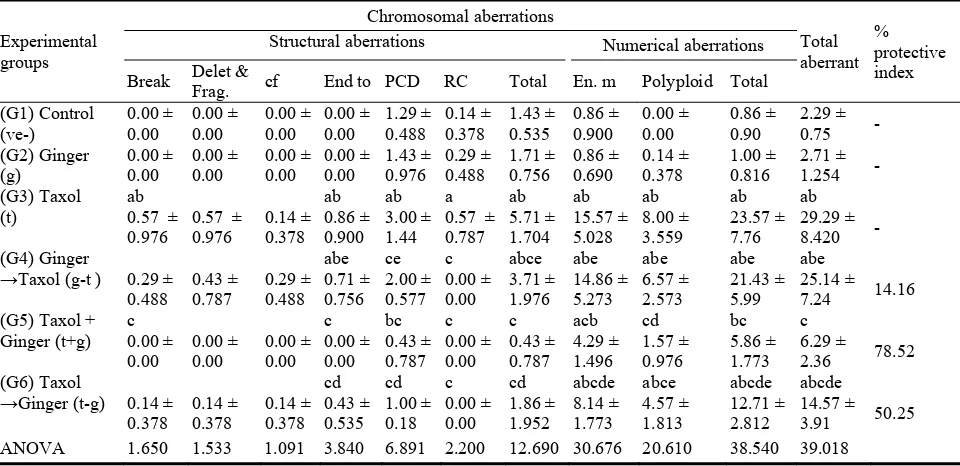
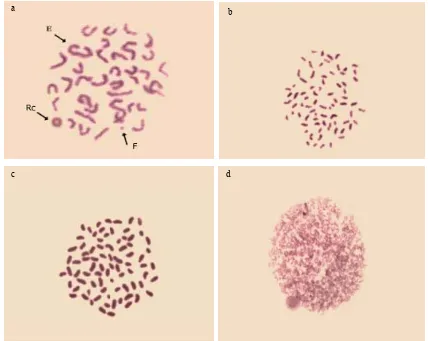
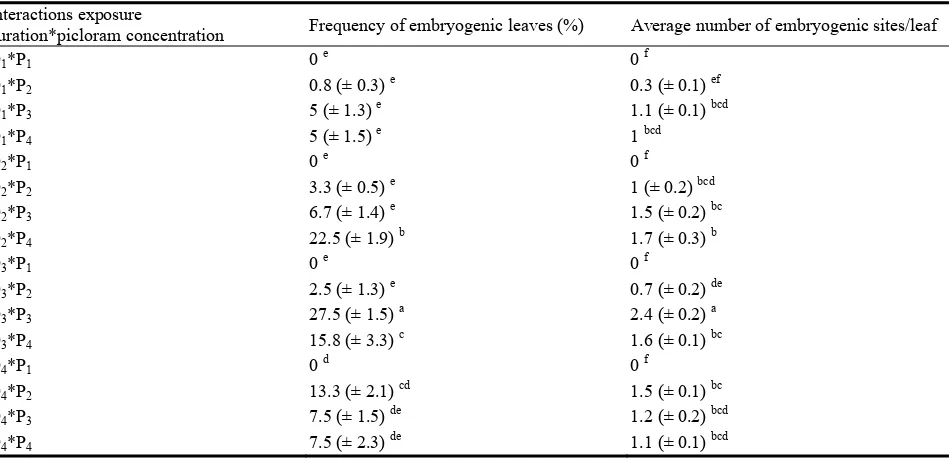
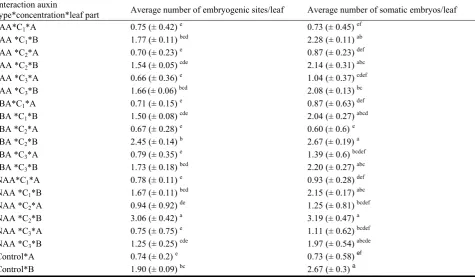
The resultsin Table 1 and Fig. 1 showed that there is an increase in the number of structural andnumerical aberrations after treatment with therapeutic dose of the Taxol drug using statistical analysis to T-test. The increase was significant at end-to-end association for structural chromosomal aberration while the increase was very highly significant in the centromeric division compared to the control sample, which in turn led to a very highly significant in the total number of structural aberrations.
Thenumerical chromosomal aberrations have also recorded significance in the end mitosis compared to control group, which also recorded high significance inpolyploidy which also caused in highly significant increase in the total number ofnumerical chromosomal aberrations. On the other hand, the results obtained haveshown that there is no significant difference in chromosomal aberrations of the grouptreated with a dose of ginger compared to control group.
For common treatments of ginger dosebefore, after and simultaneous with Taxol treatment, the number of structuraland numerical chromosomal aberrations was close to their numbers in the control group with the exception of polyploidy for numerical aberrations have
recorded asignificant in the group treated with ginger with Taxol. The treatment withginger before the drug was highly significant in numerical chromosomal aberrations (polyploidy), while the treatment of ginger after the drug has demonstrated highly significant ranges between (0.05) and highly significant(0.01)in structural chromosomal aberrations (centromeric division) andnumerical (endomitosis and polyploidy) compared to controlsample.
Table 1 Effects of different ginger (g) treatments on chromosomal aberrations induced by Taxol in bone marrow of male mice.
Experimental Structural aberrations Numerical aberrations
Break Delet & ANOVA 1.650 1.533 1.091 3.840 6.891 2.200 12.690 30.676 20.610 38.540 39.018
PCD: Premature Centromeric Division; Polyploid: 3n,4n & 5n; Delet. & Frag.: deletion & fragment; Each value represents mean ± SD; n:7 mic; a: significant difference between control (ve-) group and other groups at P < 0.05; b: significant difference between ginger (G) group and other groups at P < 0.05; c: significant difference between Taxol drug group (T) and groups take in pre or fragment with ginger at P < 0.05; d: significant difference between (g-t) group both (t-g) group at P < 0.05; e: significant difference between (t+g) group both (t-g) group and (g-t) group at P < 0.05.
Table 2 Effects of different ginger (g) treatments on MN induced by Taxol in bone marrow poly chromatic Erylrocytes (PCE) of male mice.
MN: micronuclei; No. cells examined: 1,000/mice; No. individuals examined: 7 mice; a: significant difference between control (ve-) group and other groups at P < 0.05; b: significant difference between ginger (G) group and other groups at P < 0.05; c: significant difference between Taxol drug group (T) and groups take in pre or after or with ginger at P < 0.05; d: significant difference between (g-t) group both (t-g) group at P < 0.05; e: significant difference between (t+g) group both (t-g) group and (g-t) group at P < 0.05.
in reducing the percentage of micronuclei by drug, is the treatment of ginger with the drug.
As seen from theTables 1 and 2, there are highly significant differences in the number of micronuclei of the three combined treatments through analysis of ANOVA.
From the above, theresults of the present study show that, the tests for the chromosomal aberrations or
micronuclei had shown their agreement together in reducing the induced genetic toxic effect of the drug in the simultaneous treatment whichproved that, the best times to have ginger is taken with Taxol.