www.elsevier.com / locate / bres
Research report
Failure to activate NF-
k
B promotes apoptosis of retinal ganglion cells
following optic nerve transection
a a a b d
Jun-Sub Choi , Jeong-a. Kim , Dong-Hwan Kim , Myung-Hun Chun , Byung J. Gwag ,
c a ,
*
Sungjoo Kim Yoon , Choun-Ki Joo
a
Department of Ophthalmology and Visual Science, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-ku, Seoul137-701, South Korea
b
Department of Anatomy, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-ku, Seoul 137-701, South Korea
c
Research Institutes of Medical Science, College of Medicine, The Catholic University of Korea, 505 Banpo-dong, Seocho-ku, Seoul 137-701, South Korea
d
Department of Pharmacology, Ajou University School of Medicine, Suwon, South Korea
Accepted 22 August 2000
Abstract
NF-kB is a transcription factor, which is activated by various stimuli. One of the well-known activators of NF-kB is oxidative stress, which is a cause of cell death in some tissue, or cell types. Optic nerve transection, axotomy, results in retinal cell death, because of oxidative stress, deprivation of neurotrophic factors, etc. Since it has been hypothesized that the retinal ganglion cell death after axotomy is due to the generation of reactive oxygen species, we investigated whether NF-kB is involved in the retinal cell death after axotomy. This study was performed to investigate the role of NF-kB in retinal ganglion cell death after optic nerve transection. We used double staining experiment by using anti-NF-kB antibody and ethidium bromide to observe the correlation of NF-kB activation and the cell death. NF-kB was observed only in the surviving cells. NF-kB translocation was observed 3 days after the optic nerve transection. The NF-kB inhibitor, sulfasalazine, was used to block the activation of NF-kB in the axotomized retina, and the number of ganglion cells was quantified using retrograde in the presence or absence of sulfasalazine after axotomy. Inhibition of NF-kB by sulfasalazine accelerated the degeneration of ganglion cells in the retina. The results suggest that the activated NF-kB plays a protective role from the cell death in the injured ganglion cells. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Retina and photoreceptors
Keywords: NF-kB; Ganglion cell; Retina; Degeneration; Axotomy
1. Introduction There have been numerous studies on the regeneration
of the injured optic nerves by applying neuroprotective
Optic nerve (ON) transection causes irreversible degene- reagents or by nerve transplantation [11,42]. Neurotrophic
ration of the retinal ganglion cells (RGC) [29,39]. The factors such as BDNF, NGF, or NT3 / 4 can delay the cell
optic nerve provides the retina with various neurotrophic death, but cannot make them regenerate [33,42]. In
con-factors (BDNF, NGF, and NT3 / 4) that are essential for the trast, treatment of CNTF or a peripheral nerve graft seems
RGCs to survive. After axotomy, the ganglion cells to help regeneration of the RGCs, but those treatments
ultimately die due to the deprivation of neurotrophins [33], cannot make the RGCs survive [5]. Recently, it has been
altered gene expression [18,30,39], and various reactive reported that optic nerve transection showed typical
apo-oxygen species [15,16]. ptotic cell death pattern (e.g. activation of caspase, DNA
fragmentation, TUNEL positive, and chromatin condensa-tion, etc.) [14,29], and also the previous report showing that Bcl-2 overexpression and the introduction of bax
*Corresponding author. Tel.:182-2-590-2613; fax:182-2-533-3801.
E-mail address: [email protected] (C.-K. Joo). antisense RNA protected the axotomized retina from cell
death suggest that axotomized RGCs undergo apoptic cell intraperitoneal injection of chloral hydrate (400 mg / kg).
death [12,27]. All animals were treated in accordance with the ARVO
The cell death after axotomy seems to be a slow process statement for the Use of Animals in Ophthalmic and
because a significant number of RGCs still survive several Vision Research. The ON (optic nerve) transection was
days after axotomy. It has been hypothesized that RGC performed at 5 mm from the posterior pole of the eye
death is not an immediate result of axotomy injury, and without damaging the retinal blood vessel.
