www.elsevier.com / locate / bres
Research report
Organization of vasoactive intestinal peptide-like immunoreactive
system in the brain, olfactory organ and retina of the zebrafish,
Danio rerio, during development
*
Maura Mathieu , Grazia Tagliafierro, Cristiano Angelini, Mauro Vallarino
`
Dipartimento di Biologia Sperimentale, DIBISAA, Universita di Genova, Viale Benedetto XV, 5, 16132 Genova, Italy
Accepted 3 October 2000
Abstract
The localization of vasoactive intestinal peptide (VIP)-like immunoreactivity was investigated in the brain, olfactory system and retina of the zebrafish, Danio rerio, during development and in juvenile specimens, by using the indirect immunofluorescence and the peroxidase-antiperoxidase methods. In 24 h post fertilization (hpf) embryos, VIP-like immunoreactive cells were present in the olfactory pit, the retina, and several regions of the brain, including the dorsal telencephalon, the diencephalon, the tegmentum of the mesencephalon, the caudal rhombencephalon and the anterior pituitary. In 48 hpf embryos, additional VIP-like immunoreactive cell bodies were found in the ventral telencephalon, whereas in the diencephalon VIP-like immunopositive cells were more concentrated within the ventro-caudal hypothalamus. During the 7 day larval period, a dense plexus of VIP-like immunoreactive fibers first appeared in the olfactory bulbs. In 15-day-old larvae, two new groups of positive cells were observed in the periventricular preoptic nucleus and in the dorsal rhombencephalon. In 1 month / 2 months old animals, VIP-like immunoreactive elements were confined to the olfactory organ, the olfactory bulbs, the periventricular preoptic nucleus and the pituitary, pars distalis. At 3 months stage, a large number of cells was observed in the periventricular preoptic nucleus. Western immunoblot analysis confirmed that VIP-like peptides, with molecular weight similar to that of synthetic VIP, are present early during the development of zebrafish. These results show that VIP-like immunoreactive structures appear early during ontogeny both in the olfactory pit, retina and brain. Transient expression of positive cells was found in the retina, telencephalon, diencephalon and brainstem. The location of VIP-like immunoreactivity indicates that, during development, VIP could be involved in several neuromodulatory functions, including the processing of visual and olfactory informations, as well as growth or survival promotion activities. The presence of VIP-like immunopositive cells in the pituitary, pars distalis, suggest that, during development, VIP may influence the secretion of pituitary hormones. 2001 Elsevier Science B.V. All rights reserved.
Keywords: Vasoactive intestinal peptide; Development; Brain; Immunohistochemistry; Zebrafish; Danio rerio
1. Introduction [8–11,33,56,57]. In rat, inhibition of VIP functions during the postnatal period produces retardation in the appearance Vasoactive intestinal peptide (VIP), is a 28 amino acid of several complex motor behaviours [31]. Administration peptide originally isolated from the porcine gastrointestinal of a VIP antagonist to pregnant mice resulted in mi-tract [61]. Subsequently, VIP immunoreactivity and VIP crocephaly in the newborn [29], and in sympathetic and mRNA were found in several neuronal populations in the neuroblastoma cultures, VIP stimulates neuronal mitosis, brain of rat [4,13,16,18,23,44,46,62], mouse [46,62], cat neurite extention and neuronal survival [56,57]. Studies of [55], hedgehog and sheep [2], swine [20], and man mouse early embryo have demonstrated that VIP is present [17,6,67]. In the brain, in addition to exhibiting neuro- in high concentrations in developing nervous system at modulator and neurotransmitter functions [27], VIP has critical period of cell proliferation and differentiation [33]. been shown to have growth and survival-promoting actions All these observations indicate that VIP is an important
developmental regulator in mammals.
In nonmammalian species, the presence of VIP in the *Corresponding author. Tel.: 139-10-353-8050; fax: 1
39-10-353-brain has been described in detail only in adult quail 8047.
E-mail address: [email protected] (M. Mathieu). [3,70], pigeon [54] and chick [41,42]. In fish, VIP has been
demonstrated in the brain of several species of teleosts, represents a simple model characterized by well defined including tilapia [38], sea bream [58], green molly [5], developmental stages and it is one of the major models for salmon, trout and carp [12]. To our knowledge, there are the genetic study of early brain development.
no studies on the ontogeny of VIP in the brain of fishes, in spite of developmental models notably different with
respect to: (i) mother embryo relationships; (ii) presence 2. Materials and methods
of separate developmental phases (embryonic and larval
phases). The study of different developmental models may 2.1. Animals improve our information to better understand the roles of
VIP during the various phases of the development. Specimens of zebrafish, Danio rerio, in different stages In the present study we have investigated the immuno- of development during the pharyngula period [24-h post histochemical distribution of VIP in the brain of the teleost fertilization (hpf)], the hatching period (48 hpf), the larval fish Danio rerio during development. To confirm the period (day 7, day 15), and the juvenile period (1 month, 2 presence of brain VIP-like molecules, an immunoblot months, 3 months), were sampled from different aquaria, at analysis was performed on larvae of 7 days. Danio rerio 25–288C. At least 5 animals were used for each stage.The
27
development stages were classified according to Haffter et contrast, preincubation of VIP antiserum with 10 M al. [30]. The fishes were anesthetized with tricaine synthetic pituitary adenylate cyclase activating peptide methane–sulfonate (MS 222, Sigma Chemical Co., MO), (PACAP) (Bachem) did not change the intensity of the fixed in freshly prepared Bouin’s fluid at room temperature immunostaining. No immunoreaction was observed when under vacuum for 10 min, followed by immersion in fresh the VIP antiserum was replaced by nonimmune rabbit fixative for 4 h, or in 4% paraformaldehyde in cold serum or PBS. Rat intestine sections incubated with VIP phosphate buffered saline (PBS) 0.2 M, pH 7.4. Paraffin- antiserum exhibited several immunostained nerve fibers. embedded, 4 mm thick, serial sagittal, frontal or coronal
sections were mounted on chrome alum / gelatin-coated glass slides.
