www.elsevier.com / locate / bres
Research report
Diurnal metabolism of dopamine in dystrophic retinas of homozygous
and heterozygous retinal degeneration slow
(
rds
)
mice
a ,
*
b bIzhak Nir
, Rashidul Haque , P. Michael Iuvone
a
Department of Pharmacology, University of Texas Health Science Center, San Antonio, TX, USA
b
Department of Pharmacology, Emory University School of Medicine, Atlanta, GA, USA
Accepted 15 August 2000
Abstract
Dopamine metabolism was studied in dystrophic retinal degeneration slow (rds) mice which carry a mutation in the rds / peripherin gene. RDS mutations in humans cause several forms of retinal degeneration. Dopamine synthesis and utilization were analyzed at various time points in the diurnal cycle in homozygous rds / rds retinas which lack photoreceptor outer segments and heterozygous rds /1 retinas which have short malformed outer segments. Homozygous retinas exhibited depressed dopamine synthesis and utilization while the heterozygous retina retained a considerable level of activity which was, nevertheless, significantly lower than that of normal retinas. By one year, heterozygous rds /1retinas which had lost half of the photoreceptors still maintained significant levels of dopamine metabolism. Normal characteristics of dopamine metabolism such as a spike in dopamine utilization at light onset were observed in mutant retinas. However, light intensity-dependent changes in dopamine utilization were observed in normal but not rds /1retinas. The findings of this study suggest that human patients with peripherin / rds mutations, or other mutations that result in abnormal outer segments that can still capture light, might maintain light-evoked dopamine metabolism and dopamine-dependent retinal functions during the progression of the disease, proportional to remaining levels of light capture capabilities. However, visual deficits due to reduced light-evoked dopamine metabolism and abnormal patterns of dopamine utilization could be expected in such diseased retinas. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Retina and photoreceptors
Keywords: Dopamine; Retina; Rds / peripherin mutation; Rds mouse; Retinal degeneration
1. Introduction The retinal degeneration slow (rds) mouse carrying the mutant peripherin / rds gene is extensively studied as an Retinitis Pigmentosa (RP) is a clinical diagnosis that animal model of RP. A mutation in the peripherin / rds gene encompasses a group of heterogeneous hereditary disorders in rds mice accounts for the defect in disc morphogenesis, that manifest progressive loss of photoreceptors and blind- since replacement of the gene in transgenic rds mice ness. The prevalence of the disorder is about 1 / 4000, corrects the defect [51]. RDS encodes peripherin / rds, a which makes it a common cause of visual impairment in structural glycoprotein in the rims of outer-segment discs all age groups [2]. Mutations in multiple genes have been [12,27,50].
shown to cause blindness due to photoreceptor cell-death Photoreceptors in the homozygous rds / rds mouse retina [34,52]. Over 50 mutations in the RDS gene have been differentiate normally for the first few postnatal days. Inner implicated in dominant forms of RP and macular degenera- segments project an extended cilium but outer segments tion [28,47]. fail to develop and only rudimentary discs are formed at the distal ciliary tip. Between the second and third post natal weeks the photoreceptor starts to undergo apoptotic
*Corresponding author. Tel.: 11-210-567-4974; fax: 1
1-210-567-cell death. Photoreceptor 1-210-567-cell loss proceeds slowly until
4226.
E-mail address: [email protected] (I. Nir). completed by one year [23,44]. In the heterozygous rds /1
mouse retina, photoreceptors develop a distinct outer dark / light cycle, with light on at 8 AM. Throughout this segment layer of moderate length, but disc structures paper, ‘‘day’’ refers to the light phase and ‘‘night’’ refers remain disarrayed and form irregular whorls. Photorecep- to the dark phase of the imposed dark / light cycle. Lighting tor cell loss in the rds /1 retina is first noted by 2 months, was provided by fluorescent tubes. Light intensity at cage then it progresses more slowly than in the homozygous levels was 3–5 foot candles (fc). Experiments were carried retina and by one year the photoreceptor population is out in a laboratory under selected illumination levels. reduced by half [18]. Overhead illumination was provided by cool white fluores-Reduced light responses measured in mutant rds retinas cent bulbs at a room temperature of 218C. Illumination was [10,43] are expected to affect metabolic pathways in retinal measured with a digital illuminance meter (DX-200, INS neurons that are regulated by light. In a previous study we Enterprise Inc., Taiwan). The mice were euthanized by established that light-evoked metabolism of dopamine cervical dislocation. Following enucleation, the anterior (DA) is diminished in homozygous rds / rds mice [39]. DA structures and lens were removed and the retina was is an important retinal neurotransmitter and neuro- dissected from the posterior eye cup. Procedures in the modulator [4,14,15,56]. In the mammalian retina, DA is ‘‘dark’’ were carried out under dim red light (Kodak safety synthesized and released by a subset of amacrine and light filter no. 1).
interplexiform cells [35,54]. Cellular responses to DA are
mediated by the D1 and D2 receptor families [46]. 2.2. Electron microscopy Quantitative analysis of DA receptors in the rat retina
revealed the highest concentration of D2-like receptors in Eyes were placed in 4% formaldehyde and 2% glutaral-the photoreceptor inner segments and in glutaral-the outer nuclear dehyde in 0.1 M phosphate buffer (pH 7.0) for fixation. layer [49]. In the mouse retina, D4 receptors, a subtype of After 30 min, the eyes were bisected along the vertical D2, were localized to the photoreceptor layer [11]. meridian and the two hemispheres were fixed for an DA release from dopaminergic neurons affects several additional 3 h. The tissue was then treated in 1% OsO4, processes in the retina, including: photoreceptor disc dehydrated and embedded in an epoxy resin. Thin sections shedding [4]; cone contraction and movement of melanin were cut at different sites along the central–peripheral axis in the pigment epithelium [13,42]; Na1,K1-ATPase of the retina and stained with uranyl and lead salts before activity in photoreceptors [48]; rod opsin gene expression viewing under the electron microscope.