other factors (pro-apoptotic factors) may play a role in RGC death [18,29]. Levin suggests that the destructive
molecular events by axotomy lead to the functional failure 2.2. Electron microscopy
of intrinsic protective mechanisms, which in turn brings
cell death [18]. Thus, the interplay between pro- and The retina was fixed in 0.1 M sodium phosphate buffer
anti-apoptotic factors may determine the motility of cells. (PB, pH 7.4) containing 4% glutaraldehyde. After washing
NF-kB, a transcription factor, is one of the molecules with the same buffer, the retina was fixed in 1% osmium
involved in cell death. NF-kB exists in the cytoplasm as tetroxide. The retinal tissues were washed and dehydrated
heterodimers or homodimers of Rel related proteins. The using a series of ethanol, then subsequently cleared in
amino terminal region of the Rel homology domain propylene oxide and embedded in epon mixture. The tissue
contains DNA binding, dimerization, and nuclear localiza- preparation was sectioned at 90 nm by ultramicrotome, and
tion domains. The predominant form of NF-kB is com- observed under a transmission electron microscope.
posed of NF-kB1 (p50) and RelA (p65), and is associated
with inhibitor-kB (IkB) as an inactive form. Upon
stimula-tion, I-kB is phosphorylated by IkB kanases, ubiquitinated, 2.3. Immunohistochemistry for NF-kB and Thy-1
and subsequently processed to proteolytic degradation. The
freed NF-kB translocates from the cytoplasm to the The isolated retina tissue was washed in 0.1 M
phos-nucleus by exposing the nuclear localization signal and phate buffered saline (PBS, pH 7.4), and then dehydrated
then binds to the target genes to activate transcription through a graded series of ethanol, cleared in xylene, and
[22,25]. NF-kB is activated by various stimuli, including embedded in paraffin. Eight-micrometer-thick vertical
sec-stress or injury. So far, the known inducers for NF-kB tions were made with a microtome (Leica, Nussloch,
activation are interlukin (IL)-1, TNF alpha, bacterial Germany), and the following immunohistochemical
pro-lipopolysaccharides (LPS), sphingomyelinase, oxygen free cedure was performed at room temperature. Briefly, the
radicals, ultraviolet light and g-irradiation [9,26,34]. The endogeneous peroxidase was blocked by incubating the
activation of NF-kB as a pro-apoptotic factor has been specimens in 0.3% hydrogen peroxide (Sigma, St. Louis,
shown in neuronal cell death induced by TNF alpha, MO, USA) for 10 min and rinsed with PBS prior to
glutamate, and reactive oxygen species in vitro [7,8,20,28], antibody application. Nonspecific binding was blocked by
while the anti-degenerative function also has been sug- incubation with 20% normal horse serum (Santa Cruz
gested. The mechanism for anti-apoptotic role of NF-kB is Biotech., Santa Cruz, CA, USA) for 5 min. The retina
suggested as the elevation of Bcl-2 expression by NF-kB. sections were incubated consecutively with primary
anti-Furthermore, p50 knockout mice showed increased apop- body, rabbit anti-p65 (Santa Cruz Biotech.) or anti-Thy-1
tosis and the survival pathway by growth factors or (Santa Cruz Biotech.), biotinylated goat anti-rabbit IgG
cytokines also activate NF-kB through PI-3 kinase path- and streptavidin conjugated peroxidase (Santa Cruz
way [17,23,31,36,38,41,43]. Biotech.). The immunoreactivity was detected using 3,39
-In our previous report, NF-kB was observed in the diaminobenzidine detection system (Boehringer
Mann-ganglion cell layer after axotomy with immunohistochem- heim, Mannheim, Germany) and observed under a
micro-istry [1]. However, the activation or the role of NF-kB has scope with a DIC filter (Olympus, Tokyo, Japan).
not been explored. In this study, we investigated the role of
NF-kB in axotomized ganglion cells by showing whether
the cell death is accelerated or blocked by the inhibition of 2.4. Retrograde labeling
NF-kB activation.
The degeneration was assessed morphometrically by retrograde labeling of the cell bodies. Application of the dye involved re-exposure of the nerve without damaging
2. Materials and methods the retinal blood supply. One percent of fast blue, neuro-tracer dye, was deposited 1 mm from the distal border of
2.1. Animal model the injury site. Non-injured optic nerves were similarly
labeled. Forty-eight hours later, the retinas were prepared
Adult male Sprague–Dawley rats (200–250 g) were as flattened whole mounts and the labeled ganglion cells
2.5. Western blot analysis assessed using one-way ANOVA, and comparisons be-tween groups were performed using a Student’s t-test.