Animal manipulations and experimental protocols were performed according to the recommendations of the Ethi-cal Committee at our institution and under the supervision of authorized investigators.
2.2. Immunofluorescence and peroxidase–antiperoxidase
procedures
The sections were rehydrated and processed for indirect immunofluorescence microscopy. Briefly, the sections were rinsed in cold phosphate-buffered saline, preincu-bated with normal swine serum (1:50) for 20 min to reduce non specific staining, and incubated in a dark moist chamber for 18 h at 48C with a polyclonal antiserum raised against porcine VIP (Biomeda, CA) or with a polyclonal antiserum raised against pituitary adenylate cyclase activat-ing peptide (PACAP)38 (Peninsula, Belmont, CA). The antisera were diluted 1:200 in PBS, containing 1% BSA and 0.3% Triton X-100. Rat intestine sections were incubated with the VIP antiserum as positive controls. Then, the sections were rinsed several times in PBS and incubated for 1 h at room temperature with fluorescein isothiocyanate-conjugated swine anti-rabbit gamma globulin (SAR) (Nordic Immunology, Tilburg, The Netherlands), diluted 1:100 in PBS. Finally, the sections were rinsed twice in PBS, mounted in glycerol–PBS (1:1), and examined under a Zeiss epifluorescence microscope (Oberkochen, Germany). Several sections were processed according to the peroxidase–antiperoxidase (PAP) pro-cedure. For this method, the sections were incubated with the primary antiserum as described above. The slices were washed in PBS and incubated with SAR (diluted 1:500) for 1 h at room temperature and then with the PAP complex (Dakopatts, Glostrup, Denmark) diluted 1:300 in PBS for another hour. The enzymatic activity was revealed by using 3,39-diaminobenzidine tetrahydro-chloride (DAB) (Sigma) containing 0.01% H O , in PBS solution. Nomen-2 2
clature of brain areas was based on the work of Wullimann et al. [69].
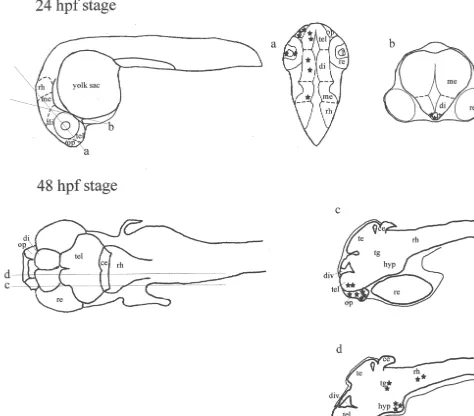
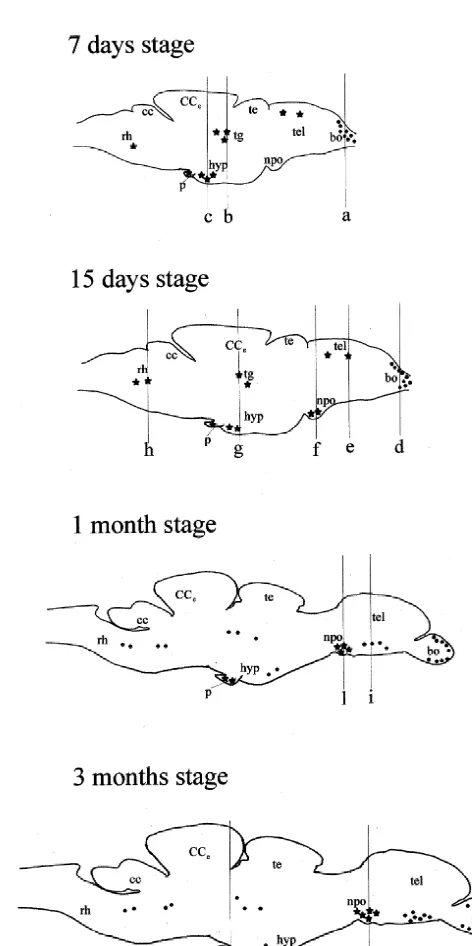
Fig. 2. Schematic sagittal sections illustrating the anatomical localization of VIP-like immunoreactive perikarya (black stars) and nerve fibers (black dots) in the central nervous system of the zebrafish, Danio rerio, 2.3. Specificity of the immunoreaction
during development: 7 days stage; 15 days stage; 1 month stage; 3 months stage. bo, olfactory bulbs; cc, crista cerebelli; CC , corpus
27 e
2.4. Western immunoblot analysis homogenized in a Teflon-glass homogenizer in 20 vol of 0.01 PBS, pH 7.4, containing proteases inhibitors: ap-2.4.1. Tissue preparation for immunoblots rotinin (20mg / ml; Sigma), 5 mM PMFS (Sigma), 50 mM Fishes, during larval period stage (7 day), were deeply NaF (Sigma), 2 mM EDTA, pH 8.0 (Sigma) and 0.2 mM anesthetized with MS 222, decapitated and the brain NaVaO (Sigma). The tissue homogenates were centrifuged3
supernatant was used for protein determination using a that the brain of zebrafish, during development and in micro BCA protein assay reagent kit (Pierce, IL). The juvenile animals, does not contain any PACAP 38-like remainder of the supernatant was diluted with 1 vol of immunoreactivity.