[1]; gap junction permeability between horizontal cells
[41] and between rods and cones [30]; glutamate-gated 2.3. Enzyme inhibitions ionic conductances in horizontal cells [29] and bipolar
cells [32], and ganglion cell receptive fields [25]. Synthesis of dopamine from L-tyrosine is a two step
In the present study we investigated the diurnal metabo- process: (1) hydroxylation by tyrosine hydroxylase (TH) lism of DA in mutant homozygous rds / rds and hetero- to produce L-3,4-dihydroxyphenylalanine (L-DOPA); and
zygous rds /1mouse retinas. The analysis included young (2) decarboxylation of DOPA by aromatic L-amino acid
and old mice to determine the consequences of a life-long decarboxylase (AAAD) to produce dopamine. DA syn-light deprivation on syn-light-evoked DA metabolism. Results thesis and utilization were analyzed after inhibition of from the present study will be relevant for the evaluation AAAD with m-hydroxybenzylhydrazine (NSD-1015; of abnormalities in DA metabolism and its effect on retinal Sigma Chemical Co.). Inhibition of AAAD results in functions in RP patients with peripherin / rds mutations and accumulation of DOPA, which is used as an estimation of also with other mutations which affect outer segment in situ TH activity and DA synthesis. Utilization of DA is integrity and light capture. reflected by the decline in DA levels following inhibition of AAAD. The drug was dissolved in phosphate-buffered saline (pH 7.0) and injected intraperitoneally (150 mg / kg
2. Material and methods body weight). Groups of 5–7 mice were injected and each mouse was placed, individually, in a clear plastic cage and 2.1. Animals illuminated for 30 min at 60 fc, unless noted otherwise. The retinas were then isolated and frozen in liquid Handling of animals conformed with principles regard- nitrogen.
ing the care and use of animals adopted by the American
Physiological Society and Society for Neuroscience. 2.4. Catecholamine analysis Normal BALB / c mice, mutant homozygous rds / rds
10mM ascorbic acid and 20 ng / ml 3,4-dihydroxybenzyl- 3.1. DA metabolism in young rds /1 and rds /rds mice
amine (DHBA), an internal standard. Homogenates were
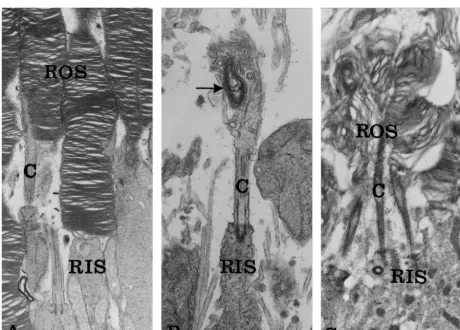
centrifuged and aliquots of each supernatant fraction were One to two month old BALB / c, rds /1 and rds / rds injected into a Beckman Ultrasphere-ODS reverse phase mice were studied. By this age, photoreceptor differentia-column. DA and metabolites were eluted with a mobile tion is completed, photoreceptor cell loss is not yet phase consisting of 100 mM phosphoric acid, 0.1 mM measurable in the rds /1 retinas [18], and only the initial EDTA, 0.45 mM sodium octylsulphate and 6% acetonitrile wave of photoreceptor cell loss occurred in the rds / rds at pH 2.6–2.7 and were detected amperometrically. Chro- retina [38,44]. The mutation phenotypes, as expressed in matographic peaks were identified by relative retention the organization of the photoreceptor outer segments, are times compared to those of external standards. Concen- depicted in Fig. 1. While normal BALB / c photoreceptors trations were determined by comparing peak areas of outer segments consist of stacks of photopigment-con-unknowns with those of standards. Values are corrected for taining disc membranes (Fig. 1A), in the homozygous the recovery of DHBA. Retinal protein was determined by rds / rds mutant photoreceptors disc membranes are not the method of Lowry [31]. formed (Fig. 1B). The heterozygous rds /1outer segments are characterized by malformed disorganized disc mem-branes (Fig. 1C).
3. Results
3.1.1. Steady state levels of DA and DOPAC in night DA metabolism in the retina was determined by the and day periods
following measurements: (a) steady state levels of DA and Steady state levels of DA at 2 h into the night period (in DOPAC, the main DA degradation product, and (b) DA darkness) and 2 h into the day period (in light) are shown utilization and synthesis (in situ TH activity) in mice in Fig. 2. There was no difference in steady state levels treated with AAAD inhibitor NSD-1015. between dark and light in each of the three genotypes.
