www.elsevier.com / locate / bres
Research report
Efficient testing of motor function in spinal cord injured rats
a ,
*
a b a aGerlinde A.S. Metz
, Doron Merkler , Volker Dietz , Martin E. Schwab , Karim Fouad
a
Brain Research Institute, University and ETH Zurich, 8057 Zurich, Switzerland b
Swiss Paraplegic Center, University Hospital Balgrist, University of Zurich, 8008 Zurich, Switzerland Accepted 2 August 2000
Abstract
In experimental spinal cord injury studies, animal models are widely used to examine anatomical and functional changes after different treatments and lesion types. A variety of behavioral paradigms exists in the literature, but definitions and criteria for motor performance vary considerably. In this study, we examined the outcome and relation of tests such as the BBB open field locomotion score, footprint analysis, kinematic analysis, placing response, grid walk and narrow beam crossing following two different lesion types. The information obtained was used to design an efficient and reliable testing strategy, which includes a broad spectrum of parameters to enhance sensitivity. This approach should help to standardize modular testing procedures across different laboratories working on spinal cord injury. 2000 Elsevier Science B.V. All rights reserved.
Theme: Motor systems and sensorimotor integration
Topic: Control of posture and movement
Keywords: Testing strategy; Combined motor score; Motor deficit
1. Introduction of the earlier 5-point Tarlov scale [46,47]. Another method is the footprint analysis, which quantifies gait coordination In spinal cord injury research, the rat has become the and placement of the feet [16,32]. Kinematic analysis of favorite animal model for testing various treatment strate- video recordings during walking allows the qualitative and gies. Several lesion techniques in rodents have been quantitative evaluation of joint and limb movements established to model human spinal cord injuries. A popular [12,34,35,50]. All these tests focus mainly on stereotyped method is the contusion of the spinal cord, which mimics movements controlled by networks and reflex pathways the situation in humans relatively closely [15,21,54]. within the spinal cord (i.e., the central pattern generator; However, with this approach it is not possible to fully CPG) [24,43]. The CPG network is driven by excitatory ablate defined spinal tracts, which can be achieved by input mainly from the ventrally located reticulospinal tract sectioning the spinal cord at specific locations [33,44,49]. [30]. Tests that reflect the integrity of the dorsal spinal To quantify the residual function in the different animal motor tracts (cortico- or rubrospinal tract), should focus on models, a variety of motor and sensory tests has been voluntary aspects of limb movements or balance [52]. One developed. Most paradigms assessing the integrity of possible paradigm is the grid walk test in which animals spinal pathways focus on hindlimb function such as have to traverse a horizontal grid elevated above the locomotor capacity and reflexes. A frequently used test to ground [23,32,45,55]. Testing the performance during assess the severity of spinal cord injury is the 21-point crossing of elevated narrow beams is another possibility to open field locomotion score (BBB scale) [4], a refinement evaluate descending motor control as well as body balance of spinal cord injured animals [29]. A reflex which is thought to be an indicator of corticospinal tract integrity is *Corresponding author. Present address: Department of Psychology the contact placing response [7,8,17].
and Neuroscience, University of Lethbridge, 4401 University Drive,
This study investigated the functional outcome of two Lethbridge, Alberta, Canada T1K 3M4. Tel.: 11-403-329-2366; fax:
widely used spinal cord injury models in adult rats, dorsal 11-403-329-2555.
E-mail address: [email protected] (G.A.S. Metz). hemisection and contusion injuries of varying severity. By 0006-8993 / 00 / $ – see front matter 2000 Elsevier Science B.V. All rights reserved.
using tests like the BBB score, footprint analysis, fixative (4% paraformaldehyde in 0.1 M phosphate buffer kinematic gait analysis, grid walk, narrow beam, open field with 5% sucrose). The spinal cords were removed and exploration and placing response we covered a broad postfixed overnight. After immersion for 2–3 days in a spectrum of behavioral and physiological parameters. The 30% sucrose solution, the spinal cords were embedded in a outcome of these paradigms was correlated in order to protein matrix [28]. The spinal cords were frozen in define their relationship and the validity of the individual isopentane at2408C and 50-mm sections were cut in the tests. Based on these results we developed a testing sagittal plane. The sequential series of sections were strategy for functional outcome, optimized for efficiency, mounted on slides.
reliability and comparability between different lesion types To visualize axons, a monoclonal antibody against the
and severities. neurofilament 200 kDa subunit (Boehringer Mannheim,
Germany) was used. Slides were treated with ethanol (95%)–acetic acid (5%) for 25 min at 48C, rehydrated in 2. Materials and methods 90% and 70% alcohol (5 min each) and incubated for 1 h at room temperature with the neurofilament antibody
2.1. Animals (1:200 in 0.1 M PBS). To localize the primary antibody,
slides were incubated for 45 min at room temperature with Two hundred and one adult rats of either sex (Lewis, a biotinylated secondary antibody (anti-mouse, rat ab-200–250 g at the beginning of experiment) were used. One sorbed, diluted 1:100) followed by the avidin–biotin– hundred and thirty-five rats received a mid-thoracic (T8) peroxidase complex (Vector, Burlingham, UK). The per-bilateral dorsal hemisection and 66 a mid-thoracic contu- oxidase activity was detected with diaminobenzidine and sion injury. They were housed in groups under a 12:12-h H O (Sigma, St. Louis, MO). For background control, the2 2 light / dark cycle with water and food ad libitum. All secondary antibody was applied in the absence of primary experiments were approved by the veterinary department antibodies.
of the canton Zurich.
2.4. Behavioral procedures 2.2. Surgery
A varying number of animals per test was analyzed For both injury types, the animals were anesthetized since data were collected from control animals in several with a combination of Fentanyl and Fluanisone (0.3 mg / kg experiments. All tests were performed before spinal cord i.p., Hypnorm, Janssen, Belgium) and Midazolam (0.6 injury and at a medium (5 weeks) or a long range (3 mg / kg i.p., Dormicum, Roche, Switzerland). Body tem- months) after injury. After this period stable neuro-perature was maintained at 378C during surgery using a pathological conditions can be expected [27].
heating pad. After shaving the skin an incision in the Except for the placing response, the results of single mid-thoracic region was made and a T8 laminectomy motor tests were also analyzed in a scoring system. For the
performed. paradigms of kinematic analysis, grid walk, narrow beam,
For dorsal hemisection, the dura mater was opened and a footprint analysis and exploration we established scores to bilateral dorsal lesion was made with iridectomy scissors, allow comparison of the single parameters.