sodium dodecyl sulfate (SDS) reducing buffer (2 ml 0.5 M
Tris–HCl, 1.6 ml glycerol, 3.0 ml 10% SDS, 0.8 M 3.1. Pharyngula period (24 hpf stage) mercaptoethanol, 0.4 ml 0.05% Bromophenol Blue) boiled
for 3 min and stored at2708C until immunoblot analysis. During this stage of development, VIP-like positive cells were observed in several regions of the brain, in the 2.4.2. Immunoblot procedure olfactory pit and retina of D. rerio. In particular, a high For VIP analyses, 20 mg brain supernatant, pepared as density of VIP-like immunopositive cells, exhibiting a described above, were separated electroforetically on a strong immunoreaction, appeared in the olfactory pit (Fig. 20% SDS–polyacrylamide gel / Tris–Tricine buffer. The 3, level a in Fig. 1). The retina contained only scattered separated proteins were transferred to a nitro–cellulose VIP-like immunopositive cells. (Fig. 4, level a in Fig. 1). membrane (Bio-Rad Laboratories, Hercules, CA) at 350 In the brain, a moderate number of VIP-like immuno-mA for 2 h at 48C. The VIP was detected by incubating the reactive cell bodies was found in the dorsal telencephalon nitrocellulose filter at 48C overnight with polyclonal VIP and within the dorsal and ventral regions of the dien-antiserum (Biomeda) diluted 1:1000 with 5% milk TBS–T. cephalon (Figs. 5 and 6, level a in Fig. 1). In the Bands were visualized by subsequent incubations with mesencephalon, a few cells, exhibiting their processes peroxidase-conjugated anti-rabbit gamma immunoglobulin directed laterally to the ventricle, were present within the (Vector Laboratories) at room temperature for 2 h and then tegmentum. Similarly, scattered VIP-like immunoreactive developed in a 1-StepE NBT / BCIP plus Suppressor-sys- cell bodies were detected in the medial region of the tem (Pierce). caudal rhombencephalon. During this stage, VIP-like im-VIP synthetic peptide (Bachem) was used as positive munoreactive cells, exhibiting a strong immunostaining, control. Prestained molecular weight markers were ob- first appeared in the anterior part of the pituitary (Fig. 7, tained by New England Biolabs (MA, USA). level b in Fig. 1). The other regions of the brain did not
show any VIP-like immunoreactivity.
3. Results 3.2. Hatching period (48 hpf stage)
The distribution of VIP-like immunoreactive cells and At this stage of development, similarly to 24 hpf stage, a fibers in the brain, olfactory pit, and retina of the zebrafish, high number of VIP-like immunoreactive cells, exhibiting
Danio rerio, was investigated in animals whose age varied a bright immunofluorescence, was found in the olfactory from the pharyngula period up to the juvenile period. pit (Fig. 8, level c in Fig. 1). A few VIP-immunoreactive Incubation of sections with the VIP antiserum revealed the cells were occasionally found in the retina. In the brain, a presence of immunopositive cell bodies in all stages moderate number of bright fluorescent VIP-like immuno-investigated. By contrast, VIP-like positive nerve fibers reactive cells was found within the telencephalon (Fig. 8, first appeared in the larval stages. No differences were level c in Fig. 1). In the diencephalon, during this stage, found between Bouin-fixed brains and paraformaldehyde- VIP-like immunopositive cell bodies were more concen-fixed brains. The anatomical distribution of VIP-like trated in the ventral region of the caudal hypothalamus immunoreactive perikarya and fibers in the brain of D. (Fig. 9, level d in Fig. 1). In the mesencephalon, a larger
rerio during the different stages of development is number of VIP-like immunoreactive cell bodies was schematically illustrated in Figs. 1 and 2. Incubations of present within the dorsal and ventral parts of the tegmen-brain sections with the antiserum agains PACAP38 showed tum (Fig. 10, level d in Fig. 1). In the rhombencephalon, a
Fig. 3. Frontal section of 24 hpf embryo of D. rerio showing the presence of VIP-like immunostained cells within the olfactory pit (heads of arrow). Level a in Fig. 1. Paraformaldehyde-fixed tissue. op, olfactory pit; tel, telencephalon. Scale bar 100mm.
Fig. 4. Frontal section of 24 hpf embryo of D. rerio showing the occurrence of scattered immunopositive cells (heads of arrow) within the retina. Level a in Fig. 1. Paraformaldehyde-fixed tissue. Scale bar 200mm.
Fig. 5. Frontal section of a paraformaldehyde-fixed tissue through the brain of 24 hpf embryo of D. rerio, showing the presence of VIP-like positive cell bodies (heads of arrow) in the dorsal part of the rostral telencephalon. Level a in Fig. 1. tel, telencephalon. Scale bar 100mm.
Fig. 6. 24 hpf stage brain of D. rerio showing the presence of strong immunolabeled cells (head of arrow) within the ventral and dorsal regions of the caudal diencephalon. Level a in Fig. 1. Transverse section of a paraformaldehyde-fixed tissue. di, diencephalon; op, olfactory pit; tel, telencephalon. Scale bar 100mm.
moderate concentration of brightly fluorescent VIP-like bulbs (Fig. 15, level d in Fig. 2) was similar to that immunoreactive perikarya was detected within the medial described in 7-day-old larvae. By contrast, during this region. Similarly to 24 hpf stage, several VIP-like im- stage of development, several positive cells were detected munoreactive cells were found in the pituitary. in the retina at level of the inner layer (Fig. 16). In the proper telencephalon, a small number of VIP-like positive 3.3. Larval period stages perikarya, exhibiting a weak immunofluorescence, was detected in the dorsal telencephalon (Fig. 17, level e in 3.3.1. Day 7 Fig. 2). In the diencephalon, a small cluster of VIP-like During this stage, the distribution of VIP-like immuno- immunoreactive cells appeared in the periventricular pre-reactivity showed some changes as compared to that optic nucleus (Fig. 17, level f in Fig. 2). A moderate described during the hatching period. A high density of number of positive cells was found in the ventral part of VIP-like immunoreactive fibers first appeared in the olfac- the caudal hypothalamus (Fig. 17, level g in Fig. 2). In the tory bulbs, within the primary olfactory fiber layer. These mesencephalon, similarly to the previous stage, positive fibers, showing a bright immunostaining, were preferen- cell bodies were located in the tegmentum (Fig. 17, level g tially distributed through the dorsal and medial region of in Fig. 2). More caudally, a population of positive the olfactory bulbs (Fig. 11, level a in Fig. 2). Similarly to perikarya was found in the dorsal region of the rostral the previous stages, a high number of strongly immuno- rhombencephalon (Fig. 17, level h in Fig. 2). As reported fluorescent VIP-like positive cells was found in the in the previous stages, a small group of immunoreactive olfactory organ. In particular, a high accumulation of cells was found in the pituitary, pars distalis.