Fig. 1. Ultrastructure of photoreceptor outer segments in normal and mutant retinas. (A) Normal BALB / c photoreceptors. The rod outer segment is characterized by stacks of photopigment-containing disc membranes.314 250. (B) Dystrophic homozygous rds / rds photoreceptors. Outer segments are not formed. Only rudimentary membranous structures are seen at the tip of the connecting cilium (arrow). 317 750. (C) Heterozygous rds /1
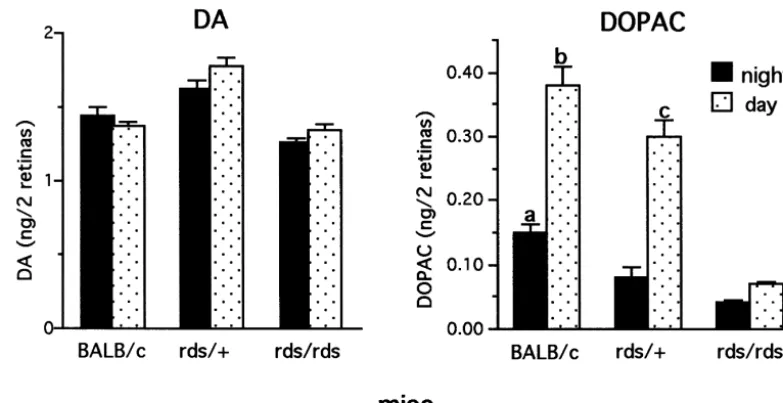
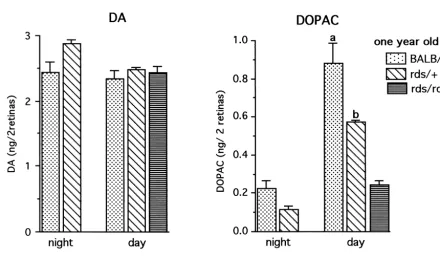
Fig. 2. Steady state levels of DA and DOPAC in 1–2 month old BALB / c, rds /1and rds / rds retinas. Mice were euthanized 2 h into the night period and 2 h into the day period. Catecholamines were determined by HPLC–EC. Each value is the mean6S.E.M. from measurements of 6–7 mice (n56–7). DA: There is no difference in steady state levels in night and day between BALB / c and rds retinas. Levels in rds /1are higher both in night and day from levels measured in BALB / c and rds / rds retinas. DOPAC: In BALB / c and rds /1retinas, levels in the day are much higher than those at night. Significant differences are seen in steady state levels of DOPAC between the 3 genotypes, both in night and day. Levels of DOPAC in rds /1retina in day are significantly higher than the daytime levels in rds / rds retinas. (a) P50.004 vs. rds /1 dark; P,0.001 vs. rds / rds dark, (b) P50.002 vs. rds /1light; P,0.001 vs. rds / rds light, (c) P,0.001 vs. rds / rds light.
Between genotypes, steady state levels in rds /1 were 3.2. DA utilization and synthesis at light onset higher both in dark (12%) and light (29%) compared to
levels measured in normal BALB / c retina. Steady state Since major cellular events such as disc shedding occur levels of DOPAC were low in the three genotypes in the at light onset at the beginning of the day, DA utilization dark and increased upon illumination (Fig. 2). Differences and synthesis in NSD-1015 treated mice were analyzed at in steady state levels of DOPAC between the 3 genotypes light onset and 2–2.5 h into the day period.
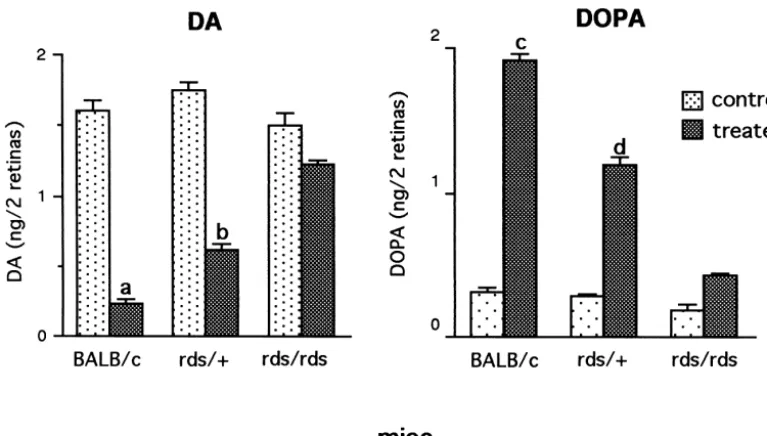
were measured both in night and day, with highest levels The highest levels of DA utilization and synthesis were measured in the BALB / c retinas both in night and day. In measured in normal mice at light onset when fully dark-comparison with normal BALB / c retina, levels of DOPAC adapted photoreceptors respond maximally to light. DA in homozygous rds / rds retina were 73% lower in the night utilization in the BALB / c retina during the first 0.5 h in and 81% lower in day. In the heterozygous rds /1 retinas, light was 73% higher than levels measured at 2.5 h into the levels of DOPAC were 47% lower in night but only 21% day period. In the rds /1 retina, DA utilization in the first lower in day, indicating a much higher response to light as 0.5 h in light was 56% higher than levels at 2.5 h into the compared with the rds / rds retina. day period (Fig. 4). In the rds / rds retina, although DA utilization in light was limited in comparison to BALB / c and rds /1 retinas, residual DA levels at 0.5 h in light 3.1.2. DA utilization and synthesis in day period (0.986 0.064 ng DA / 2 retinas, n57) are 21% lower than DA utilization and synthesis were analyzed 2–4 h into levels measured at 2 h into the day period (1.260.004 ng the day period in NSD-1015 treated mice (Fig. 3). In DA / 2 retinas, n59, P,0.001), indicating an elevated normal BALB / c retina, an 87% decline of DA levels was utilization at light onset. Similar to utilization, DA syn-measured in treated retinas from levels syn-measured in thesis was highest at light onset. In comparison with levels control, untreated retinas. In the heterozygous rds /1 at light onset, the BALB / c retinas showed a 36% decrease retina, a 65% decline was measured, while in the homo- at 2.5 h into the day and the rds /1 retina showed a 45% zygous rds / rds retina only a 19% decline in DA levels was decrease in DA synthesis (Fig. 4). No significant change in recorded. DA synthesis, as estimated from DOPA accumu- DA synthesis was measured in the rds / rds retina.