thus completely transecting the rubrospinal tract and the
main dorsal corticospinal tract component. 2.4.1. BBB open field locomotion score
The contusion injury was performed by using a stan- This open field walking score measures recovery of dardized weight-drop injury device (NYU impactor) hindlimb movements in rats during free open field locomo-[15,26]. The height of the weight drop impact was varied tion as described by Basso et al. [4]. A score of 0 was
2 2
from 6.25 g / cm to 25 g / cm . After the lesion, the given if there was no spontaneous movement, a score of 21 muscles were sutured and the skin was closed with surgical indicated normal locomotion. Plantar stepping with full clamps. Postoperative care included analgesia application weight support and complete forelimb–hindlimb coordina-(Buprenorphin, 0.03 mg / kg, s.c., Temgesic, Reckitt & tion is reached when an animal shows a score of 14 points. Colman, UK), regular bladder expression and antibiotic We used a modified version of the BBB score if the treatment (Trimethoprim, 0.83 ml / kg, i.p., Bactrim, sequence of recovering motor features was not the same as Roche, Switzerland) if infections occurred. described in the original score. If this was observed, points for the single features were added independently. For
2.3. Histology example, a rat showing incomplete toe clearance, enhanced
floor. In postoperative sessions two persons observed each rotation of the feet of more than double values as com-animal for a period of 4 min. pared to its own baseline values; 3 points were recorded if the animal showed no signs of toe dragging but foot 2.4.2. Kinematic analysis rotation; 4 points were rated if the animal showed no signs Kinematic analysis was performed for one hindlimb in of exo- or endo-rotation (less than twice the angle of the animals, which were able to perform plantar stepping on a baseline values).
treadmill after dorsal hemisection. After shaving the limb,
the iliac crest (cresta iliaca), hip (greater trochanter), ankle 2.4.4. Grid walk
(lateral malleolus) and the fifth metatarsophalangeal Deficits in descending motor control were examined by (MTP) joints were marked with ink. Because the position assessing the ability to navigate across a 1 m long runway of the knee joint is obscured by loose skin coverage, it was with irregularly assigned gaps (0.5–5 cm) between round calculated by using hip and ankle joint positions and metal bars [55]. The bar distances were randomly changed external individual measurements of femur and tibia from one testing session to the next.
lengths. Crossing this runway requires that animals accurately
Preoperatively, the animals were trained to walk on a place their limbs on the bars. In baseline training and treadmill (speed 175 mm / s) and recorded using a digital postoperative testing, every animal had to cross the grid for video camera. The limb movements of five step cycles at least three times. The number of footfalls (errors) was were analyzed frame by frame (50 frames / s) and averaged. counted in each crossing and a mean error rate was The marker coordinates were used to calculate the knee calculated. If an animal was not able to move the hin-position and to quantify flexion / extension of the limbs. dlimbs, a maximum of 20 errors was given. The numbers Parameters used were height of the MTP joint, height of of errors counted were also rated in a non-parametric grid the ankle joint and flexion / extension in the ankle during walk score: 0–1 error was rated as 3 points, 2–5 as 2 mid swing. For further analysis, data from the different points, 6–9 as 1 point and 10–20 footfalls as 0 points. phases of the step cycle were obtained: Joint angles were
measured at the initiation of the swing phase (F-phase), in
2.4.5. Narrow beam the middle swing phase (E1-phase), and in the phase in
The narrow beam test was performed according to the which the paw initially contacts the ground (E2-phase).
descriptions of Hicks and D’Amato [29]. Three types of Also, the middle stance phase (E3-phase) was analyzed,
beams were used as narrow pathways: a rectangular 2.3-cm which provides most information about the weight support.
wide beam, a rectangular 1.2-cm wide beam and a round In order to reduce inter-individual differences, a percentual
dowel with 2.5 cm diameter. All beams were 1 m long and ratio was calculated from preoperative values (100%).
elevated 30 cm from the ground. After training, normal rats were able to traverse the horizontal beams with less 2.4.3. Footprint analysis
than three footfalls. When occasionally their feet slipped Footprint analysis was modified from De Medinaceli et
off the beam, they were retrieved and repositioned precise-al. [16]. The animal’s hind paws were inked and footprints
ly. were made on paper covering a narrow runway of 1 m
A scoring system was used to assess the ability of the length and 7 cm width. This ensured that the direction of
animals to traverse the beams: 0 was counted as complete each step was standardized in line. A series of at least eight
inability to walk on the beam (the animals fell down sequential steps was used to determine the mean values for
immediately), 0.5 was scored if the animal was able to each measurement of limb rotation, stride length and base
traverse half of the beam, 1 point was given for traversing of support. The base of support was determined by
the whole length, 1.5 points when stepping with the measuring the core to core distance of the central pads of
hindlimbs was partially possible, and 2 points were noted the hind paws. The limb rotation was defined by the angle
for normal weight support and accurate foot placement. If formed by the intersection of the line through the print of
the scores of all three beams are added, a maximum of 6 the third digit and the print representing the
metatar-points can be reached. sophalangeal joint and the line through the central pad
parallel to the walking direction. Stride length was
mea-sured between the central pads of two consecutive prints 2.4.6. Exploration
on each side. To assess their exploratory behavior, rats were tested in
points and more than 200% of baseline values were rated lesions showed nearly complete disruption of dorsal,
as 5 points. lateral and ventral tracts in addition to loss of the gray
matter. The loss of gray matter was always more severe 2.4.7. Contact placing response than the loss of white matter.
Contact placing is elicited by lightly touching the skin of
the dorsal side of the foot without joint displacement 3.2. Behavioral analysis [32,42]. The animal responds by lifting the hind leg and
placing it upon the obstacle. 3.2.1. BBB open field locomotion score
The animals were held, supported by the upper body, Over-ground locomotion was rated in 137 rats (71 with the hindlimbs hanging free. The dorsum of each foot animals with dorsal hemisection, 66 with contusion) using was touched with the edge of a piece of paper. The total the BBB scoring system [4]. Weekly testing began 7 days number of placing responses of ten trials per limb was postoperatively (at the end of spinal shock) and was noted and the placing rate for individual animals was continued 5 weeks to 3 months after dorsal hemisection or determined from baseline data taken as 100%. contusion lesion, respectively. We modified the BBB score by adding earlier occurring features of the score separately 2.5. Statistical analysis (see Section 2). The results varied from 6 (no weight support) to 21 points (normal gait). Seventeen rats with Statistical analysis was performed using the Statview dorsal hemisection and 11 rats with contusion showed only software package (Abacus Concepts, CA, 1996). The data partial body weight support, absence of toe clearance and are presented in bivariate plots displaying the relationship rotated foot positions during stepping; these animals were between two variables. Regression lines and Pearson’s assigned to the group of low locomotor ability (maximal correlation coefficients were calculated. Spearman’s rank BBB score of 12.5 points in both limbs). One hundred and correlation coefficients were computed for paired com- nine animals reached a high locomotor ability. They parisons using ordinal data. For other data, Fisher’s R to Z showed consistent weight support and consistent forelimb– transformation and a ‘Z-test’ were applied to calculate the hindlimb coordination, but often still incomplete toe significance of the correlation coefficients. A P value of clearance.
less than 0.05 was chosen as the significance level for all Twelve percent of the animals with a score of 14 or 15 statistical analyses. In the present study, the variable points showed frequent to consistent toe clearance; this number of animals influences the degree of significance. function was considered to occur only at higher stages in All data are presented as the mean6standard error the original BBB scale [4]. We gave an additional point for
(S.E.M.). the clearance. Another 24% of the animals reached a score
of 17–18 points and showed a ‘tail-up’ position, again a function that was rated only at higher score in the BBB 3. Results scale. An additional point was added to include the
recovered tail position in these animals.