VIP-like immunoreactivity was observed in the external
layer of the organ (Fig. 12). As reported in the previous 3.4. Juvenile period stages stage of development, a few positive cells were found in
the retina. In the telencephalon, very few VIP-like im- 3.4.1. 1 month /2 months
munoreactive cells were found in the dorsal region. In In the brain of 1 month and 2 months old larvae, the contrast, the diencephalon contained a moderate concen- anatomical distribution and relative frequency of VIP-like tration of VIP-like immunoreactive cells at level of the immunoreactive elements was substantially similar. A ventral region of the caudal hypothalamus (Fig. 13, level c dense plexus of VIP-immunoreactive fibers, showing a in Fig. 2). In the mesencephalon, VIP-like positive cells bright fluorescence, was found in the olfactory bulbs. Also exhibiting a moderate fluorescence were present within the the olfactory organ showed a high concentration of VIP-tegmentum (Fig. 14, level b in Fig. 2). Occasional positive like immunoreactive cells. During these stages, VIP-like cell bodies were found in the ventral rhombencephalon. positive beaded nerve fibers first appeared through the Few immunoreactive cell bodies were present in the ventral telencephalon (Fig. 18, level i in Fig. 2), whereas pituitary. During this stage of development, no VIP-like no VIP-like immunoreactive perikarya were found in the immunoreactive cell bodies and fibers were found in the telencephalon. In the diencephalon, similarly to the previ-other regions of the brain. ous stage, VIP-like immunoreactive cell bodies were observed within the periventricular preoptic nucleus. In 1 3.3.2. Day 15 month / 2 months old larvae, the number of these cells was During this larval stage, the distribution of VIP-like higher if compared to that of 15-day-old larvae (Fig. 19, immunoreactivity in the olfactory pit and the olfactory level l in Fig. 2). On the contrary, no VIP-like positive
Fig. 8. Sagittal section through the 48 hpf embryo of D. rerio showing the presence of brightly immunofluorescent cells within the olfactory pit (head of arrow). VIP-immunoreactive perikarya are present in the rostral telencephalon (arrow). Level c in Fig. 1. Bouin-fixed tissue. div, diencephalic ventricle; hyp, hypothalamus; op, olfactory pit; te, tectum of the mesencephalon; tel, telencephalon. Scale bar 200mm.
Fig. 9. Sagittal section through the 48 hpf brain of D. rerio showing the occurrence of VIP-like immunopositive cells (head of arrow) in the diencephalon, at level of the ventral region of the caudal hypothalamus. Level d in Fig. 1. Bouin-fixed tissue. hyp, hypothalamus. Scale bar 200mm.
Fig. 10. Sagittal section of a Bouin-fixed tissue through the 48 hpf embryo showing the presence of immunoreactive perikarya in the tegmentum of the mesencephalon (head of arrows). Level d in Fig. 1. tg, tegmentum of the mesencephalon. Scale bar 200mm.
Fig. 11. Sagittal section through the brain of a 7-day-old larva of D. rerio showing the presence of high densities of bright fluorescent VIP-like positive nerve fibers entering the olfactory bulb at level of the glomerular layer. Level a in Fig. 2. Bouin-fixed tissue. bo, olfactory bulb. Scale bar 200mm. Fig. 12. Sagittal section of a 7-day-old larva of D. rerio showing a high accumulation of VIP-like immunoreactivity in the olfactory organ. Bouin-fixed tissue. Scale bar 100mm.
Fig. 13. Sagittal section of a Bouin-fixed tissue through the brain of a 7-day-old larva of D. rerio showing the occurrence of VIP-like immunoreactive cell bodies within the ventral region of the caudal hypothalamus (head of arrow). Level c in Fig. 2. hyp, hypothalamus. Scale bar 200mm.
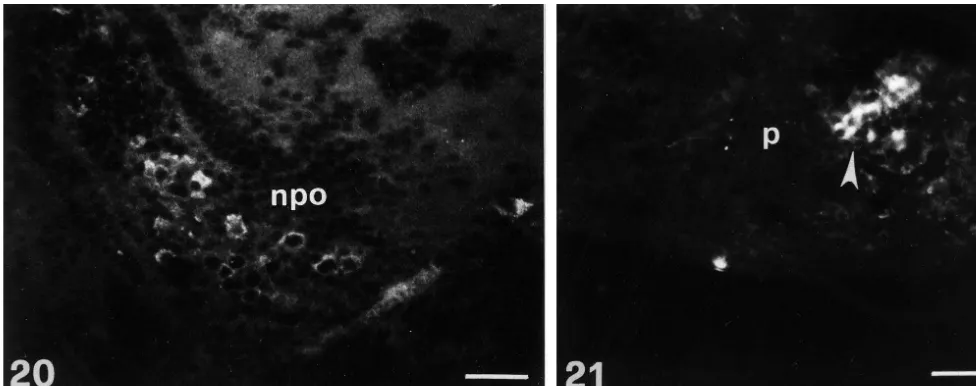
Fig. 20. Sagittal section through the brain of 3 months old larva of D. rerio showing the presence of a high number of bright fluorescent immunoreactive perikarya within the periventricular preoptic nucleus. Level m in Fig. 2. Bouin-fixed tissue. npo, preoptic nucleus. Scale bar 200mm.
Fig. 21. Sagittal section through the pituitary of 3 months old larva of D. rerio showing the location of bright fluorescent VIP-like immunopositive cells within the anterior lobe. Level n in Fig. 2. Bouin-fixed tissue. p, pituitary. Scale bar 200mm.
cells were found in the caudal hypothalamus, as well as in the mesencephalon and the rhombencephalon. These re-gions only contained few scattered positive nerve fibers. A moderate number of VIP-like immunoreactive cells, ex-hibiting a weak immunofluorescence, was observed in the pituitary, pars distalis.