lation, showed corresponding differences between the
different genotype, with treated BALB / c retinas demon- 3.3. DA utilization and synthesis under low and high strating the highest rate of synthesis. DA synthesis in illumination levels
rds /1 and rds / rds retinas was 38% and 77% lower,
measure-Fig. 3. DA utilization and synthesis in 1–2 month old normal and mutant retinas. Mice were studied 2 h into the day period. DA and DOPA levels were measured after injection of NSD-1015 (150 mg / kg) and illumination at 60 fc for 30 min. Control littermates were not treated. Each value is the mean6S.E.M from measurements of 5–8 mice (n55–8). DA: Utilization, as determined by reduction in DA levels, was high in treated normal BALB / c mice, lower in treated rds /1mice and very limited in rds / rds mice. DOPA: DA synthesis, as determined by accumulation of DOPA, was highest in treated BALB / c retinas. In the rds /1retinas, synthesis was lower than in normal retinas but significantly higher than in rds / rds retinas. (a) P,0.001 vs. rds /1
treated; P,0.001 vs. rds / rds treated, (b) P,0.001 vs. rds / rds treated, (c) P,0.001 vs. rds /1treated; P,0.001 vs. rds / rds treated, (d) P,0.001 vs. rds / rds treated.
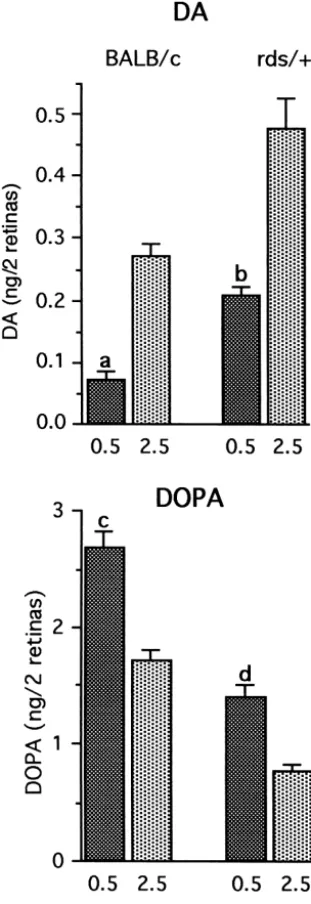
ment of DA utilization and synthesis under illumination of (14%) steady state level of DA in rds /1 in the dark 10 fc and 60 fc (Fig. 5). A divergence between DA follows a pattern similar to that measured in young rds /1
utilization and synthesis was observed between BALB / c retinas (see Fig. 2). Considerable differences in steady and rds /1 retina (Fig. 5). While in BALB / c retinas, DA state levels of DOPAC between the 3 genotypes were utilization significantly increased as light intensity in- measured in the day period, with levels in BALB / c retina creased from 10 to 60 fc, there was no difference in DA the highest. Levels of DOPAC in illuminated rds /1retinas utilization in the rds /1retina at these two light intensities. were 35% lower as compared with BALB / c retinas, while DA synthesis on the other hand showed only a 21% levels in illuminated rds / rds retinas were 72% lower. increase in BALB / c retinas between the low and high light Large night–day differences in DOPAC levels persisted in intensities, while in the rds /1 an increase of 53% was retinas of one year old rds /1 mice. Since night / day measured. differences in DOPAC levels were already shown to be very small in young rds / rds retinas (Fig. 2), night levels of 3.4. DA metabolism in one year old rds /1 and rds /rds DOPAC in old rds / rds retinas were not analyzed because
mice of limited availability of old rds / rds mice.