To detect the NF-kB transloction, the cytoplasmic and
nuclear extracts were separated. After axotomy, the retina
was isolated and stored at 2708C until further use. The 3. Results
retina was lysed in 10 ml of lysis buffer (10 mM HEPES,
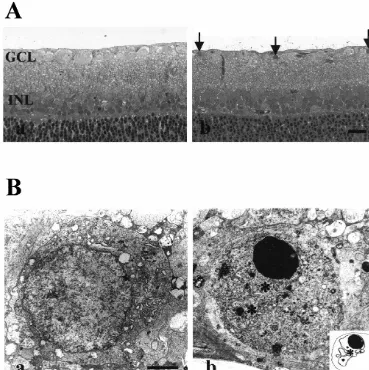
10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM 3.1. Optic nerve transection causes apoptotic cell death
dithiothreitol, and 1 mM phenylmethylsulfonyl fluoride in retinal ganglion cell layer
(PMSF)) and incubated on ice for 15 min. Six microliters
of 10% Nonidate P-40 was added to the suspension. After It has been shown that RGCs undergo apoptotic cell
centrifugation at 2000 rev. / min for 5 min, the supernatant death after optic nerve transection [29,33]. To demonstrate
was collected for cytoplasmic proteins. The nuclear pellet that our model system, an axotomized retina, is prepared
was resuspended in 10 ml of extract buffer (20 mM without damage to blood vessels near optic nerve, we
HEPES, pH 7.9, 400 mM KCl, 1 mM EDTA, 1 mM observed the retina with a light and an electron microscope
EGTA, 1 mM dithiothreitol, and 1 mM PMSF) and (Fig. 1). If the blood vessels were damaged, the ganglion
incubated for 30 min at 48C. The suspension was incubated cells die for necrosis rather than apoptosis, and also the
for 20 min at 48C and centrifuged at 15 000 rev. / min for cells in inner nuclear layer are also damaged in a day,
20 min. The supernatant was dialyzed for 3 h in dialysis which is a typical ischemic retina model [9]. The light
buffer (10 mM HEPES, 1 mM EDTA, 50 mM KCl, 20% (Fig. 1A) microscopic observation showed that there are
glycerol, 1 mM dithithreitol, and 1 mM PMSF). The still dying cells in ganglion cell layer 14 days after
supernatant was collected after centrifugation at 15 000 axotomy, which suggests that cell death seemed to be a
rev. / min for 20 min at 48C as nuclear protein and stored at slow process rather than a sudden cell death necrosis,
2708C. The amount of protein was determined using BCA which occurs mostly in a few days. Also, the cells in inner
protein assay kit (Sigma, St. Louis, MO, USA). nuclear layer were still intact at 14 days after axotomy. In
Thirty grams of protein from each sample was loaded on addition, electron microscopic observation showed that
sodium dodecyl sulfate (SDS) polyacrylamide gel electro- most of the dying ganglion cells showed apoptotic
appear-phoresis. After running the gel, the proteins were trans- ance, such as chromatin condensation and nuclear
frag-ferred to nitrocellulose membranes (hybond-C, Amersham mentation (Fig. 1B). The result showed that the RGC
Phamacia Biotech) at 300 mA for 1 h. Western blot degeneration started between 7 and 10 days after axotomy.
analysis was performed after blocking with 5% skim milk. These results showed that our model system was well
The membrane was incubated with polyclonal rabbit anti- prepared and did not cause damage to the blood vessels
NF-kB (p65) (1:1000) or anti-IkB (1:1000) (Santa Cruz next to the optic nerve.
Biotechnology, Santa Cruz, CA, USA) for 2 h and washed
three times in PBST (PBS containing 0.1% Twin 20) 3.2. Activated NF-kB co-localized with surviving cells
buffer. Then the membrane was incubated with horseradish
peroxidase (HRP) conjugated anti-rabbit IgG. After three NF-kB plays a role in cell death as a pro- or
anti-times of 10 min washing with PBST buffer, the HRP apoptotic transcription factor, depending on cell type or the
activity was visualized by applying chemiluminescent nature of injury [8,7,17,19,28,38,41]. It has been
hypoth-substrate (ECL, Amersham, Arlington Heights, IL, USA) esized that NF-kB is activated to transcribe the essential
followed by exposing the membrane to X-ray film. genes for cell death or survival [23]. Since whether or not
NF-kB is involved in axotomized retina has not been
explored, we determined the correlation between the
NF-2.6. Quantification of cell density in the ganglion cell kB activation and the cell death caused by axotomy. To
layer address the question, we performed immunohistochemistry
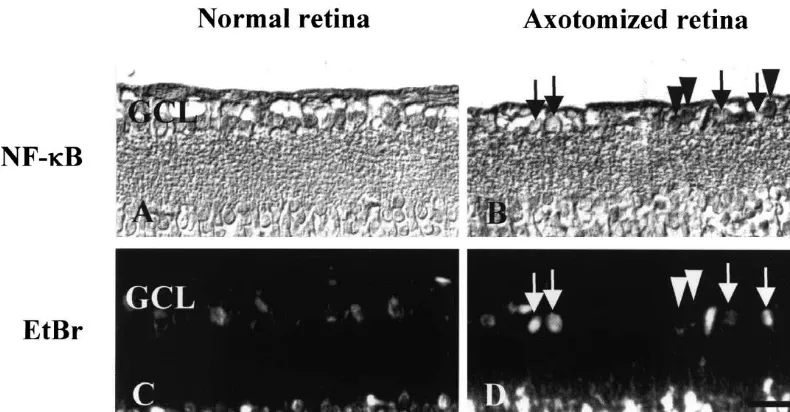
with the anti-NF-kB antibody and ethidium bromide
For each time point, five retinas from five animals were (arrows in Fig. 2B and D). Ethidium bromide (EtBr) stains
used to assess the density of the ganglion cells. The for the condensed chromosome, which is a marker for
number of cells was counted in 600 mm length of the apoptotic cell death.