3.4.2. 3 months
During this stage of development, the distribution of VIP-like immunoreactivity in the olfactory organ and olfactory bulbs was similar to that described in 1 month / 2 months stages. By contrast, a substantially larger number
Fig. 22. Western blot analysis of VIP related protein of Danio rerio. Lane of VIP-like immunoreactive fibers was found in the ventral B: brain lysates of 7 days Danio rerio larvae; lane C: synthetic VIP telencephalon as compared with previous stages. Similarly, peptide; lane A: prestained molecular weight markers.
a higher concentration of positive cell bodies was observed in the hypothalamus at level of the periventricular preoptic
stages, a moderate number of immunoreactive cells, ex-nucleus (Fig. 20, level m in Fig. 2). High densities of
hibiting a bright fluorescence, was present in the pituitary, immunoreactive fibers were distributed in the ventral
pars distalis (Fig. 21, level n in Fig. 2). Outside these region of the caudal hypothalamus. As reported in previous
Fig. 15. Sagittal section through a 15-day-old larva brain of D. rerio showing the presence of a high density of VIP-like immunoreactive fibers in the olfactory bulb. Level d in Fig. 2. Bouin-fixed tissue. bo, olfactory bulb. Scale bar 200mm.
Fig. 16. Sagittal section showing the presence of several VIP-like immunoreactive cells within the most internal layer of the retina (head of arrow). Bouin-fixed tissue of a 15-day-old larva of D. rerio. Scale bar 200mm.
Fig. 17. Sagittal section through the brain of a 15-day-old larva of D. rerio showing the occurrence of scattered immunoreactive cell bodies (head of arrows) within the dorsal telencephalon (level e in Fig. 2), the periventricular preoptic nucleus (level f in Fig. 2), the ventral hypothalamus (level g in Fig. 2), the mesencephalon (level g in Fig. 2) and the dorsal part of the rostral rhombencephalon (level h in Fig. 2). Bouin-fixed tissue. cc, crista cerebelli; CC ,e corpus cerebelli; hyp, hypothalamus; npo, preoptic nucleus; te, tectum of the mesencephalon; tel, telencephalon; tg, tegmentum of the mesencephalon; rh, rhombencephalon. Scale bar 400mm.
Fig. 18. Sagittal section through the brain of 1 month old larva of D. rerio showing the occurrence of strong immunofluorescent nerve fibers within the ventral telencephalic area. Level i in Fig. 2. Bouin-fixed tissue. Scale bar 200mm.
regions, no VIP-like immunopositive material was found in and salmon [12] and VIP immunoreactive structures have the brain of zebrafish. been described in the olfactory bulbs of rat [21,24], hedgehog and sheep [2], brown bat [43] and chick [42].
In the retina of zebrafish, a transient expression of 3.5. Western immunoblot VIP-like immunoreactive cells occurred during pharyngula, hatching and larval stages. These cells ap-A western immunoblot analysis of VIP extracts from the peared early in the retina of 24 hpf embryos as scattered brain of embryos of zebrafish at 7 day of development elements and their number increased significantly in 15-revealed a protein band with a mobility corresponding to day-old larvae. By contrast, no VIP-like immunoreactive that of synthetic VIP (Fig. 22). structures were found in the retina of juvenile fishes. Previously, VIP immunoreactivity has been demonstrated in amacrine cells of rabbit [15] and in the retina of snail [37] and highly selective VIP receptors have been
de-4. Discussion scribed in the retina of rat [64]. In rat, it has been suggested that VIP protects retinal tissue from lipid This study provides the first anatomical description of peroxidation [65] and stimulates glial cells to release VIP-like immunoreactivity in the brain, olfactory organ factors that are important for the neuronal survival of the and retina of a teleost fish, the zebrafish Danio rerio, retina [39]. At present, if VIP has similar functions in the during development. In addition, the immunobloting re- retina of fish during development is unknown.
nucleus, respectively [28]. In 15-day-old zebrafish larvae, during development, was further confirmed by western VIP immunoreactivity appeared in the preoptic-hypo- immunoblot analyses of brain extracts.
thalamus complex, suggesting that, early during develop-ment, VIP-related peptides may act as hypophysiotropic factors in fish. Previous studies have demonstrated that in
Acknowledgements
mammals VIP stimulates the secretion of various hypo-physial hormones such as growth hormone [7] and
prolac-This work was supported by grants from the University tin [1,14,35,36,60]. VIP has a potent stimulatory effect on
of Genova, Italy. prolactin secretion also in birds [47,45,53] and amphibians
[40]. By contrast, in fish VIP has an inhibitory action on prolactin secretion [38]. One of the most striking new
finding in this study was the demonstration of VIP-like References
immunoreactive cells in the pituitary during development
of embryos, larvae and in juvenile animals. [1] H. Abe, D. Engler, M.E. Molitch, F. Bollinger-Gruber, S. Reichlin, Vasoactive intestinal peptide is a physiological mediator of prolactin To our knowledge, this finding is the first evidence of
release in the rat, Endocrinology 116 (1985) 1383–1390. VIP-like peptides in the pituitary during development.
[2] J. Antonopoulos, G.C. Papadopoulos, A.N. Karamanlidis, J.G. Previously, VIP immunoreactivity and VIP gene expression Parnavelas, A. Dinopoulos, H. Michaloudi, VIP- and CCK-like-have been demonstrated in the anterior pituitary of several immunoreactive neurons in the hedgehog (Erinaceus europaeus) and adult mammalian species (for review, [52]. Although VIP sheep (Ovis aries) brain, J. Comp. Neurol. 263 (1987) 290–307.
[3] N.C. Aste, C. Viglietti-Panzica, A. Fasolo, G.C. Panzica, Mapping of immunoreactivity was localized in rat lactotrope cells
neurochemical markers in quail central nervous system: VIP- and [49,50,63] and human corticotrope cells [34], the cells type
SP-like immunoreactivity, J. Chem. Neurol. 8 (1995) 87–102. involved in the production of VIP is still a matter of debate [4] F. Baldino Jr, M. Fitzpatrick, S. Elligott, I. Gozes, J.P. Card, [52]. In zebrafish, studies are in progress to detect which Localization of VIP and PHI-27 messenger RNA in rat thalamic and class of pituitary cells syntesize VIP-related peptides cortical neurons, J. Mol. Neurosci. 1 (1989) 199–207.