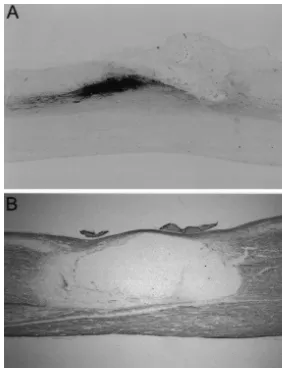
3.1. Lesion size Typical time courses of recovery of open field
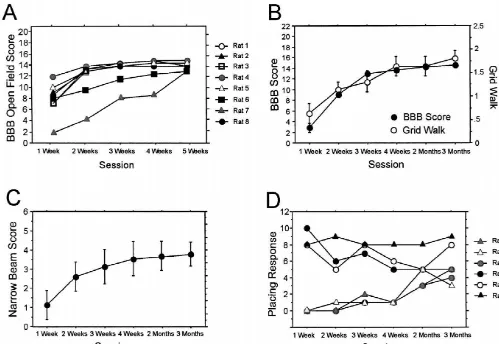
locomo-tion over 3 months are shown in Fig. 2A for eight 3.1.1. Dorsal hemisection individual animals with dorsal hemisection lesions. Ani-The histological analysis of the lesion site 5 weeks or 3 mals with initially low locomotor performance showed months after injury revealed that in all animals the dorsal greater relative recovery than animals that started with funiculi of both sides were transected, as well as both weight support already at 1 week postoperatively. All these dorsal horns of the gray matter. The depth of the lesion rats reached a maximum BBB score of 13–14 points, often reached the ventral funiculus. Secondary tissue which reflects the ability of plantar stepping with partial or damage massively enlarged the originally well localized full weight support. A time course for a group of ten scissors cut (Fig. 1A). animals with contusion lesions is shown in Fig. 2B. Also, here most animals reached a plateau at 14 points by 3–5
3.1.2. Contusion weeks. This plateau value was typically seen in rats with
The lesion site was characterized by a central cavity moderate lesion severity [36]. The initially lower BBB surrounded by a rim of spared white matter (Fig. 1B). The values of rats with contusion lesion as compared to dorsal cyst walls were of variable thickness, surrounded by tissue hemisection lesions probably reflect the greater damage of containing activated microglia and macrophages. Trabecu- the ventral tracts.
lar structures, often with infiltrated cells, were present at
the lesion site in many animals. In a few rats, the cyst was 3.2.2. Kinematic analysis
Fig. 1. Extents of midthoracic lesions. (A) Photograph illustrating a typical hemisection lesion on a sagittal view of the spinal cord. The dorsal, main components of the corticospinal tract and the laterally situated rubrospinal tract were disrupted in all animals. In most animals, also the grey matter was completely transected. (B) Photograph of a sagittal section through the spinal cord with a mid-grade contusion lesion; immunostaining for neurofilament. Remaining dorsal and ventral tissue bridges surround a central cavity. Magnification:340; Scale bar50.7 mm.
analyzed. All showed larger angles of the hip, knee and angle of foot exo-rotation, base of support and stride ankle joint movements as compared to baseline values. length. The preoperative analysis revealed a mean angle of Values of the F-phase (transition from stance to swing) rotation of the hindlimbs of 11.361.98. Postoperatively, were 48.366% larger than in the baseline, demonstrating this parameter changed to a mean of 23.261.58 after that the initiation of the swing phase was delayed and the hemisection and 22.161.18 after contusion injury. The joints were more extended at swing initiation in the base of support, measured as the distance between the left lesioned animals. In the E1 (middle swing) phase, a mean and right leg central pads, showed a baseline value of increase to 12666.6% was found. These observations 2.260.1 cm. The base of support increased to 3.860.2 cm strengthen the finding of the BBB score that most of the after hemisection and to 3.760.2 cm after the contusion. animals showed toe dragging and incomplete weight The third parameter measured was stride length, which support. No significant changes were found for the E2 showed a mean of 11.960.3 cm in normal animals and (initial ground contact) and the E3 phase (middle stance). 12.260.7 cm after a dorsal hemisection and 1260.8 cm
following the contusion injury.
Fig. 2. Time course of motor recovery in different behavioral tests. (A) Time course of eight animals after midthoracic dorsal hemisection lesion in the BBB score starting from 1 week up to 5 weeks postoperatively. At the end of the testing period, all animals have reached a stable outcome with a mean of 14 points. (B) Time course of a group of ten animals with contusion injury showing BBB scores, grid walk scores and (C) narrow beam scores over a postoperative interval of 3 months. BBB scores improved mostly within the first 3 weeks after injury. The score on the grid and narrow beam scores increased steadily, reflecting motor recovery and training effects throughout the observation period. (D) Number of placing responses of six animals with contusion injury from 1 week up to 3 months postoperatively. The time course of this task illustrates the variability of measurements in single animals. Mean values6S.E.M.
score of 1.760.3 points. Nine animals had a score of 0 The animals’ performance on the grid was also rated in points, only one animal reached a score of 4 points. a scoring system ranging from 0 (worst) to 3 points. Normal animals showed a score of 3. Five weeks to 3
3.2.4. Grid walk months postinjury animals showed a reduction of scoring
The ability of 137 animals (71 with dorsal hemisection, points to a mean of 1.960.1 points (1.760.1 in rats with 66 with contusion injury) to cross a grid with randomly dorsal hemisection, 2.160.1 in rats with contusion injury). spaced bars was assessed by counting the number of errors Eight animals stayed at a score of 0 points (8% of rats with
(missed bars). dorsal hemisection, 3% of rats with contusion lesion), and
In preoperative baseline measurements, the animals 27 animals reached the maximum score of 3 (17% of showed a mean error rate of 0.2760.05. Five weeks or 3 animals with dorsal hemisection, 23% of animals with months after surgery, 12 animals were able to cross the contusion).
grid with a mean of less than one error per crossing (six
rats with dorsal hemisection and six with contusion). Five 3.2.5. Narrow beam score
animals, all of them with dorsal hemisection, showed a The ability of the animals to cross three different types maximum error value of 20. The improvement in 19 of beams was scored. Preoperatively, the animals achieved contusion injury animals over the subsequent 3 months is a score of 2 points per beam, giving a total of 6 points. illustrated in Fig. 2B, showing that the most substantial Sixty-two animals were evaluated in their narrow beam improvement was found in the first three weeks after performance at postoperative week 5 or at 3 months (57
lesion). Seven animals were not able to cross at least one overview of all correlations for the two lesion paradigms of the three beams (0 points), and three animals were able hemisection and contusion is given in Table 1.