ganglion cell layer. Twenty sections per retina were The results showed that the cells stained with EtBr were
observed for the cell counting. To inhibit the activation of not stained with NF-kB. On the other hand, the EtBr
NF-kB, 10 ml of 5 mM sulfasalazine (Sigma, St. Louis, negative cells were positively stained with the anti-NF-kB
MO, USA) in 25% dimethyl sulfoxide (DMSO) was antibody (arrowheads in Fig. 2B and D). This indicates
injected into the vitreous body 30 min prior to axotomy. that the surviving cells after axotomy were stained with
The control animals were injected with 25% DMSO only. NF-kB, but the dead or dying cells were not stained with
Fig. 1. (A) Light microscopy of axotomized retina. Fourteen days after axotomy, the retinas were collected and subjected to the light microscopic observation. Non-treated retina was healthy and normal (a), while axotomized retina (b) showed pyknotic nucli (arrows) in ganglion cell layer, but the cells in inner nuclear layer were intact. Bar520mm. (B) Electron microscopy of axotomized retina. Seven days after axotomy, the retinas were collected and subjected to the electron microscopic observation. Non-treated retinal ganglion cells were healthy and normal (a), while axotomized cells (b) showed the apoptotic cell death such as chromatin (arrows) condensation, nuclear fragment formation (asterisks, insert). Bar52mm.
more resistant to cell death when the retina is injured by to the translocation from the cytoplasm to the nucleus. We
axotomy. This suggests that NF-kB may play an anti- concluded that NF-kB is activated when the retina is
apoptotic role in axotomized retinas. injured by axotomy.
3.3. NF-kB activation 3.4. Inhibition of NF-kB activation
Since the translocation of NF-kB from the cytoplasm to In order to ascertain the role of NF-kB activation in
the nucleus is essential for NF-kB activation, we examined degenerating RGCs, we used sulfasalazine to inhibit the
its activation with Western blot analysis after separation of translocation of NF-kB [4,40]. The inhibitory effect of
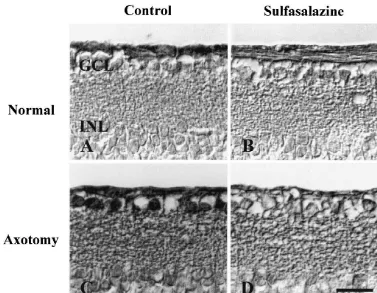
nucleic extract from cytoplasmic extract (Fig. 3). Fig. 3 sulfasalazine was confirmed with Western blot (Fig. 3C, D)
shows that a greater amount of NF-kB was observed in the and immunohistochemistry (Fig. 4C, D). The Western blot
nucleus than in the cytoplasm with the injury. The results showed that the treatment of axotomized retina with
translocated NF-kB increased maximally 3 days after 5 mM sulfasalazine did not increase the NF-kB in the
axotomy, and the activation of NF-kB persisted until 14 nuclear compartment while the axotomized retina showed
days after axotomy, even though the activation gradually a dramatic increase of NF-kB in the nuclear extract. The
decreased (data not shown). These results confirm that the immunohistochemistry results showed that the
Fig. 2. Immunohistochemistry of retina with anti-NF-kB antibody. The retina was collected 7 days after optic nerve transection. Positive immunoreactivity for NF-kB (arrow heads) appeared as dark staining in the cells (A, B), and EtBr (arrows) stained for the condensed chromosome which was shown by bright white dots (C, D). Neither the NF-kB activation, nor the nuclear condensation (A, C) was observed in the control. In ON transected retina, NF-kB activation (B) or nuclear condensation (D) was clearly observed. NF-kB activated cells were not morphologically changed yet in 7 days after the ON transection (B, D). GCL: Ganglion cell layer, Bar520mm.
