Essential roles of noise in neural coding and in studies of
neural coding
Edwin R. Lewis
a,*, Kenneth R. Henry
b, Walter M. Yamada
caDepartment of EECS,Uni
6ersity of California,Berkeley,CA94720-1770,USA bDepartment of Psychology,Uni
6ersity of California,Da6is,CA95616,USA cDepartment of Physiological Science,Uni
6ersity of California,Los Angeles,CA90095,USA
Abstract
We present examples of results from our studies of auditory primary afferent nerve fibers and populations of such fibers in the frog and gerbil. We take advantage of the natural dithering effect of internal noise, where it is sufficient, to construct highly predictive descriptive models (based on the Wiener series with kernels derived from white-noise analysis). Where the internal noise is insufficient, we enhance dithering by applying external acoustic noise together with our stimuli. Using acoustic noise as a background sound, orthogonal to the stimulus waveform, we show that under some circumstances such background sound can enhance the ability of individual fibers and populations of fibers to encode the stimulus waveform. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Wiener series; Dithering with noise; Auditory spectrotemporal filters; Coding in auditory afferents
www.elsevier.com/locate/biosystems
1. Introduction
In his theoretical studies of action potentials, FitzHugh (1955, 1961) concluded that the under-lying dynamics were represented well by a state-plane divided by a single line segment (a separatrix) into two regions. In one of the two regions, all trajectories corresponded to full am-plitude spikes; all trajectories originating in the other region corresponded to graded responses. The resting state of the system was represented by a stable point in the graded-response region. All
trajectories, both full-spike and graded-response, converged on that point. Thus, full-spike trajecto-ries eventually carried the system back into the region of graded responses. This required that the full-spike region and the graded-response region merge, beyond the end of the separatrix. A brief stimulus that moved the state of the system from the resting state to any point on the near side of the separatrix produced a graded response; one that moved the state of the system to any point on the far side of the separatrix produced a full spike. For both classes of trajectory, the closer the tra-jectory was to the separatrix, the farther along the separatrix the trajectory coursed before diverging away from it. Thus, FitzHugh’s state-plane model described a system capable of graded responses
* Corresponding author. Tel.: +1-510-6426954; fax: + 1-510-6438426.
E-mail address:[email protected] (E.R. Lewis).
with amplitudes ranging from zero to that of a full spike.
A commonly-cited feature of action potentials in real neurons, however, is the all-or-none (threshold) property. The amplitudes of graded responses at spike triggers usually are limited to values very much less than the amplitude of a full spike. Responses with intermediate amplitudes usually are not seen.
In the region of the state plane reachable from the resting state by means of brief stimuli, both classes of trajectories (full spike and graded re-sponse) diverged strongly from the separatrix. This divergence gradually became weaker toward the far end of the separatrix, but it remained strong well along the way. Thus, where they could be reached by brief stimuli, the trajectories close to the separatrix were concentrated on a state-plane structure very much like a knife-edge ridge. A tiny nudge to one side or the other would move
the state of the system off of the ridge and onto a trajectory moving rapidly away, toward a full spike or toward the resting point. FitzHugh con-cluded that the noise in the real system (but not included explicitly in the state-plane model) would provide such nudges and would thus make it virtually impossible for the system to remain long on the knife-edge ridge. Thus, the presence of noise would create what FitzHugh called a ‘‘no-man’s land,’’ a large area into which the system was denied entry by the presence of noise. Be-cause all trajectories corresponding to graded re-sponses of intermediate or large amplitude passed through this area, such responses were not avail-able. Thus, FitzHugh concluded that the spike trigger owes its all-or-none (threshold) property to the presence of noise.