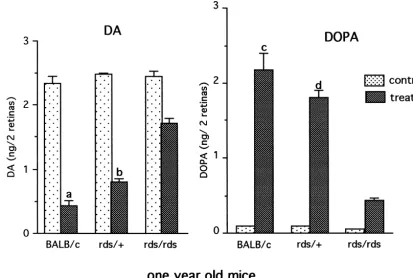
Eleven to twelve month old BALB / c, rds /1 and rds / 3.4.2. DA utilization and synthesis in day period
rds mice were studied. By this age most of the photo- DA Utilization and synthesis were studied 3–5 h into the receptors were lost in the rds / rds retina and about 50% of day period (Fig. 7). Both utilization and synthesis were photoreceptors were lost in the rds /1 retina. The remain- maintained at relatively high levels in the old rds /1
ing photoreceptors in the rds /1retinas carry abnormal rod mutant (Fig. 7). In normal retina, an 81% decline in DA outer segments similar to those seen in young rds /1mice from steady state levels was measured in NSD-1015 (Fig. 1). treated retina. A considerable decline (68%) was measured also in the heterozygous rds /1 retina whereas in the 3.4.1. Steady state levels of DA and DOPAC in night homozygous rds / rds retina only a 27% decline in DA
and day periods levels was measured. DA synthesis, as depicted by DOPA
photoreceptor cell death [39]. In the present study of the rds /1 retina we have found that the heterozygous retina, which is characterized by the presence of short and disorganized outer segments, is capable of considerable levels of DA turnover in comparison with the homozygous rds retina. However, DA metabolism is still significantly reduced in comparison to normal retinas. The reduced DA utilization and synthesis as measured in the present study in the 1–2 month old rds /1 retinas, prior to the onset of significant photoreceptor cell loss, is an indication of reduced light capture by the mutant retina. Electroretinog-ram (ERG) measurements of rds /1 retina revealed re-duced rod ERG in 2 month old mice which might be a reflection of abnormalities in outer segment size and organization [10]. Thus, there appears to be a general correlation between structural integrity of outer segments, light capture as measured by the electroretinogram and light-evoked DA metabolism.
The correlation between outer segment structural integri-ty, the amount of light capture and DA turnover is a result of light-evoked regulation of key enzymes in DA syn-thesis. Increased DA synthesis in light is due to activation of TH, the rate limiting enzyme in DA synthesis [21,22], and induction of AAAD [17]. TH activation and DA release are linked to light by the removal of tonic inhibition of dopaminergic cells by GABA and direct light-dependent activation of dopaminergic cells via gluta-mate released by bipolar cells [5,6,16,26,33]. Interestingly, in spite of the large differences in DA turnover in the normal, rds / rds and rds /1 retinas, steady state levels of DA were similar in the three genotypes both in dark and light. Moreover, normal steady state levels of DA were maintained in old rds /1and rds / rds retina, which suffered major photoreceptor loss. This might be a result of an efficient feedback mechanism by which reduced light-evoked synthesis and utilization are calibrated to maintain the DA storage at full capacity.
Further insight into the relationship between outer segment integrity and light sensitivity as related to DA
Fig. 4. DA metabolism during the first 0.5 h in light, at the beginning of
turnover was obtained by measurements of DA utilization
the day period, and after 2 h into the day period. Mice, 2 month old, were
and synthesis at a lower (10 fc) and higher (60 fc)
treated with NSD-1015 and illuminated for 30 min at 60 fc before
illumination level. In the normal retina, increased
illumina-sacrifice. The first group of mice was treated with NSD-1015 in the dark,
at the end of the night period, illuminated for 30 min at light onset and tion resulted in elevated utilization indicating that at the
sacrificed after 0.5 h into the day period. Other groups were treated at 2 h lower intensity the light evoked release mechanism was into the day period and sacrificed 30 min later, at 2.5 h into the day
not fully activated. Under the same conditions, the rds /1
period. Each value is the mean6S.E.M from measurements of 6–7 mice
mutant did not respond to increased illumination by
(n56–7). DA: In BALB / c and rds /1retinas, utilization at light onset, at
elevation of DA utilization. However, unlike utilization,
the beginning of the day period, was much larger than that measured at
2.5 h into the day period. DOPA: In BALB / c and rds /1retinas, DOPA DA synthesis in the rds /1 retina showed a significant
accumulation was higher in the first 0.5 h into the day period compared to increase upon increase in illumination intensity. It is not that at 2.5 h. (a) P50.006 vs. 2.5 h, (b) P,0.001 vs. 2.5 h, (c) P,0.001
clear how the difference in light requirements for synthesis
vs. 2.5 h, (d) P,0.001 vs. 2.5 h.
and utilization in the rds /1retina is related to the presence of truncated photoreceptor outer segments in this mutant.
Fig. 5. DA utilization and synthesis in BALB / c and rds /1retinas at 10 and 60 fc illumination. Two month old mice were kept under 10 fc illumination for the first 3–4 h of the day period. They were subsequently treated with NSD-1015 and exposed for 30 min to either 10 fc or 60 fc illumination. Each value is the mean6S.E.M. from measurements of 6–7 mice (n56–7). DA: While utilization in BALB / c retinas increased significantly as light intensity increased from 10 to 60 fc, there was no difference in DA utilization in the rds /1retina. DOPA: Exposure to higher illumination levels resulted in a significant increase in DOPA accumulation in the rds /1retina but not in the BALB / c retina. (a) P,0.001 vs. 10 fc, (b) P50.016 vs. 10 fc.