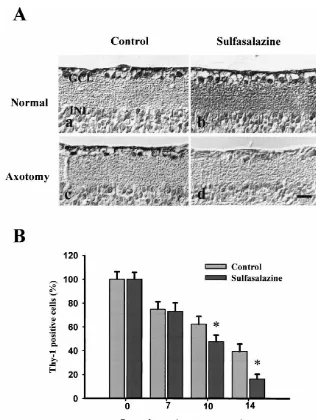
NF-kB labeled ganglion cells by 20% of the non-sul- fasalazine exhibited a marked decrease in the number of
fasalazine treated cells. These results showed that sul- Thy-1 labeled ganglion cells after axotomy (Fig. 5A).
fasalazine inhibits NF-kB activation in our model system. The difference in the number of Thy-1 positive ganglion
cells between the control and the sulfasalazine-treated
3.5. Role of NF-kB in axotomized retina group was observed from 10 days after axotomy. The
sulfasalazine-treated group contained less than 50% of the
To understand the role of NF-kB in degenerating number of Thy-1 positive cells found in the non-treated
retinas, we quantified the surviving cells with or without group. The most significant difference in the number of
sulfasalazine after axotomy. We stained the retina with the Thy-1 positive ganglion cells was shown at 14 days after
anti-Thy-1 antibody, specific for retinal ganglion cells. The the ON transection, when only 16.264.1% of Thy-1
Thy-1 labeled ganglion cells were counted at various time positive ganglion cells remained in the
sulfasalazine-points (0, 7, 10, and 14 days) after the ON transection. treated group compared to 39.366.4% in the non-treated
Compared to the control, the retinas treated with sul- group (Fig. 5B).
Fig. 4. Inhibition of NF-kB translocation by sulfasalazine 3 days after axotomy. (A) DMSO only; (B) sulfasalazine only; (C) DMSO treated axotomized retina; and (D) sulfasalazine treated axotomized retina. The translocation of NF-kB was inhibited by the treatment of sulfasalazine 3 days after optic nerve transection. GCL: Ganglion cell layer, INL: Inner nuclear layer, Bar520mm.
Even though Thy-1 stains only in RGCs, there is a or anti-apoptotic factor is determined by cell type or stress
¨
report that Thy-1 stains in Muller cells in some instances conditions [19,25,38].
[6,24]. To confirm the Thy-1 staining result, we tried Recently, an anti-apoptotic function of NF-kB has been
retrograde staining (Fig. 6), which stains neurons only in described by Van Antwerp et al., Wang et al. and
central nervous system. The data showed a similar result to Kaltshmidt et al. [13,23,41]. Anti-apoptotic role of NF-kB
the Thy-1 staining experiment, where the NF-kB active is largely substantiated by the report that Rel A (NF-kB
cells were shown to survive longer than the NF-kB p65) 2/2 knockout mice die at embryonic day 15–16
inactive cells. with extensive apoptosis in the liver and in tumor cells
[41]. Also, NF-kB inhibition by the overexpression of I-kB
potentiates amyloid-beta induced cell death [13]. In
addi-tion, NF-kB inhibition by a proteasome inhibitor was
4. Discussion reported to induce neuronal cell death in the hippocampus
[35].
In this study, we observed NF-kB activation in the On the other hand, protection from apoptosis may not be
surviving cells after optic nerve transection, and the a universal effect of NF-kB activation. Some reports have
inhibition of NF-kB promoted ganglion cell death. Our demonstrated the NF-kB activation in the cell death
results demonstrated that NF-kB plays a role as an anti- induced by glutamate-mediated excitotoxicity or reactive
apoptotic factor in the axotomized retinal ganglion cells. oxygen species, and showed that its inhibition protects the
NF-kB plays multiple roles in cell death, survival, cells from the cell death [3,7,8,28]. Schneider et al. have
proliferation and differentiation. It has been reported that reported that NF-kB activation promotes cell death in
NF-kB is activated by various stimuli [2,7,22,23,25]. NF- ischemic brain [32]. In addition, NF-kB activation was
kB activation has also been observed in the dopaminergic reported in the apoptosis induced by TNF alpha as well as
and hippocampal neurons in the patients with Parkinson’s p75NTR and oxidative stress [19,25,26]. Thus, the role of
and Alzheimer’s disease, respectively [10,20,28,37]. De- NF-kB in the programmed cell death as a pro-apoptotic or
spite numerous observations of NF-kB activation in the anti-apoptotic factor may depend on the nature of the cell
damaged neuronal cells, its role in cell death is not clearly type or stimulation [7,21].