Attacking this problem quantitatively with the state-plane model, Clay (1976) reached the same
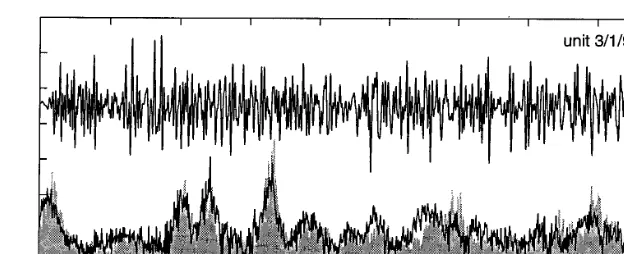
Fig. 1. Prediction of the PSTH by the first-order term of the Wiener series. In auditory units tuned to low frequencies, the instantaneous spike rate typically exhibits a large component of linear response to a stimulus waveform. For a given unit, this response component can be predicted by the first-order term of the Wiener series (convolution of the first-order Wiener kernel with the stimulus waveform). (A) First-order Wiener kernel for an auditory unit from the American bullfrog (Rana catesbeiana). Horizontal axis shows time in ms. (B) Amplitude component of the discrete Fourier transform of the waveform in A.Vertical axis:amplitude in decibels.Horizontal axis:frequency in Hertz. (C) A 200-ms segment of band-lim-ited white noise (50 – 500 Hz) (smooth line) that served as a stimulus waveform of arbitrary complexity, along with the PSTH (ragged line) generated from 6798 spikes in response to 3000 repetitions of that waveform, presented without a back-ground of continuous noise stimulus. Note the lack of corre-spondence between the peaks and troughs of the stimulus waveform and those of the instantaneous spike rate. (D) PSTH shown again, along with response prediction (smooth line) produced by convolution of the first-order Wiener kernel (panel A) with the stimulus waveform in panel C. Note the close correspondence between the peaks and troughs of these two functions. Although this unit had a low background spike rate, dithering by its internal noise clearly was sufficient to give the model predictive power. Because it represents only the linear responsiveness of the system, the first-order Wiener kernel cannot describe non-linearities, such as the clipping of the instantaneous spike rate at 0 spikes per second. Clipping could have been eliminated in the PSTH by presenting the repeated waveform in a background of non-repeating white noise.
conclusion as FitzHugh, but he also showed that the amplitudes available to the graded responses should increase conspicuously as the speed of the channel kinetics involved in spike triggering in-creased. If the state of the system could move more rapidly along the knife edge ridge, its chances of being nudged off of the ridge were reduced. Furthermore, the farther it was able to move along the ridge, the less steep were the ridge edges it faced, making further motion along the ridge more probable. One way to increase the speed of channel kinetics is to increase tempera-ture. Cole et al. (1970) in fact had already shown that the all-or-none spike property of the squid giant axon gives way to completely graded re-sponse (all amplitudes between zero and full-spike amplitude are available) when the axon is warmed about 20°C above its normal temperature. This increased the channel kinetics by about an order of magnitude, evidently without increasing the noise amplitude enough to compensate. All of this suggests that FitzHugh was correct, the spike trigger does owe its threshold properties to the presence of noise.
At nearly the same time that FitzHugh was drawing his conclusions, Lowenstein (1956) was asserting that with respect to external sensory input, randomly firing, spontaneously-active pri-mary sensory neurons have no threshold. In other words, he was asserting that the response of a primary sensory axon (a change in the instanta-neous spike rate) to an arbitrarily weak sensory input could be observed if one applied the stimu-lus repeatedly and had the patience to wait for the averaged response to emerge from the noisy back-ground spike rate. This notion subsequently was explored quantitatively by Stein and coworkers (Stein, 1970; French and Stein, 1970), and later by others, including our laboratories (e.g. Yu and Lewis, 1989). The conclusion reached by all is the following: independent noise at the spike trigger of each member of a population of axons can counteract the effects of threshold (the all-or-none property) and allow the population to encode continuous input signals in a continuous manner, to signal levels well below the threshold of the
individual spike trigger. This was essentially Lowenstein’s conclusion as well. It is analogous to dithering, which is employed by engineers to soften the effects of the lowest-level bit in digitally recorded waveforms (Vanderkooy and Lipshitz, 1984). In dithering (which relies on a very high sampling rate in a single channel rather than on a large number of parallel channels), added noise makes the system responsive to signal amplitudes that otherwise would be below the threshold for that lowest-level bit. The process also has ap-peared in the literature as the essential ingredient in a phenomenon labeled ‘‘stochastic resonance’’ (Wiesenfeld and Moss, 1995).
One can summarize all of these results as fol-lows: regarding threshold, noise giveth and noise taketh away. It is the taking away part that has been especially interesting to people in the field (hearing research) of the three authors of this paper.
2. Making the spike trigger invisible
The temporal coding of complex acoustic wave-forms by peripheral auditory nerve fibers (individ-ually and collectively) has been a central theme of many investigations (e.g. Young and Sachs, 1979; Bodnar and Capranica, 1994). Probably because frogs are known to be especially responsive to the temporal aspects of acoustic stimuli, most such studies in recent years have been focussed on those animals. A major goal of these studies has been the derivation of a descriptive model of the primary auditory unit (afferent axon along with its peripheral tuning structures) that can predict the temporal patterns of its responses to acoustic waveforms of arbitrary complexity (e.g. Lim, 1990; Kumerasen et al., 1996).