Fig. 7. DA Utilization and synthesis in one year old BALB / c, rds /1and rds / rds retinas. Mice were analyzed 2–3 h into the day period. Each value is the mean6S.E.M. from measurements of 5–6 mice (n55–6). DA: Utilization levels, as determined by decline in DA levels in NSD-1015 treated mice, were highest in BALB / c retinas. Significant utilization was seen also in treated rds /1retinas but relatively limited utilization in treated rds / rds retinas was observed. DOPA: Synthesis, as reflected by DOPA accumulation in treated retinas, was considerable in the old rds /1retinas, whereas that in treated rds / rds retinas was small. (a) P50.002 vs. rds /1treated; P,0.001 vs. rds / rds treated, (b) P,0.001 vs. rds / rds treated, (c) P50.016 vs. rds /1treated; P,0.001 vs. rds / rds treated, (d) P,0.001 vs. rds / rds treated.
regulation of target retinal neurons in an illumination- coincides with peak outer segment disc shedding in the dependent manner. mouse retina [3]. Although direct evidence for a role for Previous analysis of DA metabolism in normal BALB / c DA in regulation of disc shedding in the mammalian retina retina revealed a dramatic spike in DA synthesis and is not available, studies of amphibian retinas suggested that utilization at light onset, at the beginning of the day period changes in DA release during the day period might be [40]. In the present study, significantly higher levels of DA involved in regulation of disc shedding [4]. In the rds /1
synthesis and utilization at light onset were measured also retina, unlike normal albino mice, a peak in disc shedding in the rds /1 retina. Furthermore, even the rds / rds retina was recorded near the end of the light period [45]. Hence, with very diminished light capture and a comparatively if DA is involved in regulation of disc shedding, the sharp low level of DA utilization during the light period, showed change in DA turnover which was measured in the rds /1
a significant increase in DA utilization at light onset. Thus, at light onset might not correspond with peak shedding in in mutant retinas with major outer segment abnormalities, this retina.
the transition between the night and day phases of the By one year, steady state levels of DA in the mutant diurnal cycle is translated into a transient increase in DA rds /1 and rds / rds retinas were maintained at the same release that separates the initial period in light from the levels as in 1 yr old normal BALB / c retina. Furthermore, rest of the day period. It appears that a residual signal the ratios of synthesis and utilization in the mutants as transduction capability, mediated by remaining photopig- compared to normal retinas at 1 yr (Fig. 7), was close to ment molecules in the rudimentary discs and the plasma that measured in young mice (Fig. 2). Thus, in 1 yr old membrane of rds / rds photoreceptors [37,53], might be rds /1 and rds / rds mice, loss of photoreceptors and life-sufficient to elicit a light-evoked spike of DA release in long light deprivation did not exacerbate the deficiencies dopaminergic amacrine cells at light onset. Alternatively, which were already measured in young mutant mice. The non-traditional photoreceptors, such as those thought to maintenance of relatively high levels of light-evoked DA regulate circadian photoreceptions [55], might influence turnover in 1 yr old rds /1 retina is of interest since, by 1 DA neuronal activity. yr, about half of the photoreceptors were lost in the rds /1
[2] E.L. Berson, Retinitis Pigmentosa, Invest. Ophthalmol. Vis. Sci. 34
in synaptic connectivity. Increase in the areas of synaptic
(1993) 1659–1676.
contact between rod terminals and second order neurons
[3] J.C. Besharse, J.G. Hollyfield, Turnover of mouse photoreceptor
was observed in the rds /1 mutant [24]. It was suggested outer segments in constant light and darkness, Invest. Ophthal. Vis. that the increased synaptic contact might compensate for Sci. 18 (1979) 1019–1024.
chronic cell loss and help maintain a threshold level of [4] J.C. Besharse, P.M. Iuvone, M.E. Pierce, Regulation of rhythmic photoreceptor metabolism: A role for post-receptoral neurons, in:
signal transmission [24].
N.N. Osborne, G.J. Chader (Eds.), Progress in Retinal Research,
An important issue is whether the course of the disease
Pergamon Press, Oxford, 1988, pp. 21–61.
in the homozygous rds / rds retinas is affected by the very [5] J.H. Boatright, J.R. Gordon, P.M. Iuvone, Inhibition of endogenous low release of DA from dopaminergic cells. In view of the dopamine release in amphibian retina by L-2-amino-4-phos-possible role of DA in photoreceptor metabolism either phonobutyric acid (L-AP4) and
trans-2-aminocyclopentane-1,3-di-carboxylate (ACPD), Brain Res. 649 (1994) 339–342.
directly through interaction with D2 / D4 receptors on the
[6] J.H. Boatright, N.M. Rubim, P.M. Iuvone, Regulation of endogenous
photoreceptor cell [11], or indirectly through its interaction
dopamine release in amphibian retina by melatonin: the role of
with other neurotransmitters [19] and growth factors [9], GABA, Vis. Neurosci. 11 (1994) 1013–1018.
reduced availability of DA in dystrophic retinas might [7] I. Bodis-Wollner, Visual deficits related to dopamine deficiency in affect photoreceptor viability. Previously, a survival pro- experimental animals and Parkinson’s disease patients, Trends in
Neurosci. 13 (1990) 296–302.
moting effect of DA was demonstrated in a study where
[8] G.A. Bubenik, R.A. Purtill, The role of melatonin and dopamine in
administration of a D2 receptor agonist, bromocriptine,
retinal physiology, Can. J. Physiol. Pharmacol. 58 (1980) 1457–
protected photoreceptors against light damage in normal
1462.
rat retinas and degenerative photoreceptor cell death in [9] F. Bussolino, G. Pescarmona, G. Camussi, F. Gremo, Acetylcholine dystrophic RCS rat retinas [8]. However, similar protection and dopamine promote the production of platelet activating factor in immature cells of chick embryonic retina, J. Neurochem. 51 (1988)
was not observed in rds / rds retinas that were treated daily,
1755–1759.
between postnatal day 14 and 28, with D2 receptor
[10] T. Cheng, N.S. Peachey, S. Li, Y. Goto, Y. Cao, M.I. Naash, The
agonists (bromocriptine; quinpirole) or D1 receptor
agon-effect of peripherin / rds haploinsufficiency on rod and cone
photo-ists (SKF 38393; 6-Chloro-PB-hydrobromide). None of receptors, J. Neurosci. 17 (1997) 8118–8128.
these prevented the 30–40% photoreceptor cell death [11] A.I. Cohen, R.D. Todd, S. Harmon, K.L. O’Malley, Photoreceptors
observed in 1 month old rds / rds retinas [Nir, unpublished of mouse retinas possess D4 receptors coupled to adenylate cyclase, Proc. Natl. Acad. Sci. USA 89 (1992) 12093–12097.
findings].