to cross all three beams with full weight support (6 points). The results of these correlations showed that the per-Often animals were able to cross the beam by sliding over formance in most of the motor tests is correlated to the without using the hindlimbs. The mean narrow beam BBB score. Two examples are illustrated in Fig. 3. performance of lesioned animals was 2.260.3 points Animals with low locomotor ability (BBB score,13 (2.360.3 after dorsal hemisection, and 2.160.3 after points) differ greatly in grid walk and footprints from
contusion lesion). animals with high locomotor ability (BBB score $13
A postoperative time course over 3 months after contu- points; Fig. 3A,B). Animals with less than 13 points show sion is shown in Fig. 2C, illustrating that the main no or only partial weight support, a feature necessary for recovery took place in the first 3 weeks after the surgery. performance in tasks requiring body balance. Therefore, This observation is similar to that made for the open field we subdivided the animals into two groups: low locomotor
locomotion scale. capacity with no or partial weight support (BBB score
from 1 to 13 points) and high locomotor capacity and full weight support (13–21 points). This procedure allowed a 3.2.6. Exploratory activity in the open field
more specific subsequent quantification. Exploration in an open field was assessed in 28 rats
Table 2 summarizes the correlation coefficients for preoperatively and 5 weeks after dorsal hemisection.
animals with high and low locomotor outcome. Some of Before the operation, the mean number of crossed fields
these correlations are described in more detail in the was 27.663.6 in 5 min (range: 8–52 fields). Five weeks
following. after spinal cord lesion, the animals showed a similar
exploration rate (25.364 fields; range: 2–59 fields). How-ever, in an analysis for each animal compared to its
3.3.1. BBB score individual baseline activities, 56% of the animals showed
As shown in Table 1, the BBB score correlated closely reduced exploration, whereas the activity of the remaining
to parameters of the kinematic analysis in animals with rats was increased to 126.5629.8%. From these data a
dorsal hemisection. The best correlation coefficient was 5-point activity score was calculated and the mean score
found for the E2 and E3 phase, which provide valuable was 2.4 points.
information about weight support and limb movements of individual animals.
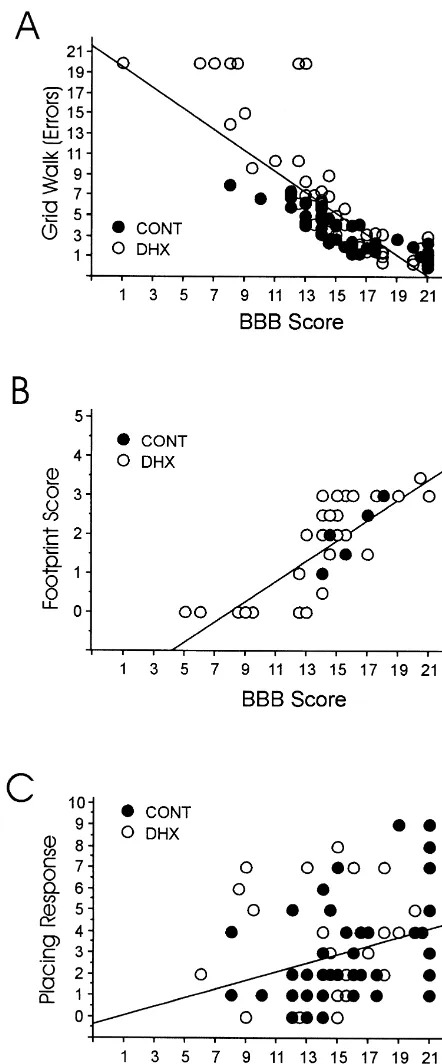
3.2.7. Placing response A positive relationship was also found between the BBB The contact placing response was assessed in 16 animals score and quantified data from footprint analysis and the with dorsal hemisection and 59 rats with contusion lesion. footprint score. Of these parameters, the footprint score As compared to their baseline values (mean number of and the base of support showed the highest correlation to responses: 6.560.5 per 10 trials), the animals showed a the BBB score in both lesion types. The correlation for postoperative reduction in their placing response to a mean animals with a low BBB score (,13 points) to the of 360.3 per hindlimb (range: 0 to 9; 2.760.5 in rats with footprint score was weaker than for animals with a high dorsal hemisection, 3.260.3 in rats with contusion). In BBB score ($13 points; see Table 2). This is illustrated in nine animals, no placing reflex was elicitable after lesion Fig. 3B.
(31% of dorsal hemisection, 7% of contusion animals). In the grid walk, the BBB score was highly correlated to Overall, the number of placing responses 5 weeks to 3 the number of errors and to the error score. A similar months after spinal cord injury was reduced to a mean of correlation was found for both lesion types (Table 1), but 57611.7% of baseline values (51.4630.6% after dorsal animals with low and high locomotor capacity showed hemisection, 72.869% after contusion injury). Interesting- different results: in rats with low locomotor ability, the ly, three animals showed an enhanced placing response, correlation was weaker than for animals with high indicating a facilitated reflex. locomotor ability (Table 2 and Fig. 3A).
A time course of the recovery of the placing responses The BBB score showed a positive correlation to the over 3 months in six animals with contusion injury is performance on the narrow beam, which was higher for shown in Fig. 2D. This graph shows large inter-animal hemisected animals than for those with a contusion lesion, differences with late improvements in three rats and a and also higher for animals with high locomotor outcome decreased performance over time in two animals. than for animals with low motor ability (Table 2). In a
comparison to the open field exploratory activity, the
3.3. Correlations correlation was closer for animals with low locomotor
Table 1
a Summary of correlations of different behavioral tests in rats
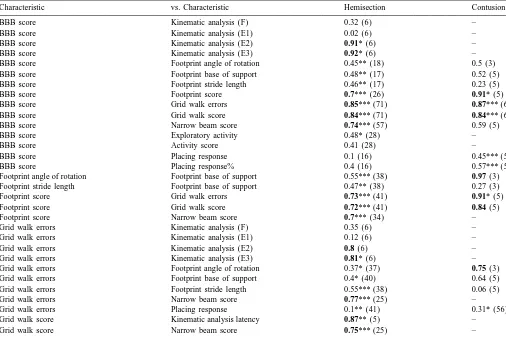
Characteristic vs. Characteristic Hemisection Contusion
BBB score Kinematic analysis (F) 0.32 (6) –
BBB score Kinematic analysis (E1) 0.02 (6) –
BBB score Kinematic analysis (E2) 0.91* (6) –
BBB score Kinematic analysis (E3) 0.92* (6) –
BBB score Footprint angle of rotation 0.45** (18) 0.5 (3)
BBB score Footprint base of support 0.48** (17) 0.52 (5)
BBB score Footprint stride length 0.46** (17) 0.23 (5)
BBB score Footprint score 0.7*** (26) 0.91* (5)
BBB score Grid walk errors 0.85*** (71) 0.87*** (66)
BBB score Grid walk score 0.84*** (71) 0.84*** (66)
BBB score Narrow beam score 0.74*** (57) 0.59 (5)
BBB score Exploratory activity 0.48* (28) –
BBB score Activity score 0.41 (28) –
BBB score Placing response 0.1 (16) 0.45*** (59)
BBB score Placing response% 0.4 (16) 0.57*** (59)
Footprint angle of rotation Footprint base of support 0.55*** (38) 0.97 (3)
Footprint stride length Footprint base of support 0.47** (38) 0.27 (3)
Footprint score Grid walk errors 0.73*** (41) 0.91* (5)
Footprint score Grid walk score 0.72*** (41) 0.84 (5)
Footprint score Narrow beam score 0.7*** (34) –
Grid walk errors Kinematic analysis (F) 0.35 (6) –
Grid walk errors Kinematic analysis (E1) 0.12 (6) –
Grid walk errors Kinematic analysis (E2) 0.8 (6) –
Grid walk errors Kinematic analysis (E3) 0.81* (6) –
Grid walk errors Footprint angle of rotation 0.37* (37) 0.75 (3)
Grid walk errors Footprint base of support 0.4* (40) 0.64 (5)
Grid walk errors Footprint stride length 0.55*** (38) 0.06 (5)
Grid walk errors Narrow beam score 0.77*** (25) –
Grid walk errors Placing response 0.1** (41) 0.31* (56)
Grid walk score Kinematic analysis latency 0.87** (5) –
Grid walk score Narrow beam score 0.75*** (25) –
Grid walk score Placing response 0.19** (41) 0.19 (56)
a
The table shows correlation coefficients (r-value) and significance levels, and the correlation coefficients are noted with asterisks: *P#0.05; **P#0.01; ***P#0.001. The number of animals per correlation is given in parentheses. The best correlations, indicated by the correlation coefficient, are marked in boldface.