[5] T.F.C. Batten, M.L. Cambre, L. Moons, F. Vandesande, Comparative during development. In the pituitary of zebrafish, as well
distribution of neuropeptide-immunoreactive systems in the brain of as in the pituitary of mammals, VIP could influence the
the green molly, Poecilia latipinna, J. Comp. Neurol. 302 (1990) secretion of pituitary hormones by means of autocrine and 893–919.
paracrine mechanisms. [6] C. Bouras, P.J. Magistretti, J.H. Morrison, An immunohistochemical VIP-like immunoreactive cells were found during the study of six biologically active peptides in the human brain, Hum.
Neurobiol. 5 (1986) 213–226. earlier development stages of zebrafish also in the
tegmen-[7] P. Brazeau, W. Vale, R. Burgus, N. Ling, M. Butcher, J. Rivier, R. tum of the mesencephalon. The highest number of positive
Guillemin R, Hypothalamic polypeptide that inhibits the secretion of cells was detected in larval period stages. A few cells were
immunoreactive pituitary growth hormone, Science 179 (1973) also found in 24 hpf and 48 hpf embryos, whereas no 77–79.
immunoreactivity was observed in juvenile animals. In rat, [8] D.E. Brenneman, L.E. Eiden, Vasoactive intestinal peptide and electrical activity influence neuronal survival, Proc. Nat. Acad. Sci. VIP gene expression has been demonstrated late during
USA 83 (1986) 1159–1162. development in E20 embryos in the central gray, colliculus
[9] D.E. Brenneman, L.E. Eiden, R.E. Siegel, Neurotrophic action of superior and near the intermediate reticular zone of the
VIP on spinal cord cultures, Peptides 6 (1985) 35–39.
mesencephalon [28]. In E21 rat embryos, this last group of [10] D.E. Brenneman, E.A. Neale, G.A. Foster, S. d’Autremont, G.L. cells did not express VIP mRNA suggesting that also in rat Westbrook, Nonneuronal cells mediate neurotrophic actions of vasoactive intestinal peptide, J. Cell. Biol. 104 (1987) 1603–1610. exists a transient expression of VIP in the mesencephalon.
[11] D.E. Brenneman, T. Nicol, D. Warren, L.M. Bowers, Vasoactive A similar feature was also showed in the rhombencephalon
intestinal peptide: a neurotrophic releasing agent and an astroglial of D. rerio, which exhibited VIP-like immunoreactive cell
mitogen, J. Neurosci. Res. 25 (1990) 386–394.
bodies from 24 hpf embryos to 15-day-old larvae. By [12] P. Canciglia, J.L. Martin, C.L. Bolis, D. Randall, P.J. Magistretti, contrast, in juvenile animals, this region only contained Regional distribution of vasoactive intestinal peptide immuno-reactivity in the brain of salmon, trout and carp, Biol. Signals 4 few positive fibers.
(1995) 86–93. In summary, the present results indicate that VIP-like
[13] J.P. Card, N. Brecha, H.J. Karten, R.Y. Moore, Immunocytochemical immunoreactive structures appear early in the olfactory
localization of vasoactive intestinal polypeptide-containing cells and organ, retina and brain of the teleost, Danio rerio, during processes in the suprachiasmatic nucleus of the rat: light and development. In the brain, VIP-like immunoreactivity is electron microscopic analysis, J. Neurosci. 1 (1981) 1289–1303. more widely distributed during the first stages of the [14] A.J. Carrillo, P.C. Doherty, X.B. Guan, J.R. Sturtevant, D.G. Walro,
Preferential increase in pituitary prolactin versus vasoactive intesti-ontogeny than that reported in juvenile animals. Transient
nal peptide as a function of estradiol benzoate dose in the ovariec-expression of VIP-like immunoreactivity was found both
tomized rat, Endocrinology 128 (1991) 131–138.
[16] J. R Connor, A. Peters, Vasoactive intestinal polypeptide-immuno- [35] H. Kaji, K. Chihara, H. Abe, T. Kita, Y. Kashio, Y. Okimura, T. reactive neurons in rat visual cortex, Neuroscience 12 (1984) 1027– Fujita, Effect of passive immunization with antisera to vasoactive
1044. intestinal polypeptide and peptide histidine isoleucine amide on
5-hydroxy-L-tryptophan-induced prolactin release in rats, Endo-[17] J.P. Dai, D.F. Swaab, R.M. Bujis, Distribution of vasopressin and
crinology 117 (1985) 1914–1919. vasoactive intestinal polypeptide (VIP) fibers in the human
hypo-thalamus with special emphasis on suprachiasmatic nucleus efferent [36] Y. Kato, Y. Iwasaki, J. Iwasaki, H. Abe, N. Yanaihara, H. Imura, projection, J. Comp. Neurol. 383 (1997) 397–414. Prolactin release by vasoactive intestinal polypeptide in rats,
Endo-crinology 103 (1978) 554–558. [18] M. Dussaillant, A. Sarrieau, I. Gozes, A. Berod, W. Rostene,
Distribution of cells expressing vasoactive intestinal peptide / peptide [37] W. Kaufmann, H.H. Kerschbaum, C. Hauser-Kronberger, G.W. histidine-amide precursor messenger RNA in the rat brain, Neuro- Hacker, A. Hermann, Distribution and seasonal variation of vasoac-science 50 (1992) 519–530. tive intestinal (VIP)-like peptides in the nervous system of Helix
pomatia, Brain Res. 695 (1995) 125–136. [19] P.C. Emson, R.F.T. Gilbert, I. Loren, J. Fahrenkrug, F. Sundler, O.B.