[12] G. Connell, R. Bascom, R. Molday, D. Reid, R.R. McInnes, R.S.
Since human RP patients with rds mutations are mostly
Molday, Photoreceptor peripherin is the normal product of the gene
heterozygous, data obtained with the rds /1 mice might be responsible for retinal degeneration in the rds mouse, Proc. Natl. predictive of the levels of DA metabolism of RP patients Acad. Sci. USA 88 (1991) 723–726.
with abnormal photoreceptors. Indications for the direct [13] B. Dearry, Burnside, Light-induced dopamine release from teleost retinas acts as a light-adaptive signal to the retinal pigment
role of DA deficiency on visual functions in humans were
epithelium, J. Neurochem. 53 (1989) 870–878.
obtained from observations of Parkinson patients. Depleted
[14] M.B.A. Djamgoz, H.J. Wagner, Localization and function of
dopa-retinal DA stores as measured in post mortem eyes [36], mine in the adult vertebrate retina, Neurochem. Inl. 20 (1992) were linked to impaired retinal processing as determined 139–191.
by psychophysical and electrophysiological studies [7,20]. [15] J.E. Dowling, Retinal neuromodulation: The role of dopamine, Vis. Neurosci. 7 (1991) 87–97.
It can be expected, therefore, that in RP patients, in
[16] S. Gustincich, A. Feigenspan, D.K. Wu, L.J. Koopman, E. Raviola,
addition to direct loss of visual functions due to
photo-Control of dopamine release in the retina: a transgenic approach to
receptor pathology, further visual deficits might be caused neural networks, Neuron 18 (1997) 723–736.
by abnormal light-evoked retinal DA synthesis and utiliza- [17] M. Hadjiconstantinou, Z. Rossetti, C. Silvia, D. Krajnc, N.H. Neff,
tion. Aromatic L-amino acid decarboxylase activity of the rat retina is modulated in vivo by environmental light, J. Neurochem. 51 (1988) 1560–1564.
[18] R.K. Hawkins, H.G. Jansen, S. Sanyal, Development and degenera-Acknowledgements tion of retina in rds mutant mice: Photoreceptor abnormalities in the
heterozygotes, Exp. Eye Res. 41 (1985) 701–720.
[19] J.G. Hensler, D.J. Cotterell, M.L. Dubocovich, Pharmacological and
Supported by grants EY10286 (I.N.) and
RO1-biochemical characterization of the D-1 dopamine receptor
mediat-EY04864 (P.M.I.) from the National Institutes of Health.
ing acetylcholine release in rabbit retina, J. Pharmacol. Exp.
The authors thank Joseph Harrison for careful reading of Therapeu. 243 (1987) 857–867.
the manuscript and fruitful discussion. [20] H. Ikeda, G.M. Head, C.J.K. Ellis, Electrophysiological signs of retinal dopamine deficiency in recently diagnosed Parkinson’s disease and a follow up study, Vis. Res. 34 (1994) 2629–2638. [21] P.M. Iuvone, C.L. Galli, N.H. Neff, Retinal tyrosin hydroxylase: References Comparison of short-term and long term stimulation by light, Mol.
Pharmacol. 14 (1978) 1212–1219.
[23] H.G. Jansen, S. Sanyal, Development and degeneration of retina in [40] Nir, R. Haque, P.M. Iuvone, Diurnal metabolism of dopamine in the rds mutant mice: electron microscopy, J. Comp. Neurol. 224 (1984) mouse retina, Brain Res. 870 (2000) 118–125.
71–84. [41] M. Piccolino, J. Neyton, H.M. Gerschenfeld, Decrease of gap
[24] H.G. Jansen, S. Sanyal, Synaptic plasticity in the rod terminals after junction permeability induced by dopamine and cyclic adenosine 39: partial photoreceptor cell loss in heterozygous rds mutant mouse, J. 59-monophosphate in horizontal cells of turtle retina, J. Neurosci. 4 Com. Neurol. 316 (1992) 117–125. (1984) 2477–2488.
[25] R.J. Jensen, N.W. Daw, Effects of dopamine and its agonists and [42] M.E. Pierce, J.C. Besharse, Circadian regulation of retinomotor antagonists on the receptive field properties of ganglion cells in the movements. I. Interaction of melatonin and dopamine in the control rabbit retina, Neuroscience 17 (1986) 837–855. of cone length, J. Gen. Physiol. 86 (1985) 671–689.
[26] W. Kamp, W.W. Morgan, GABA antagonists enhance dopamine [43] J.H. Reuter, S. Sanyal, Development and degeneration of retina in turnover in the rat retina in vivo, Eur. J. Pharmacol. 69 (1981) rds mutant mice: The electroretinogram, Neurosci. Lett. 48 (1984)
273–279. 231–237.