individual animals with the same BBB score also differed 4. Discussion in a broad range in the placing response.
The aim of the present study was to compare outcome and relationships of different locomotor tests in order to 3.3.2. Grid walk performance establish an efficient testing procedure for spinal cord The number of errors made while crossing the grid as injured rats. We collected data from experiments in adult well as the grid walk score were highly correlated with the rats after dorsal hemisection or contusion lesion using parameters obtained by kinematic analysis, especially with several motor tasks and scaling systems. The results of the the joint angles in the E2 and E3-phase (Table 1). different motor tests were correlated to evaluate the extent Correlations to the parameters obtained by footprint analy- to which the parameters relate to each other. The per-sis were weaker and revealed a heterogeneous result for formance of animals with high locomotor performance the different lesion types: a close relationship was found to showed a close relationship between tests requiring sup-angles of foot rotation and base of support in contusion raspinal motor control such as grid walk or narrow beam. animals, but not in animals with dorsal hemisection. On In contrast, animals with low locomotor capacity showed a the other hand, the correlation to stride length was better in closer relationship between the BBB score and activity in animals with a hemisection lesion. In comparison to other the open field.
motor tasks, a close relationship to the narrow beam score
was found in animals with dorsal hemisection (data for 4.1. Methodological considerations contusion lesion injury were not obtained) whereas the
relationship to placing responses was very weak for both 4.1.1. Open field locomotion
locomo-tion is the use of rating scales, based on observalocomo-tions of defined leg movements. This provides information on the activation of spinal networks that are able to produce a coordinated stepping pattern [24,25,43]. These pattern generating networks are activated by the ventrally located reticulospinal tract [11,30]. Therefore, lesions restricted to dorsal tracts of rat spinal cords, such as the corticospinal tract, result in almost complete recovery of hindlimb function [35,38].
In order to allow inter-laboratory comparisons of results, various rating systems were developed. One of the most commonly used scales was the Tarlov score [46,47], which ranks hindlimb movements and weight support in five categories. However, this method has been described to be more sensitive when the animal is capable of hindlimb weight support and is less reliable when used to score hindlimb movements without weight support [10]. This scale was improved by increasing the number of categories for all hindlimb motor features in the BBB score [4]. The BBB score is now widely used and has been shown to provide reproducible results [5]. One advantage of this scale is that preoperative training of the animals is not necessary. It was originally designed for contusion injuries and is thought to be less sensitive to clip compression injuries [48]. However, for spinal hemisection we found that the BBB score is sensitive as well. Potential limita-tions of the BBB score are due to the subjective scoring system, e.g. for determination of forelimb–hindlimb coordination [10].
An important drawback is the fact that the ordinal BBB rating system is not linear: the lower part of the scale concerns gross aspects of locomotion, while the upper part of the scale includes rather discrete movement aspects that do not represent major improvements for the animal’s motor ability. Motor training usually performed by the animals in the cages and during the tests may affect motor improvement especially in the lower part of the scale. This may be one of the reasons why the BBB score correlates closely to exploratory activity for animals with low motor ability. In the upper part of the scale ($13 points), the sequence of recovery is often not related to the scaling hierarchy. If animals reach this level of performance, we suggest the addition of points for single features like toe clearance and tail position independently. Also, the motor recovery after any different lesion type and treatment strategies may require a new order of the scaling hierarchy. Fig. 3. Correlations of behavioral parameters. (A) Scattergram of a Especially in animals with a high locomotor outcome correlation between the BBB score and the number of errors produced on (BBB score.13) and a stable degree of performance, the a grid in animals with hemisection (DHX) or contusion injury (CONT).
difference between single animals is very subtle. There-(B) Correlation between the BBB score and the footprint score. (C)
fore, for detailed inter-individual differences, additional Scattergram showing the correlation between BBB score and the number
of placing responses in animals with dorsal hemisection and contusion appropriate motor tests have to be used. lesion. The correlation reveals a positive relationship between both
paradigms, although the distribution of single values was very inhomoge- 4.1.2. Footprint analysis neous. For both lesion types, the distribution was similar. All these graphs
Different methods were previously used to measure the illustrate that the inter-animal variation was larger in animals with low
placement of the hind paws [16,50]. In addition to the BBB scores (,13 points) than in animals with high BBB scores ($13
analy-Table 2
a Summary of the two animal groups, subdivided into low and high locomotor outcome based on the BBB score
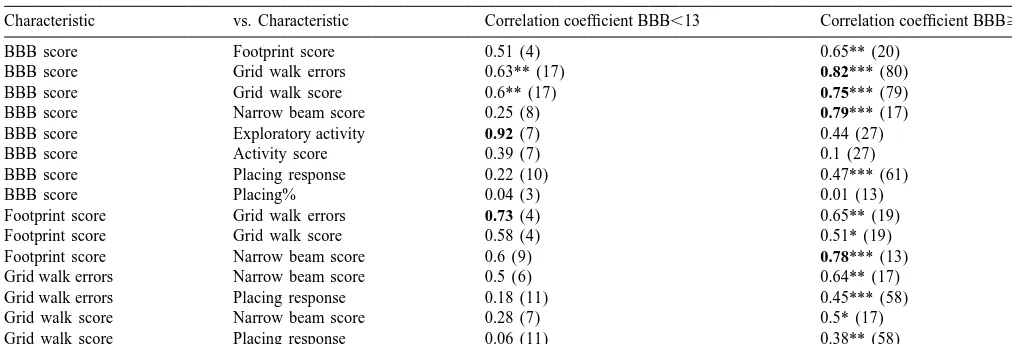
Characteristic vs. Characteristic Correlation coefficient BBB,13 Correlation coefficient BBB$13
BBB score Footprint score 0.51 (4) 0.65** (20)
BBB score Grid walk errors 0.63** (17) 0.82*** (80)
BBB score Grid walk score 0.6** (17) 0.75*** (79)
BBB score Narrow beam score 0.25 (8) 0.79*** (17)
BBB score Exploratory activity 0.92 (7) 0.44 (27)
BBB score Activity score 0.39 (7) 0.1 (27)
BBB score Placing response 0.22 (10) 0.47*** (61)
BBB score Placing% 0.04 (3) 0.01 (13)
Footprint score Grid walk errors 0.73 (4) 0.65** (19)
Footprint score Grid walk score 0.58 (4) 0.51* (19)
Footprint score Narrow beam score 0.6 (9) 0.78*** (13)
Grid walk errors Narrow beam score 0.5 (6) 0.64** (17)
Grid walk errors Placing response 0.18 (11) 0.45*** (58)
Grid walk score Narrow beam score 0.28 (7) 0.5* (17)
Grid walk score Placing response 0.06 (11) 0.38** (58)
a
The correlation coefficients (r-value) are listed and the number of animals per correlation is listed in brackets. Significant levels are given with asterisks: *P#0.05; **P#0.01; ***P#0.001. The best correlations, as indicated by the correlation coefficient, are marked in boldface.