Fig. 5. (A) Immunohistochemistry for Thy-1 positive ganglion cells in the retina. (a) Normal retina; (b) sulfasalazine injected normal retina; (c) axotomized retina; and (d) axotomized retina with the treatment of sulfasalazine. All the retinas were collected 14 days after axotomy. DMSO was injected for the control of sulfasalazine. The number of Thy-1 positive cells (dark staining) was decreased with the treatment of sulfasalazine in the axotomized retina, while sulfasalazine did not affect to the normal retina. Bar520mm. (B) Quantification of the immuhistochemistry of Thy-1 staining. The number of Thy-1 positive cells was dramatically decreased in the sulfasalazine treated retina compared to that of the retina treated with DMSO only in axotomized retina. The statistical analysis showed that sulfasalazine treated group is significatly lower in the surviving cell number compared to the non-treated group (asterisks, P50.0005).
cell layer 3 days after axotomy in our model system. In the transduction pathway [22,34]. The NF-kB activation
path-case of ischemic or traumatic injury, NF-kB translocation way in axotomy remains to be elucidated, since the
occurred at earlier time points within 2 h [32]. Since mechanism for NF-kB activation in axotomized cell death
ischemic injury releases various kinds of reactive oxygen has not been studied in axotomized retinal cell death.
species [20], NF-kB activation may not be surprising In this study, we demonstrated that NF-kB was activated
because NF-kB is the general transcription factor activated in the RGCs after axotomy and the inhibition of NF-kB
by reactive oxygen species. In contrast, the mechanism for translocation resulted in an increased number of
degenerat-the cell death after axotomy has not been clearly eluci- ing retinal ganglion cells. This indicates the protective role
dated. It was reported that nitric oxide synthase is stimu- of NF-kB in the ganglion cells from degeneration after
lated after axotomy [15,16], so nitric oxide might be one of axotomy. Our results in conjunction with those of other
the reactive oxygen species which activates NF-kB. In reports [3,7,19,21,41,43] suggest that the modulation of
many other studies, NF-kB was activated by the activation NF-kB signaling pathway may be important for regulating
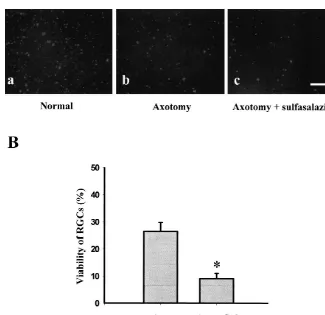
Fig. 6. (A) Retrograde staining of RGCs in retina. Unleisioned normal retina (a); 14 days after axotomy (b); 5 mM sulfasalazine injected 14 days after axotomy (c). One percent fast blue was loaded 1 mm from the distal border of the injury site. Then h later, the retinas were prepared as flattened mounts and the number of labeled RGCs were counted using fluorescence microscope. Bar5100mm. (B) Quantification of the retrograde staining after axotomy with or without treatment of 5 mM sulfasalazine (* P50.0002).
[6] I. Dabin, C.J. Banstable, Rat retinal Muller cells express Thy-1 Acknowledgements
following neuronal cell death, Glia 14 (1995) 23–32.
[7] M. Grilli, M. Memo, Possible role of NF-kB and p53 in the
This work was supported in part by the Korea Science glutamate-induced pro-apoptotic neuronal pathway, Cell Death
and Engineering Foundation (KOSEF) through the Brain Differ. 6 (1999) 22–27.
[8] M. Grilli, M. Pizzi, P.F. Memo, Spano. Neuroprotection by aspirin
Disease Research Center at Ajou University (to C.-K. Joo).
and sodium salicylate through blockade of NF-kB activation, Science 274 (1996) 1383–1385.
[9] W.F. Hughes, Quantitation of ischemic damage in the rat retina,
References Exp. Eye Res. 53 (1991) 573–582.
[10] S. Hunot, B. Brugg, D. Ricard, P.P. Michel, M.P. Muriel, M. Ruberg, B.A. Faucheux, Y. Agid, E.C. Hirsch, Nuclear translocation of [1] J.S. Choi, S. Kim-Yoon, C.K. Joo, NF-kB activation following optic
NF-kB is increased in dopaminergic neurons of patients with nerve transection, Korea J. Ophthalmol. 12 (1998) 19–24.
Parkinson disease, Proc. Natl. Acad. Sci. USA 94 (1997) 7531– [2] J.A. Clemens, D.T. Stephenson, E.P. Dixon, E.B. Smalstig, R.E.