Schaffalitzky de Muckadell, Development of vasoactive intestinal [38] K.M. Kelley, R.S. Nishioka, H.A. Bern, Novel effect of vosoactive polypeptide (VIP) containing neurons in the rat brain, Brain Res. intestinal polypeptide and peptide histidine isoleucine: inhibition of 177 (1979) 437–444. in vitro secretion of prolactin in the tilapia, Oreochromis
mossam-bicus, Gen. Comp. Endocrinol. 72 (1988) 97–106. [20] J. Fahrenkrug, O.B. Schaffalitzky de Muckadell, Distribution of
vasoactive intestinal polypeptide (VIP) in the porcine central [39] M.S.W. Koh, G.F. Roberge, VIP modulation of cultured glial cells of nervous system, J. Neurochem. 31 (1978) 1445–1451. the rat retina, Curr. Eye Res. 8 (1989) 1207–1210.
[21] J. Fahrenkrug, Vasoactive intestinal polypeptide, Trends Neurosci. 3 [40] R. Koiwai, S. Kikuyama, T. Seki, H. Yanaihara, In vitro effect of (1980) 1–4. vasoactive intestinal polypetide and peptide histidine isoleucine of prolactin secretion by the bullfrog pituitary gland, Gen. Comp. [22] J. Fink, M.R. Montminy, T. Tsukada, H. Hoefler, L.A. Specht, R.M.
Endocrinol. 64 (1986) 254–259. Lechan, H. Wolfe, G. Mandel, R.H. Goodman, In situ hybridization
¨
of somatostatin and VIP mRNA in the rat nervous system, in: G.R. [41] W.J. Kuenzel, S. Blahser, Vasoactive intestinal polypeptide (VIP)-Uhl (Ed.), In Situ Hybridization in Brain, Plenum Press, New York, containing neurons: distribution throughout the brain of the chick 1986, pp. 181–191. (Gallus domesticus) with focus upon the lateral septal organ, Cell.
¨ Tissue Res. 275 (1994) 91–107.
[23] K. Fuxe, T. Hokfelt, S. Said, V. Mutt, Vasoactive intestinal
poly-peptide and the nervous system: Immunohistochemical evidence for [42] W.J. Kuenzel, S.K. McCune, R.T. Talbot, P.J. Sharp, J.M. Hill, Sites localization in central and peripheral neurons, particularly intracorti- of gene expression for vasoactive intestinal polypeptide throughout cal neurons of the cerebral cortex, Neurosci. Lett. 5 (1977) 241– the brain of the chick (Gallus domesticus), J. Comp. Neurol. 381
246. (1997) 101–118.
[24] C. Gall, K.B. Seroogy, N. Brecha, Distribution of VIP- and NPY- [43] L.K. Laemle, J.R. Cotter, Immunocytochemical localization of like immunoreactivities in rat main olfactory bulb, Brain Res. 374 vasoactive intestinal polypeptide (VIP) in the brain of the little (1986) 389–394. brown bat (Myotis lucifugus), J. Neurocytol. 17 (1988) 117–129.
`
[25] I. Gozes, Y. Shani, W.H. Rostene, Developmental expression of the [44] L.I. Larsson, J. Fahrenkrug, O.B. Schaffalitzky de Muckadell, F. VIP-gene in brain and intestine, Mol. Brain Res. 2 (1987) 137–148. Sundler, R. Hakanson, J.F. Rehfeld, Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons, Proc. [26] I. Gozes, P. Schachter, Y. Shani, E. Giladi, Vasoactive intestinal
Natl. Acad. Sci. USA 73 (1976) 3197–3200. peptide gene expression from embryos to aging rats,
Neuroen-docrinology 47 (1988) 27–31. [45] R.W. Lea, D.M. Vowles, Vasoactive intestinal polypeptide stimulates prolactin release in vivo in the ring dove (Streptopelia risoria), [27] I. Gozes, D.E. Brennemann, VIP: molecular biology and
neuro-Experientia 42 (1986) 420–422. biological function, Mol. Neurobiol. 3 (1989) 201–236.
´
[46] I. Loren, P.C. Emson, J. Fahrenkrug, A. Bjorklund, A. Alumets, R. [28] M. Graber, J.M. Burgunder, Ontogeny of vasoactive intestinal
Hakanson, F. Sundler, Distribution of vasoactive intestinal poly-peptide gene expression in rat brain, Anat. Embryol. 194 (1996)
peptide in the rat and mouse brain, Neuroscience 4 (1979) 1953– 595–605.
1976. [29] P. Gressens, J.M. Hill, B. Paindaveine, I. Gozes, M. Fridkin, D.E.
[47] M.C. MacNamee, P.J. Sharp, R.W. Lea, R.J. Sterling, S. Harvey, Brenneman, Severe microcephaly induced by blockade of vasoactive
Evidence that vasoactive intestinal polypeptide is a physiological intestinal peptide function in the primitive neuroepithelium of the
prolactin-releasing factor in the bantam hen, Gen. Comp. Endo-mouse, J. Clin. Invest. 94 (1994) 2020–2027.
crinol. 62 (1986) 470–478. [30] P. Haffter, M. Granato, M. Brand, M.C. Mullins, M.
Ham-[48] G.P. McGregor, P.L. Woodhams, D.J. O’Shaughnessey, M.A. Ghat-merschmidt, D.A. Kane, J. Odenthal, F.J. van-Eeden, Y.J. Jiang, C.P.
tei, J.M. Polak, S.R. Bloom, Developmental changes in bombesin, Heisenberg, R.N. Kelsh, M. Furutani-Seiki, E. Vogelsang, D.
Beuch-substance P, somatostatin and vasoactive intestinal polypeptide in le, U. Schach, C. Fabian, C. Nusslein-Volhard, The identification of
the rat brain, Neurosci. Lett. 28 (1982) 21–27. genes with unique and essential functions in the development of the
zebrafish, Danio rerio, Development 123 (1996) 1–36. [49] G. Morel, J. Besson, G. Rosselin, P.M. Dubois, Ultrastructural evidence for endogenous vasoactive intestinal peptide-like immuno-[31] J.M. Hill, I. Gozes, J.L. Hill, M. Fridkin, D.E. Brenneman,
Vasoac-reactivity in the pituitary gland, Neuroendocrinology 34 (1982) tive intestinal peptide antagonist retards the development of neonatal
85–89. behaviors in the rat, Peptides 12 (1991) 87–192.