sis gives precise measurements for the foot rotation, which [31,32,45,55]. This task requires forelimb–hindlimb is an indicator of walking stability and body balance. We coordination, which is mediated by ventrolateral tracts used a simple method with paper stripes to record foot- [11], a functioning reticulospinal system to initiate the prints and for further categorization of the parameters into stepping rhythm, as well as voluntary movement control, a 4-point scoring system. Individual and motivational which is predominantly mediated by the corticospinal factors can greatly interfere with the quantification. Previ- [53,55] and the rubrospinal systems in rats [39,51]. There-ous studies describe an influence of walking velocity on fore, complex tasks like the grid walk paradigm can reveal the length of single steps [13,14,50]. In animals that have deficits that are not apparent during normal locomotion. only partial weight support and little plantar placement, In the present study, we used a grid walk in which bars measurements of angle and stride length are almost were placed at variable distances. This allows modifying
impossible. the sensitivity according to the severity of injury.
Addi-Correlations of other tests to footprint analysis parame- tionally, a variably spaced grid can be used if animals are ters revealed heterogeneous results. For instance, stride tested frequently in order to reduce training effects. length measurements correlated better for hemisection However, gait velocity and stress can influence the out-lesions than for contusion out-lesions, probably due to a slight come of this task [37] and more foot faults may occur asymmetry that may occur in a section lesion. when animals are crossing the grid faster or inattentively. Because of partial overlap with measurements in other By using a 4-point rating scale and categorizing the motor tests like the BBB score, footprint analysis can serve animals’ performance, this interference can be filtered out. to refine observations made in those tests for weight
support, trunk stability and foot placement. 4.1.5. Narrow beam
The performance on a narrow beam has been analyzed 4.1.3. Kinematic analysis both qualitatively [29], and quantitatively by using error The kinematic measurement of step cycles provides counting and time measurements [32] or scores [35]. assessment of single components of limb movement in two Quantitative assessment of narrow beam performance is a or three dimensions [35,50]. Although time-consuming, very sensitive tool to monitor even discrete deficits in foot this method allows detection of discrete deficits in gait and placement and body balance including tail movements. can be performed for normal and treadmill walking. If Another advantage of this paradigm is that the difficulty of animals show no weight support but limb movements, a this task can be varied via the narrowness and the shape of qualitative assessment can be made. Otherwise, this meth- the beams. The ability to cross a narrow beam in rats is od can quantify foot placement, limb coordination and dependent upon the function of spinal networks as well as exact joint angles. This technique adds precise information on supraspinal motor control from the cortico-, rubro-to other locomorubro-tor tests and provides detailed outcome on ([29,35]; Metz, unpublished observations) and possibly the step cycle duration and phase relations. vestibulospinal tracts [40]. In paralyzed animals such a scoring system can be useful since rats can be trained to
4.1.4. Grid walk traverse the beam without use of the hindlimbs by crawling
4.1.6. Open field activity more stable result. As compared to the original quantitative Open field activity is a good measure of gross motor assessment, the scoring system divides distinct measure-behavior and general health in spinal cord injured animals. ments into gross categories.
The exploratory activity is especially sensitive to
indi-vidual differences among animals with a low locomotor 4.3. Testing strategy and procedure capacity and even severely damaged rats can show
signifi-cant locomotor activity. Thus, this simple test is sensitive One of our aims was to develop a sensitive testing to a wide range of injuries. However, spontaneous ex- strategy that evaluates gross, spontaneous behavior as well ploratory activity is influenced by motivational factors as more refined aspects of locomotion. This is necessary such as anxiety, which can cause freezing behavior in since locomotion on a flat surface, as assessed in the BBB rodents thus reducing the rate of exploration [22]. It is also score, is affected only by certain types of lesions. Sponta-important to leave appropriate interval periods between neous recovery and compensatory mechanisms can mask single testing sessions to avoid habituation to the testing and / or mimic treatment effects. An important issue in environment. Furthermore, locomotor training after spinal combining tests is the partial overlap in the outcome cord lesion can enhance the exploratory activity [20]. between the parameters due to a common underlying neural basis. For many of these tests, the pathways
4.1.7. Placing response involved in mediating the respective behavior is not
The placing response is a reflex response elicited by completely known. Therefore, tests have to be selected slight touch on the foot dorsum [42]. In the present study, carefully to cover a broad spectrum of parameters and for this flexion–extension movement is poorly correlated to yielding reproducible data over days or weeks. The effects motor tasks and shows a wide distribution of values within of training must also be considered. Because most tests are and between single animals. Placing reactions in spinal differentially sensitive to the degree of injury, a combina-cats were variable, thus confirming our own observations tion of tests allows a more complete and precise evaluation of variable reflex responses in spinal and even normal rats of the overall deficit than any individual test alone. [18]. Placing responses might also depend on the muscle Based on the present results, we suggest a testing tone: after acute spinal cord injury, paralyzed animals often procedure consisting of four priority levels (Fig. 4). At the showed a more clear placing reaction than normal animals. first priority level, the assessment of BBB scores enables One way to reduce intra-individual changes is to calculate the subdivision into groups of low and high locomotor a ratio of postoperative data compared to preoperative ability. The BBB score is reliable and most independent
values. from motivational aspects [5]. It is easy and fast to perform
The anatomical substrate for placing responses is under and thus it can serve as a ‘baseline’ before further tests are debate. Some studies described the contact placing re- done. The subdivision of the animals in two groups was sponse to be dependent on the integrity of the corticospinal based on the results obtained in the comparison of different tract and cortical control [2,3,7–9,17]. In cats and in rats, tests. This strategy allows to use appropriate tests for this reflex has been shown to be dependent upon a spinal animals with more or less severe lesions that require either circuitry [1,18,19], which normally is under supraspinal whole body movements or specific limb placement control but remains elicitable in decerebrate animals [53]. abilities.