Fig. 2. In auditory units tuned to very high frequencies, the instantaneous spike rate typically exhibits no linear response to a stimulus waveform. The upper line shows a complex stimulus waveform that was presented 6392 times to such a unit in the frog ear. It is a 100-ms segment of band-limited (600 – 3200 Hz) white noise. The stimulus amplitude was 79 dB SPL. Dithering was accomplished in this case by presenting the stimulus in a background of non-repeating noise (81 dB SPL, 300 – 5,400 Hz). Along with the stimulus is shown the PSTH (solid gray figure) of the unit’s response (derived from 9,270 spikes) and the predicted PSTH (ragged dark line along the upper edge of the PSTH). The prediction was derived by adding the zero-order and second-order terms of the Wiener series (Yamada and Lewis, 1999). Note that the presence of the non-repeating background acoustic noise in this case was sufficient to prevent the sort of clipping (at zero spikes per second) seen in the PSTH of Fig. 1. We also made predictions (not shown) from a simplified version of the second-order term, computed by convolving the stimulus waveform with a linear-filter function (the highest-ranking eigenvector from singular-value decomposition of the second-order kernel), then taking the square of the envelope of the result (the filtered waveform). In fact, the predictions obtained in this way consistently were more faithful (in terms of rms error) than those obtained from the entire kernel (Yamada and Lewis, 1999).
ear, that impose randomness (dithering) on the responses to the stimulus waveform. In such cases, prediction with a model is limited to re-sponse statistics. The statistic employed most widely by hearing researchers is the average tem-poral pattern of spikes, or peristimulus time his-togram (PSTH), taken over many presentations of the stimulus waveform. Each PSTH bin represents a segment of time whose duration is equal to the unit of temporal resolution (the bin sampling time during each stimulus presentation), which is an indivisible instant of time in the histogram’s dis-crete-time presentation of the data. Division of the total spike count in a bin by the total time represented by that bin (bin sampling time during each stimulus presentation times the number of presentations used to construct the PSTH) yields the instantaneous spike rate for the corresponding time in the stimulus cycle. Thus, the PSTH yields a discrete-time estimate of the temporal pattern of the modulation of spike rate, instant by instant, through the time course of the stimulus wave-form. When the dithering imposed by the internal noise of the ear is sufficient, this temporal models (French and Stein, 1970; Yu and Lewis,
1989). When a stimulus waveform is applied re-peatedly to an undithered spike-trigger model, the spikes occur at precisely the same place in the stimulus cycle each time the stimulus is presented. The timing is determined by the particulars of the spike-trigger model (e.g., dynamics of accommo-dation and refractoriness). Thus, evidently, in most frog auditory units, the timing of spikes in response to an acoustic stimulus waveform de-pends on the particulars of the spike trigger as much as it does on the properties of the peripheral tuning structure. This seems to have made predic-tive modeling very difficult for these units. To date, nobody has succeeded in creating a model that will predict the temporal responses of such a unit to a waveform of arbitrary shape.
In mammalian auditory units and in a few frog auditory units, including all of those with substan-tial background spike activity, the temporal
pat-terns of spike responses tend to vary
Fig. 3. Responses of a gerbil cochlear unit (Lewis and Henry, 1995) to a probe stimulus (a 73-dB (SPL), 700-Hz tone burst). The unit was tuned to 8 kHz, where its threshold was 34 dB SPL. The upper PSTH shows the smoothed response to 260 presentations of the probe stimulus alone; the unit clearly responded to the abrupt onsets and offsets of the tone burst. The lower PSTH shows the smoothed response to 840 presen-tations of the same probe stimulus together with a 51-dB (SPL) noise burst of the same duration. The noise bandwidth was 9.7 kHz. In the presence of the noise-burst, the probe elicited an unclipped sinusoidal modulation of instantaneous spike rate strongly phase-locked to the 700 Hz tone.
be predictive. Figs. 1 and 2 show examples of the power of the model to predict PSTHs in response to an arbitrarily complex stimulus (a repeated segment of band-limited white noise).