Mincy, K.S. Rash, S.P. Little, Global cerebral ischeimia activates 7536.
¨ ¨
nuclear factor-kappa B prior to evidence of DNA fragmentation, [11] M. Hull, M. Bahr, Regulation of immediate-Early gene expression Mol. Brain Res. 48 (1997) 187–196. in rat retinal ganglion cells after axotomy and during regulation [3] J.A. Clemens, D.T. Stephenson, T. Yin, B. Smalstig, J.A. Panetta, through a peripheral nerve graft, J. Neurobiol. 25 (1994) 92–105.
¨
S.P. Little, Drug-induced neuroprotection from global ischemia is [12] S. Isenmann, S. Engel, F. Gillardon, M. Bahr, Bax antisense associated with prevention of persistent but not transient activation oligonucleotides reduce axotomy-induced retinal ganglion cell death of nuclear factor-kB in rat, Stroke 29 (1998) 677–682. in vivo by reduction of Bax protein expression, Cell Death Differ. 6 [4] B.N. Cronstein, C. Montesinos, G. Weissmann, Salicylates and (1999) 673–682.
sufaalazine, but not glucocorticoids, inhibit leukocyte accumulation [13] B. Kaltschmidt, M. Uherek, H. Wellmann, B. Volk, C. Kaltschmidt, by an adenosine-dependent mechanism that is independent of Inhibition of NF-kB potentiates amyloid b-mediated meuronal inhibition of prostaglandin synthesis and p105 of NF-kB, Proc. Natl. apoptosis, Proc. Natl. Acad. Sci. USA 96 (1999) 9409–9411.
¨ ¨
Acad. Sci. USA 96 (1999) 6377–6381. [14] P. Kermer, N. Klocker, M. Labes, M. Bahr, Inhibition of CPP32-[5] Q. Cui, Q. Lu, K.F. So, H.K. Yip, CNTF, not othertrophic factors, Like proteases rescues axotomized retinal ganglion cells from promotes axonal regeneration of axotomized retinal ganglion cells in secondary cell death in vivo, J. Neurosci. 18 (1997) 4656–4662.
¨
inhibition of nitric oxide synthase potentiates the neurotrophic [30] G.A. Robinson, Change in the expression of transcription factors effects of brain-derived neurotrophic factor on axotomized retinal ATF-2 and Fra-2 after axotomy and duering regeneration in at retina ganglion cells in vivo, J. Neurosci. 18 (1998) 1038–1046. ganglion cells, Mol. Brain Res. 41 (1996) 57–64.
[16] P.D. Koeberle, A.K. Ball, Nitric oxide synthase inhibition delays [31] J.A. Romashkova, S.S. Makarov, NF-kappa B is a target of AKT in axonal degeneration and promotes the survival of axotomized retinal anti-apoptotic PDGF signalling, Nature 401 (1999) 86–90. ganglion cells, Exp. Neurol. 158 (1999) 366–381. [32] M. Schneider, A. Martin-Villalba, F. Weih, J. Vogel, T. Wirth, M. [17] S.Y. Lee, D.R. Kaufman, A.L. Mora, A. Santana, M. Boothby, Y. Schwaninger, NF-kB is activated and promotes cell death in focal
Choi, Stimulus-dependent synergism of the antiapoptotic tumor cerebral ischemia, Nat. Med. 5 (1999) 554–559.
necrosis fantor receptor-associated factor 2 (TRAF2) and nuclear [33] S. Shen, A.P. Wiemelt, F.A. McMorris, B.A. Barres, Retinal factorkB pathway, J. Exp. Med. 188 (1998) 1381–1384. ganglion cells lose trophic responsiveness after axotomy, Neuron 23 [18] L.A. Levin, Intrinsic survival mechanisms for retinal ganglion cells, (1999) 285–295.
Eur. J. Ophthalmol. Suppl. 1 (1999) S12–S16. [34] I. Stancovski, D. Baltimore, NF-kB activation: The IkB kinase [19] F. Lezoulalc’h, Y. Sagara, F. Holsboer, C. Behl, High constitutive revealed?, Cell 91 (1997) 299–302.
NF-kB activity mediates resitance to oxidative stress in neuronal [35] G. Taglialatela, J.A. Kaufmann, A. Trevino, J.R. Perez-Polo, Central cells, J. Neurosci. 18 (1998) 3224–3232. nervous system DNA fragmentation induced by the inhibition of [20] M.P. Mattson, International review of Neurobiology, Free Radicals, nuclear factor kappa B, Neuroreport 9 (1998) 489–493.