[27] W. Kedzierski, J. Weng, G.H. Travis, Analysis of the rds / [44] S. Sanyal, H.G. Jansen, Absence of receptor outer segments in the peripherin–rom1 complex in transgenic photoreceptors that express retina of rds mutant mice, Neurosci. Lett. 21 (1981) 23–26. a chimeric protein, J Biol. Chem. 274 (1999) 29181–29187. [45] S. Sanyal, R.K. Hawkins, Development and degeneration of retina in [28] T.J. Keen, C.F. Inglehearn, Mutations and polymorphisms in the rds mutant mice: altered disc shedding pattern in the albino human peripherin-RDS gene and their involvement in inherited heterozygotes and its relation to light exposure, Vis. Res. 28 (1988) retinal degeneration, Human Mutation 8 (1996) 297–303. 1171–1178.
[29] A.G. Knapp, J.E. Dowling, Dopamine enhances excitatory amino [46] M. Schorderet, J.Z. Nowak, Retinal dopamine D1 and D2 receptors: acid-gated conductances in cultured retinal horizontal cells, Nature Characterization by binding or pharmacological studies and physio-325 (1987) 437–439. logical functions, Cell. Mol. Neurobiol. 10 (1990) 303–325. [30] D. Krizaj, R. Gabriel, W.G. Owen, P. Witkovsky, Dopamine D2 [47] B.S. Shastry, Signal transduction in the retina and inherited
re-receptor-mediated modulation of rod–cone coupling in the Xenopus tinopathies, Cell Mol. Life Sci. 53 (1997) 419–429.
retina, J. Comp. Neurol. 398 (1998) 529–538. [48] L.M. Shulman, D.A. Fox, Dopamine inhibits mammalian photo-[31] O.H. Lowry, N.J. Rosebrough, A.L. Farr, R.J. Randall, Protein receptor Na1,K1-ATPase activity via a selective effect on thea3
measurement with the folinphenol reagent, J. Biol. Chem. 193 isozyme, Proc. Natl. Acad. Sci. USA 93 (1996) 8034–8039. (1951) 265–275. [49] V.T. Tran, M. Dickman, Differential localization of dopamine D1 [32] G. Maguire, F. Werblin, Dopamine enhances a glutamate-gated ionic and D2 receptors in rat retina, Invest. Ophthalmol. Vis. Sci. 33
current in OFF bipolar cells of the tiger salamander retina, J. (1992) 1620–1626.
Neurosci. 14 (1994) 6094–6101. [50] G.H. Travis, J.G. Sutcliffe, D. Bok, The retinal degeneration slow [33] P.B. Marshburn, P.M. Iuvone, The role of GABA in the regulation of (rds) gene product is a photoreceptor disc membrane-associated
the dopamine / tyrosine hydroxylase-containing neurons of the rat glycoprotein, Neuron 6 (1991) 61–70.
retina, Brain Res. 214 (1981) 335–347. [51] G.H. Travis, K.R. Groshan, M. Lloyd, D. Bok, Complete rescue of [34] S.M. Molday, Photoreceptor membrane proteins, phototransduction, photoreceptor dysplasia and degeneration in transgenic retinal
and retinal degenerative diseases, Invest. Ophthalmol. Vis. Sci. 39 degeneration slow (rds) mice, Neuron 9 (1992) 113–119. (1998) 2493–2513. [52] G.H. Travis, Mechanism of cell death in the inherited retinal [35] J. Nguyen-Legros, B. Berger, A. Vigny, C. Alvarez, Tyrosine degenerations, Am. J. Hum. Genet. 62 (1998) 503–508.
hydroxylase-like immunoreactive interplexiform cells in the rat [53] J. Usukura, D. Bok, Changes in the localization and content of opsin retina, Neurosci. Lett. 27 (1981) 255–259. during retinal development in the rds mutant mouse: immuno-[36] J. Nguyen-Legros, C. Harnois, T. Di Paolo, A. Simon, The retinal cytochemistry and immunoassay, Exp. Eye Res. 45 (1987) 501–515. dopamine system in Parkinson’s disease, Clin. Vis. Sci. 8 (1993) [54] C. Versaux-Botteri, E. Martin-Martinelli, J. Nguyen-Legros, M.
1–12. Geffard, A. Vigny, L. Denoroy, Regional specialization of the rat
[37] Nir, D.S. Papemaster, Electron microscopical localization of opsin in retina: catecholamine-containing amacrine cell characterization and the inner segment plasma membrane of photoreceptors in retinas of distribution, J. Comp. Neurol. 243 (1986) 422–433.
rds mutant mice, Invest. Ophthalmol. Vis. Sci. 27 (1986) 836–840. [55] M. von Schantz, I. Provencio, R.G. Foster, Recent developments in [38] Nir, N. Agarwal, D.S. Papermaster, Opsin gene expression during circadian photoreception: more than meets the eye, Invest.
Ophthal-early and late phases of retinal degeneration in rds mice, Exp. Eye mol. Vis. Sci. 41 (2000) 1605–1607.
Res. 51 (1990) 257–267. [56] P. Witkovsky, A. Dearry, Functional role of dopamine in the [39] Nir, P.M. Iuvone, Alterations in light-evoked dopamine metabolism vertebrate retina, Prog. Retinal Res. 11 (1992) 247–292.