The present study revealed that the motor performance of Rats with low locomotor ability, rated from 0 to 12 contusion animals is more closely related to the placing points in the BBB scale, show no or only partial weight response than that of hemisection animals, suggesting that support and no or partial limb coordination. In these tracts running in the ventrolateral part of the spinal cord
spared in the contusion lesion may play a role in mediating this reflex response.
4.2. Quantitative assessment or rating scales?
From the present data the question arises whether absolute values or scores are more sensitive and useful for data processing. Scoring methods are established in the field of human neurological examination in order to allow
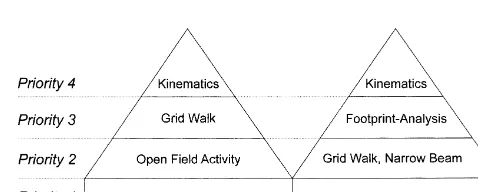
comparative evaluation and classification of symptoms in Fig. 4. Schematic illustration of an efficient behavioral testing design. It shows a pyramidal structure with four priority categories of tests: based individuals. The parametric data arising from direct
quanti-on the outcome in the BBB score, animals can be divided into groups of fication are most objective and reflect lesion and treatment
behavior in rats with neonatal unilateral cortical damage involves animals, an analysis of open field activity can be done as
the remaining hemisphere, J. Neurosci. 10 (1990) 3449–3459. second priority in order to gain further information about
[4] D.M. Basso, M.S. Beattie, J.C. Bresnahan, A sensitive and reliable the motility. Kinematic monitoring can help to specify the locomotor rating scale for open field testing in rats, J. Neurotrauma BBB scorings. To include a specific test for more complex 12 (1995) 1–21.
functions and descending control a grid walk test can be [5] D.M. Basso, M.S. Beattie, D.K. Bresnahan, D.K. Anderson, A.I. Faden, J.A. Gruner, T.R. Holford, C.Y. Hsu, L.J. Noble, R. Nockels, performed.
P.L. Perot, S.K. Salzman, W. Young, MASCIS evaluation of open For animals showing high locomotor ability (BBB of 13
field locomotor scores: effects of experience and teamwork on and higher), second priority tests are grid walk and narrow reliability, J. Neurotrauma 13 (1996) 343–359.
beam scores. To assess foot rotation and toe clearance, [6] G. Bignami, Economical test methods for developmental neuro-footprints and quantitative kinematic analysis of joint behavioral toxicity, Env. Health Perspect. 104 (1996) 285–298.
[7] B.S. Bregman, M.E. Goldberger, Infant lesion effect. I. Develop-angles are recommended for supplementary analysis.
ment of motor behavior following neonatal spinal cord damage in Combinations of two of these tests like kinematic or open
cats, Dev. Brain Res. 9 (1983) 103–118.
field locomotion with footprint analysis, respectively, were [8] B.S. Bregman, M.E. Goldberger, Infant lesion effect. II. Sparing and used before by Westerga and Gramsbergen [50] and recovery of function after spinal cord damage in newborn and adult Popovich et al. [41], showing that this procedure can refine cats, Dev. Brain Res. 9 (1983) 119–135.
[9] B.S. Bregman, E. Kunkel-Bagden, L. Schnell, H.-N. Dai, D. Gao, the analysis by taking into account limb position or gait
M.E. Schwab, Recovery from spinal cord injury mediated by patterns.
antibodies to neurite growth inhibitors, Nature 378 (1995) 498–501. The testing strategy, once started, should not be changed [10] J.G. Broton, Z. Nikolic, S. Suys, B. Calancie, Kinematic analysis of during the experiment. Before choosing the tests the limb position during quadrupedal locomotion in rats, J. Neurotrauma degree of spontaneous recovery during the first postopera- 13 (1996) 409–416.
[11] E. Brustein, S. Rossignol, Recovery of locomotion after ventral and tive weeks has to be taken into account, e.g., animals
ventrolateral spinal lesions in the cat. II. Effects of noradrenergic which are expected to reach a certain group within the first
and serotoninergic drugs, J. Neurophysiol. 81 (1999) 1513–1530. week postoperatively have to be placed from the testing [12] H. Cheng, S. Almstrom, L. Gimenez-Llort, R. Chang, S.O. Ogren,¨ ´ ¨ start on into the corresponding locomotor ability group. B. Hoffer, L. Olson, Gait analysis of adult paraplegic rats after
spinal cord repair, Exp. Neurol. 148 (1997) 544–557.
[13] K.A. Clarke, A.J. Parker, A quantitative study of normal locomotion
4.4. Conclusion in the rat, Phys. Behav. 38 (1986) 345–351.
[14] A.H. Cohen, C. Gans, Muscle activity in rat locomotion: movement The approach shown here allows an efficient and analysis and electromyography of the flexors and extensors of the
elbow, J. Morphol. 146 (1974) 177–196. transparent quantification of motor deficits after spinal cord
[15] S. Constantini, W. Young, The effects of methylprednisolone and the injury in rats and should optimize the testing procedure for
ganglioside GM1 on acute spinal cord injury in rats, J. Neurosurg. the experimenter as well as the inter-laboratory com- 80 (1994) 97–111.
parability of the results. [16] L. de Medinaceli, W.J. Freed, R.J. Wyatt, An index of the functional condition of rat sciatic nerve based on measurements made from walking tracks, Exp. Neurol. 77 (1982) 634–643.
[17] J.M. Donatelle, Growth of the corticospinal tract and the
develop-Acknowledgements ment of placing reactions in the postnatal rat, J. Comp. Neurol. 175 (1977) 207–232.
¨ ¨
[18] H. Forssberg, S. Grillner, A. Sjostrom, Tactile placing reactions in ¨
We wish to thank Christian Brosamle for helpful
chronic spinal kittens, Acta Physiol. Scand. 92 (1974) 114–120. comments on the manuscript. We also thank Eva
Hoch-[19] H. Forssberg, Stumbling corrective reaction: a phase dependent ¨
reutener and Roland Schob for help with illustrations. This compensatory reaction during locomotion, J. Neurophysiol. 42 study was supported by grants of the Swiss National (1979) 936–953.
Science Foundation (Grant no. 4038-043918.95), by the [20] F. Fouad, D. Merkler, V. Dietz, M.E. Schwab, G.A.S. Metz, Negligible effects of treadmill training in spinal cord injured rats, International Research Institute for Paraplegia (Grant no.
Behav. Brain Res. 115 (2000) 107–113. P38 / 97) and by the Spinal Cord Consortium of the
[21] K. Gale, H. Kerasidis, J.R. Wrathall, Spinal cord contusion in the rat: Christopher Reeve Paralysis Foundation (Springfield, NJ). behavioral analysis of functional neurologic impairment, Exp.
Neurol. 88 (1985) 123–134.
[22] R. Gerlai, N.S. Clayton, Analysing hippocampal function in trans-genic mice: an ethological perspective, Trends Neurosci. 22 (1999)
References 47–51.
[23] M.E. Goldberger, B.S. Bregman, C.J. Vierck, M. Brown, Criteria for assessing recovery of function after spinal cord injury: behavioral [1] V.E. Amassian, R. Ross, Development in the kitten of control of
methods, J. Neurotrauma 8 (1990) 3–9. contact placing by sensorimotor cortex, J. Physiol. 230 (1973)
[24] S. Grillner, Neural networks for vertebrate locomotion, Sci. Am. 274 55–56.