Fig. 4. The left-hand panel shows the extracellular voltage taken over approximately the first two millimeters of the cochlear nerve of the gerbil, averaged over approximately 500 repetitions of a near-threshold (13-dB SPL) probe stimulus. The stimulus comprised two frequency components (10.00 and 10.93 kHz) of equal amplitude. The adjacent right-hand panels show the corresponding amplitude spectra (from discrete Fourier transform). The 930-Hz sinusoidal component of the voltage has been shown to be the consequence of the summed sodium currents from all of the spiking zones (nodes of Ranvier) along the two-millimeter nerve segment (Henry, 1995). It thus corresponds to a 930-Hz modulation of instanta-neous spike rate over a population of units tuned to the neighborhood of the probe-stimulus frequency (10 – 11 kHz). Cochlear units are unable to respond linearly at frequencies as high as 10 kHz. Like high frequency frog units, however, they are presumed to produce a component of instantaneous spike rate that is proportional to the square of the (positive) envel-ope of the linearly filtered stimulus waveform. In this case, that would be a distorted 930-Hz sinusoid. The top waveform in the figure contains the response to the probe stimulus alone. The middle waveform contains the response to the probe stimulus plus a 10-ms noise burst (10 dB SPL, 300 – 17 000 Hz) added in the midst of the probe. The bottom waveform contains the response to the probe stimulus plus a 30-ms noise burst (also 10 dB SPL, 300 – 17 000 Hz). The 930-Hz compo-nent of instantaneous spike rate, taken over the entire popula-tion of cochlear-nerve axons, clearly was increased by the presence of the noise. This implies that the noise increased the number of units responding to the envelope of the filtered probe stimulus and/or increased the responsiveness of the units that already had been responding to the envelope of the filtered probe in the absence of the noise.
pattern can be predicted remarkably faithfully by Wiener series (de Boer and de Jongh, 1978; Wolodkin et al., 1996). When the dithering is not sufficient, as is true of many frog auditory units, it often can be made sufficient by addition of external (acoustic) noise to the stimulus waveform (Yamada, 1997; Yamada and Lewis, 1999). For primary auditory units, the kernels of the Wiener
series traditionally are based on reverse
3. Making peripheral axons more sensitive to pure sine waves
The inspiration for Lowenstein’s conclusions regarding the effects of dithering came largely from experiments in which simple stimuli, such as velocity ramps and sinusoids, were employed. The effects are especially clear with sinusoids. These have been applied widely in physiological studies of acoustic sensors and sensors of head orienta-tion and moorienta-tion. When the frequency of such a stimulus is sufficiently low (less than about 4 kHz in mammals, less than about 800 Hz in amphibi-ans, reptiles and fish), the instantaneous spike rate of an inner-ear axon responsive to that frequency will show a component that is modulated at that frequency and very easy to detect (by phase-locked analysis). If the sinusoidal stimulus is suffi-ciently small and there is a suffisuffi-ciently large background component of firing (i.e. a sufficiently large d.c. component of instantaneous spike rate), then the modulation can be very close to linear. Sufficiency for the d.c. spike rate component in that case is slightly greater than half of the peak-to-peak linear amplitude of the nearly linear mod-ulation. As a consequence of the circuit-theory metamodel and the extensive body of knowledge associated with it (e.g. Lewis, 1996), nearly linear dynamics are richly interpretable in terms of un-derlying biophysics. Thus, where they lead to such dynamics, sinusoids are excellent probe stimuli.
For gerbil cochlear units, a small-amplitude sinusoidal stimulus was used as probe to measure the changes in inner-ear auditory sensitivity in response to changes in ambient sound levels. The background sound was band-limited white noise. Sudden increases in the amplitude of that noise typically produced sudden transient increases in the linear responsiveness of the system to the probe stimulus (Lewis and Henry, 1995). The most conspicuous examples occurred in units tuned to high frequencies (e.g. the unit in Fig. 3). The noise was turned off and on and the axons exhibited no linear response at all to a low-fre-quency probe sinusoid when the noise was off. When the noise was on, the probe produced close to 100% sinusoidal modulation of instantaneous spike rate. Thus, axons that were unresponsive to
the probe in the absence of background sound became very responsive when that sound was added. This means that adding the background sound (a noise signal in this case) increased the population of axons in the auditory nerve that responded to the probe stimulus. Whole-nerve measurements (Fig. 4) strongly support this conclusion.
4. Uncited reference
Yamada et al. (1996)
Acknowledgements
Research supported by Grant DC00112 from the National Institute of Deafness and Commu-nicative Disorders. We thank Eva Hecht Poinar for help with the illustrations.
References
Bodnar, D.A., Capranica, R.R., 1994. Encoding of phase spectra by the peripheral auditory system of the frog. J. Comp. Physiol. A 174, 157 – 171.