[50] G. Nagy, J.J. Mulchahey, J.D. Neill, Autocrine control of prolactin [32] J.M. Hill, D.V. Agoston, P. Gressens, S. McCune, Distribution of
secretion by vasoactive intestinal peptide, Endocrinology 122 (1988) VIP mRNA and two distinct binding sites in the developing rat
364–366. brain: relation to ontogenic events, J. Comp. Neurol. 342 (1994)
186–205. [51] F. Nobou, J. Besson, W. Rostene, G. Rosselin, Ontogeny of
vasoactive intestinal peptide and somatostatin in different structures [33] J.M. Hill, S.K. McCune, R.J. Alvero, G.W. Glazner, D.E.
Bren-of the rat brain: effects Bren-of hypo- and hypercorticism, Dev. Brain neman, VIP regulation of embryonic growth, in: A. Arimura, S.I.
Res. 20 (1985) 296–301. Said (Eds), VIP, PACAP, and related peptides, Ann. N.Y. Acad. Sci.
805 (1996) 259–279. [52] G.G. Nussdorfer, L.K. Malendowicz, Role of VIP, PACAP, and related peptides in the regulation of the hypothalamo-pituitary-[34] D.W. Hsu, P.N. Riskind, E.T. Hedley-Whyte, Vasoactive intestinal
adrenal axis, Peptides 19 (1998) 1443–1467. peptide in the human pituitary gland and adenomas:an
by vasoactive intestinal peptide, Proc. Soc. Exp. Biol. Med. 187 [63] J.H. Steel, G. Gon, D.J. O’Halloran, P.M. Jones, N. Yanaihara, H. (1988) 455–460. Ishikawa, S.R. Bloom, J.M. Polak, Galanin and vasoactive intestinal
´
[54] P. Peczely, J.Z. Kiss, Immunoreactivity to vasoactive intestinal polypeptide are colocalized with classic pituitary hormones and polypeptide (VIP) and thyreotropin-releasing hormone (TRH) in show plasticity of expression, Histochemistry 93 (1989) 183–189. hypothalamic neurons of the domesticated pigeon (Columbia livia). [64] A.P. Swedlund, A.S. Rosensweig, Characterization of vasoactive Alterations following lactation and exposure to cold, Cell. Tissue intestinal peptide receptors in retina, Exp. Eye Res. 51 (1990)
Res. 251 (1988) 485–494. 317–323.
[55] A. Peters, D.L. Meinecke, A.N. Karamanlidis, Vasoactive intestinal [65] N. Tunc¸el, H. Basmak, K. Uzuner, M. Tunc¸el, G. Altiokka, V. ¨
polypeptide immunoreactive neurons in the primary visual cortex of Zaimoglu, A. Ozer, F. Gurer, Protection of rat retina from ischemia-¨ the cat, J. Neurocytol. 16 (1987) 23–38. reperfusion injury by vasoactive intestinal peptide (VIP): the effect [56] D.W. Pincus, E.M. DiCicco Bloom, I.B. Black, Vasoactive intestinal of VIP on lipid peroxidation and antioxidant enzyme activity of peptide regulation of neuroblast mitosis and survival: role of cAMP, retina and choroid, in: A. Arimura, S.I. Said (Eds.), VIP, PACAP, and Brain Res. 514 (1990) 355–357. Related Peptides, Vol. 805, Ann. NY Academy Science, 1996, pp. [57] D.W. Pincus, E.M. DiCicco Bloom, I.B. Black, Vasoactive intestinal 489–498.
peptide regulate mitosis, differentiation and survival of cultured [66] P. Wahle, G. Meyer, Early postnatal development of vasoactive sympathetic neuroblasts, Nature 343 (1990) 564–567. intestinal peptide- and peptide histidine isoleucine-immunoreactive [58] D.M. Power, P.M. Ingleton, Distribution of vasoactive intestinal structures in the cat visual cortex, J. Comp. Neurol. 282 (1989)
peptide in the brain and hypothalamo-hypophysial system of the sea 215–248.
bream (Sparus aurata), in: H. Vaudry, M.C. Tonon, E.W. Roubos, A. [67] A. Walter, J.K. Mai, L. Lanta, T. Gorcs, Differential distribution of De Loof (Eds.), Trends in Comparative Endocrinology and Neuro- immunohistochemical markers in the bed nucleus of the stria biology, From Molecular to Integrative Biology, Vol. 839, NY terminalis in the human brain, J. Chem. Neuroanat. 4 (1991) Academy Science, New York, 1998, pp. 357–358. 281–298.
[59] S. Ramon y Cajal Agueras, P. Contamina, P. Parra, L. Martinez- [68] J.A. Waschek, J. Ellison, D.T. Bravo, V. Handley, Embryonic Millan, The distribution of VIP-immunoreactive neurons in the expression of vasoactive intestinal peptide (VIP) and VIP receptors visual cortex of the adult rabbits and during postnatal development, genes, J. Neurochem. 66 (1996) 1762–1765.
Brain Res. 370 (1986) 333–337. [69] M.F. Wullimann, B. Rupp, H. Reichert, Neuroanatomy of the [60] W.H. Rotsztejn, M. Dussaillant, F. Nobou, G. Rosselin, Rapid Zebrafish Brain: A Topological Atlas, Birkhauser Verlag, Basel,¨
glucocorticoid inhibition of vasoactive intestinal peptide induced 1996.
cyclic AMP accumulation and prolactin release in rat pituitary cells [70] S. Yamada, S. Mikami, N. Yanaihara, Immunohistochemical locali-in culture, Proc. Natl. Acad. Sci. USA 78 (1981) 7584–7588. zation of vasoactive intestinal polypeptide (VIP)-containing neurons [61] S.I. Said, V. Mutt, Polypeptide with broad biologic activity: isolation in the hypothalamus of the Japanese quail, Coturnix coturnix, Cell.
from small intestine, Nature 225 (1970) 863–864. Tissue Res. 266 (1982) 13–26. [62] K.B. Sims, D.L. Hoffmann, S.I. Said, E.A. Zimmermann, Vasoactive