(1996) 64–69. [2] P. Bard, Studies on the cerebral cortex. I. Localized control of
[25] S. Grillner, R. Dubuc, Control of locomotion in vertebrates: spinal placing and hopping reactions in the cat and their normal
manage-and supraspinal mechanisms, Adv. Neurol. 47 (1988) 425–453. ment by small cortical remnants, Arch. Neurol. Psychiatry 30 (1933)
[26] J.A. Gruner, A monitored contusion model of spinal cord injury in 40–74.
[27] G. Guizar-Sahagun, I. Grijalva, I. Madrazo, R. Franco-Bourland, H. [41] P.G. Popovich, Z. Guan, P. Wei, I. Huitinga, N. van Rooijen, B.T. Salgado, A. Ibarra, E. Oliva, A. Zepeda, Development of post- Stokes, Depletion of hematogenous macrophages promotes partial traumatic cysts in the spinal cord of rats subjected to severe spinal hindlimb recovery and neuroanatomical repair after experimental cord contusion, Surg. Neurol. 41 (1994) 241–249. spinal cord injury, Exp. Neurol. 158 (1999) 351–365.
¨
[28] A. Herzog, C. Brosamle, ‘Semifree-floating’ treatment: a simple and [42] G.G. Rademaker, Das Stehen, Springer, Berlin, 1931.
fast method to process consecutive sections for immunohistochemis- [43] S. Rossignol, R. Dubuc, Spinal pattern generation, Curr. Opin. try and neuronal tracing, J. Neurosci. Methods 72 (1997) 57–63. Neurol. 4 (1994) 894–902.
¨
[29] S. Hicks, C.J. D’Amato, Motor-sensory cortex-corticospinal system [44] M.E. Schwab, C. Brosamle, Regeneration of lesioned corticospinal and developing locomotion and placing in rats, Am. J. Anat. 143 tract fibers in the adult rat spinal cord under experimental
con-(1975) 1–42. ditions, Spinal Cord 35 (1997) 469–473.
[30] L.M. Jordan, Initiation of locomotion in mammals, Ann. N. Y. Acad. [45] J.S. Soblosky, L.L. Colgin, D. Chorney-Lane, J.F. Davidson, M.E. Sci. 860 (1998) 83–93. Carey, Ladder beam and camera video recording system for [31] E. Kunkel-Bagden, H.N. Dai, B.S. Bregman, Recovery of function evaluating forelimb and hindlimb deficits after sensorimotor cortex
after spinal cord hemisection in newborn and adult rats: Differential injury in rats, J. Neurosci. Methods 78 (1997) 75–83.
effects on reflex and locomotor function, Exp. Neurol. 116 (1992) [46] I.M. Tarlov, Spinal cord compression studies. III. Time limits for
40–51. recovery after gradual compression in dogs, Arch. Neurol.
Psychi-[32] E. Kunkel-Bagden, H.N. Dai, B.S. Bregman, Methods to assess the atry 71 (1954) 588–597.
development and recovery of locomotor function after spinal cord [47] I.M. Tarlov, H. Klinger, S. Vitalae, Spinal cord compression studies: injury in rats, Exp. Neurol. 119 (1993) 153–164. I. experimental techniques to produce acute and gradual compres-[33] Y. Liu, D. Kim, B.T. Himes, S.Y. Chow, T. Schallert, M. Murray, A. sion, Arch. Neurol. Psychiatry 70 (1953) 813–819.
¨
Tessler, I. Fischer, Transplants of fibroblasts genetically modified to [48] M. von Euler, A. Seiger, E. Sundstrom, Clip compression injury in express BDNF promote regeneration af adult rat rubrospinal axons the spinal cord: A correlative study of neurological and morphologi-and recovery of forelimb function, J. Neurosci. 19 (1999) 4370– cal alterations, Exp. Neurol. 145 (1997) 502–510.
¨
4387. [49] J. von Meyenburg, C. Brosamle, G.A.S. Metz, M.E. Schwab,
[34] D. Merkler, G.A.S. Metz, V. Dietz, M.E. Schwab, K. Fouad, Regeneration and sprouting of chronically injured corticospinal tract Improved recovery of locomotion in spinal cord injured rats treated fibers in adult rats promoted by NT-3 and the mAB IN-1, which with an antibody neutralizing myelin-associated neurite growth neutralizes myelin-associated neurite growth inhibitors, Exp. Neurol.
inhibitors, in preparation. 154 (1998) 583–594.
[35] G.A.S. Metz, V. Dietz, M.E. Schwab, H. van de Meent, The effects [50] J. Westerga, A. Gramsbergen, The development of locomotion in the of unilateral pyramidal tract section on hindlimb motor performance rat, Dev. Brain Res. 57 (1990) 163–174.
in the rat, Behav. Brain Res. 96 (1998) 37–46. [51] I.Q. Whishaw, S.M. Pellis, V.C. Pellis, A behavioral study of the [36] G.A.S. Metz, H. van de Meent, I. Klusman, A. Curt, M.E. Schwab, contributions of cells and fibers of passage in the red nucleus of the V. Dietz, Validation of the weight-drop contusion model in rats: a rat to postural righting, skilled movements, and learning, Behav. comparative study to human spinal cord injury, J. Neurotrauma 17 Brain Res. 52 (1992) 29–44.
(2000) 1–17. [52] M. Wiesendanger, The pyramidal tract: its structure and function, in: [37] G.A.S. Metz, M.E. Schwab, H. Welzl, The effects of acute and A.L. Towe, E.S. Luschei (Eds.), Motor Coordination, Plenum, New
chronic stress on motor and sensory performance, Physiol. Behav., York, 1981, pp. 401–491.
in press. [53] C.J. Woolf, Long term alterations in the excitability of the flexion [38] G.D. Muir, I.Q. Whishaw, Complete locomotor recovery following reflex produced by peripheral tissue injury in the chronic decerebrate
corticospinal tract lesions: Measurement of ground reaction forces rat, Pain 18 (1984) 325–343.
during overground locomotion in rats, Behav. Brain Res. 103 (1999) [54] J.R. Wrathall, R.K. Pettegrew, F. Harvey, Spinal cord contusion in
45–53. the rat: production of graded, reproducible, injury groups, Exp.
[39] G.D. Muir, I.Q. Whishaw, Red nucleus lesions impair overground Neurol. 88 (1985) 108–122.
locomotion in rats: A kinetic analysis, Behav. Brain Res. 103 (1999) [55] W.J. Z’Graggen, G.A.S. Metz, G.L. Kartje, M.E. Schwab, Func-45–53. tional recovery and enhanced cortico-fugal plasticity in the adult rat [40] J.F. Pflieger, T. Cabana, The vestibular primary afferents and the after unilateral pyramidal tract section and blockade of myelin-vestibulospinal projections in the developing and adult opossum, associated neurite growth inhibitors, J. Neurosci. 18 (1998) 4744–