Clay, J.R., 1976. A stochastic analysis of the graded excitatory responses of nerve membrane. J. Theoret. Biol. 59, 141 – 158.
Cole, K.S., Guttman, R., Bezanilla, F., 1970. Nerve membrane excitation without threshold. Proc. Natl. Acad. Sci. USA 65, 884 – 891.
de Boer, E., de Jongh, H.R., 1978. On cochlear encoding: potentialities and limitations of the reverse-correlation technique. J. Acoust. Soc. Am 63, 115 – 135.
FitzHugh, R., 1955. Mathematical models of threshold phe-nomena in the nerve membrane. Bull. Math. Biophys. 17, 257 – 278.
FitzHugh, R., 1961. Impulses and physiological states in theo-eretical models of nerve membrane. Biophys. J. 1, 445 – 466.
French, A.S., Stein, R.B., 1970. A flexible neural analog using integrated circuits. IEEE Trans. Biomed. Eng. BME-17, 248 – 253.
Henry, K.R., 1995. Auditory nerve neurophonic recorded from the round window of the Mongolian gerbil. Hear. Res. 90, 176 – 184.
C.R., Hecht-Poinar, E. (Eds.), Diversity in Auditory Me-chanics. World Scientific, Singapore, pp. 190 – 196. Lewis, E.R., Henry, K.R., 1995. Nonlinear effects of noise on
phase-locked cochlear-nerve responses to sinusoidal stim-uli. Hear. Res. 92, 1 – 16.
Lewis, E.R., 1996. A brief introduction to network theory. In: Berger, S.A., Goldsmith, W., Lewis, E.R. (Eds.), Introduc-tion to Bioengineering. Oxford University Press, Oxford, pp. 261 – 338.
Lim, D., 1990. Representation of complex sounds in the peripheral auditory nervous system of the green treefrog. Ph.D. Dissertation, Cornell University, pp. 1 – 153. Lowenstein, O., 1956. Peripheral mechanisms of equilibrium.
Br. Med. Bull. 12, 114 – 118.
Stein, R.B., 1970. The role of spike trains in transmitting and distorting sensory signals. In: Schmitt, F.O. (Ed.), The Neurosciences. Rockefeller Press, New York, pp. 597 – 604. Vanderkooy, J., Lipshitz, P., 1984. Resolution below the least significant bit in digital systems with dither. J. Audio Eng. Soc. 32, 106 – 113.
van Dijk, P., Wit, H.P., Segenhout, J.M., Tubis, A., 1994. Wiener kernel analysis of inner ear function in the Ameri-can bullfrog. J. Acoust. Soc. Am. 95, 904 – 919.
Wiesenfeld, K., Moss, F, 1995. Stochastic resonance and the benefits of noise from ice ages to crayfish and SQUIDs. Nature 373, 33 – 36.
Wolodkin, G., Yamada, W.M., Lewis, E.R., Henry, K.R., 1996. Spike rate models for auditory fibers. In: Lewis, E.R., Long, G.R., Lyon, R.F., Narins, P.M., Steele, C.R., Hecht-Poinar, E. (Eds.), Diversity in Auditory Mechanics. World Scientific, Singapore, pp. 104 – 110.
Yamada, W.M., 1997. Second-order Wiener-kernel analysis of auditory afferent axons of the North American bullfrog and Mongolian gerbil responding to noise. Doctoral Dis-sertation, Graduate Group in Neurobiology, University of California, Berkeley, pp. 1 – 280.
Yamada, W.M., Lewis, E.R., 1999. Predicting the temporal responses of non-phase-locking bullfrog auditory units to complex acoustic waveforms. Hear. Res. 130, 155 – 170. Yamada, W.M., Wolodkin, G., Lewis, E.R., Henry, K.R.,
1996. Wiener kernel analysis and the singular-value decom-position. In: Lewis, E.R., Long, G.R., Lyon, R.F., Narins, P.M., Steele, C.R., Hecht-Poinar, E. (Eds.), Diversity in Auditory Mechanics. World Scientific, Singapore, pp. 111 – 118.
Young, E.D., Sachs, M.B., 1979. Representation of steady-state vowels in the temporal aspects of the discharge patterns of populations of auditory-nerve fibers. J. Acoust. Soc. Am. 66, 1381 – 1403.
Yu, X.Y., Lewis, E.R., 1989. Studies with spike intitiators: linearization by noise allows continuous signal modulation in neural networks. IEEE Trans. Biomed. Eng. 36, 36 – 43.