Calcium, and the Synaptic Plasticity-cell Death Continuum: Emerg- [36] M. Tamatani, Y.H. Che, H. Matsuzaki, S. Ogawan, H. Okado, S. ing Roles of the Transcription Factor NF-kB, Vol. 42, Academic Miyake, T. Mizuno, M. Tohyama, Tumor necrosis factor induces Press, San Diego, CA, 1998. Bcl-2 and Bcl-x expression through NFkB activation in primary [21] M.W. Mayo, C.Y. Wang, P.C. Cogswell, K.S. Rogers-Graham, S.W. hippocampal neurons, J. Biol. Chem. 274 (1999) 8531–8538.
Lowe, C.J. Der, A.S. Baldwin, Requirment of NF-kB activation to [37] K. Terai, A. Matsuo, P.L. McGeer, Enhancement of immuno-suppress p53-independent apoptosis induced by oncogenic ras, reactivity for NF-kappa B in the hippocampal formation and Science 278 (1997) 1812–1815. cerebral cortex of Alzheimer’s disease, Brain Res. 735 (1996) [22] F. Mercurio, A.M. Manning, Multiple signals converging on NF-kB, 159–168.
Curr. Opin. Cell Biol. 11 (1999) 226–232. [38] D.J. Van Antwerp, S.J. Martin, I.M. Verma, D.R. Green, Inhibition of [23] G. Middleton, M. Hamanoue, Y. Enokido, S. Wyatt, D. Pennica, E. TNF-induced apoptosis by NF-kB, Trends Cell Biol. 8 (1998)
Jaffray, R.T. Hay, A.M. Davies, Cytokine-induced nuclear factor 107–111.
kappa B activation promotes the survival of developing neurons, J. [39] M.P. Villegas-Perez, M. Vidal-Sanz, M. Rasminsky, G.M. Bray, A.J. Cell Biol. 148 (2000) 325–332. Aguayo, Rapid and protracted phases of retinal ganglion cell loss [24] M.S. Nash, N.N. Osborne, Assessment of Thy-1 mRNA leveled as follow axotomy in the optic nerve of adult rats, J. Neurobiol. 24
an index of retinal ganglion cell damage, Invest. Ophthalmol. Vis. (1993) 23–36.
Sci. 40 (1999) 1293–1298. [40] C. Wahl, S. Liptay, G. Alder, R.M. Schimid, Sulfasalazine: a potent [25] L.A.J. O’Neill, C. Kaltshmidt, NF-kB: a crucial transcription factor and specific inhibitor of neuclear factor kappa B, J. Clin. Invest. 101
for glial and neuronal cell function, Trends Neurosci. 20 (1997) (1998) 1163–1174.
252–258. [41] C.Y. Wang, M.W. Mayo, R.G. Korneluk, D.V. Goeddel, A.S. Bal-[26] B. Pettmann, C.E. Henderson, Neuronal cell death, Neuron 20 dwin, NF-kB antiapoptosis: Induction of TRAF1 and TRAF2 and (1998) 633–647. c-IAP1 and c-IAP2 to suppress caspase-8 activation, Science 281 [27] V. Porciatti, T. Pizzorusso, M.C. Cenni, L. Maffei, The visual (1998) 1680–1683.
response of retinal ganglion cells is altered by optic nerve transec- [42] Q. Yan, J. Wang, C.R. Matheson, J.L. Urich, Glial cell line-derived tion in transgenic mice overexpressing Bcl-2, Proc. Natl. Acad. Sci. neurotrophic factor (GDNF) promotes the survival of axotomized USA 93 (1996) 14955–14959. retinal ganglion cells in adult rats: comparison to and combination [28] A. Post, F. Holsboer, C. Behl, Induction of NF-kB activity during with brain-derived neurotrophic factor (BDNF), J. Neurol. 38
haloperidol-induced oxidative toxicity in clonal hippocampal cells: (1999) 382–390.
suppression of NF-kB and neuroprotection by antioxidants, J [43] Z. Yu, D. Z, A.J. Bruce-Keller, M.S. Kindy, M.P. Mattson, Lack of Neurosci. 18 (1998) 8236–8246. the p50 subunit of nuclear factor-kB increases the vulnerability of [29] H.A. Quigley, R.W. Nickells, L.A. Kerrigan, M.E. Pease, D.J. hippocampal neurons to excitotoxic injury, J. Neurosci. 19 (1999)
Thibault, D.J. Zack, Retinal ganglion cell death in experimental 8856–8865. glaucoma and after axotomy occurs by apoptosis, Invest.