www.elsevier.com / locate / bres
Research report
A comparison of morphine analgesic tolerance in male and female
mice
a,b,c ,
*
c b,cBenjamin Kest
, Christina Palmese , Eileen Hopkins
a
Department of Psychology(4S-223), The College of Staten Island /City University of New York, 2800 Victory Blvd., Staten Island, NY 10314, USA
b
CSI /IBR Center for Developmental Neuroscience, Staten Island, NY 10314, USA
c
Neuropsychology Doctoral Subprogram, Queens College /City University of New York, Flushing, NY 11367, USA Accepted 5 July 2000
Abstract
Studies comparing morphine tolerance in males and females are rare, and all studies to date have utilized the rat. To generalize from findings with rats morphine tolerance was investigated in male and female mice using the tail-withdrawal test. Three and 7 days of systemic morphine injections produced significant but unequal rightward shifts in the morphine dose–response curve such that females displayed greater increases in analgesic ED50 values when compared to males. In a separate experiment, males and females displayed similar reductions in morphine analgesic sensitivity when %MPE (maximum possible effect) and %total (area under the curve) were compared after 3 days of morphine. Differences in initial morphine sensitivity between sexes were not observed in either study. The data demonstrate that, in contrast to rats, female mice undergo greater reductions in morphine analgesia relative to males following chronic morphine, but this sex difference may depend on the method of assessing analgesia. Furthermore, the duration and / or cumulative dose of morphine treatment does not affect the expression of sex differences in morphine tolerance. 2000 Elsevier Science B.V. All rights reserved.
Theme: Sensory systems
Topic: Pain modulation: pharmacology
Keywords: Morphine; Tolerance; Sex differences; Analgesia
1. Introduction morphine administration is analgesic tolerance, or loss in the relative analgesic potency of morphine. Tolerance It is becoming increasingly appreciated that sex may results from a myriad of complex molecular and biochemi-determine opioid analgesic sensitivity in both human and cal changes whose relative contributions are as yet not rodent populations (see reviews [5,28]). With regard to clearly understood [10,14,16,34]. The relative participation morphine, males typically display greater analgesic sen- of any or several of these variables in tolerance may sitivity than females across several nociceptive assays depend on several interacting methodological factors such following systemic administration in both rats and mice as dose and dosing schedules, route of administration, and [2,7–9,19,21–24]. A strong case that sex differences in the nociceptive response under study [3,15,35]. It is morphine analgesia may be mediated by differential central therefore not surprising that differences in initial sensitivity nervous system (CNS) mechanisms can be made based on to morphine in rats and mice are often not predictive of the the observations that males of both species display greater magnitude in tolerance subsequent to chronic morphine analgesic effect than females following central administra- administration [17,18,30,32,36,37]. Thus, despite apparent
tion [6,25,29,31]. sex differences in morphine analgesia, it is not possible to
One of the most noticeable consequences of repeated predict whether male and female rodents will undergo similar changes in morphine analgesic sensitivity following chronic administration. Badillo-Martinez [3] reported a
*Corresponding author. Tel.: 11-718-982-4070; fax: 1
1-718-982-greater attenuation of morphine analgesia on the hot-plate
3794.
E-mail address: [email protected] (B. Kest). in male relative to female rats treated daily with morphine
over 14 days. A similar pattern of greater tolerance in Abuse (Rockville, MD, USA), and was dissolved in 0.9% males relative to females on the hot-plate test was ob- physiological saline. All morphine and saline control served after twice daily or once weekly morphine in- injections were delivered via subcutaneous (s.c.) injection jections as well [11]. Studies using the tail-flick test in a volume of 10 ml / kg.
however present inconsistent findings. Whereas repeated
administration of morphine at weekly intervals for 3 weeks 2.2. Tail-withdrawal assay resulted in a greater loss in total morphine analgesia (area
under the curve; AUC) in males relative to females [11], Mice were assessed for nociceptive sensitivity on the no significant sex differences in morphine ED50 estimates 498C tail-withdrawal test. In this assay of acute, thermal were found following twice daily morphine injections for 7 nociception, the mouse is gently restrained and the distal days in either of the two strains of rats tested [20]. These half of the tail is immersed in water maintained at conflicting findings may be attributable to differences in 49.060.28C by an immersion circulator pump (Fisher methodology between the two studies. Although the identi- Isotemp Model 71). Latency to reflexive withdrawal of the cal nociceptive assay was employed, they differed in the tail was measured twice by an experimenter to the nearest frequency (weekly vs. twice daily injections) and duration 0.1 s, with each determination separated by 20 s. The two (3 weeks vs. 7 days) of morphine injections, as well as in determinations were averaged to reflect each animal’s the method of quantifying analgesia (AUC vs. ED50 mean withdrawal latency. The tail-withdrawal test was
estimates). chosen because of its stability even after repeated exposure
The present study had three aims. First, we wanted to to this noxious water temperature [12]. A cut-off latency of assess whether sex differences in morphine tolerance are 15 s was employed to prevent the possibility of tissue also observed in mice. Although it is always useful to other damage, and was thus used to calculate %MPE (see investigators when findings from one species can be below).
generalized to others, the increasing use of inbred and
transgenic mouse models in genetic approaches to the 2.3. Tolerance induction problems of chronic opioid use makes our understanding
of sex differences in this species particularly vital. Second, Mice in the morphine treatment groups were injected we attempted to determine whether sex differences in three times daily (tid) for either 3 (10, 20, and 40 mg / kg tolerance depend on duration of morphine exposure and / or on Days 1, 2, and 3, respectively) or 7 (10, 20, 40, 40, 80, cumulative dose administered by comparing male and 80, and 100 mg / kg, respectively) days. Mice in saline female for analgesia after both 3 and 7 days of injections control groups received an equal number of saline in-using a single analgesic measure (ED50 values), nocicep- jections but were not tested on Day 1.
tive assay (tail-withdrawal), and repeated injection (three
times daily) paradigm. Third, we assessed whether sex 2.4. Dose–response studies differences in tolerance to morphine depends on the
method of quantifying analgesia by comparing dose–re- To reduce the number of mice, ED50 values were sponse data (i.e., ED50 estimates) with time–response data derived from cumulative dose–response curves as previ-(peak analgesia and AUC) in both sexes following identi- ously described [26]. Briefly, mice were injected with a 1.0
cal morphine administration paradigms. mg / kg dose of morphine and received increasing doses
(|0.25 log units) until each became analgesic. Analgesia was operationally defined as a doubling of each subject’s 2. Materials and methods mean baseline withdrawal latency on consecutive determi-nations at a given dose. Mice were tested 30 min following The following study was conducted in accordance with each morphine injection. ED50 values were determined for approved protocols of The College of Staten Island / CUNY all mice prior to (Day 1) and following (Day 4 and 8) Institutional Animal Care and Use Committee. tolerance induction except for mice in the vehicle control group who received only an equal number of saline
2.1. Subjects /drugs injections on Day 1. To insure that all mice in the
morphine treatment group received the same cumulative Adult male and female CD-1 mice (25–35 g) 6–8 weeks dose of drug on Day 1, responders were injected with the of age were housed four to a cage with same-sex litter- same subsequent morphine doses as non-responders mice mates and maintained on a 12 h light / 12 h dark cycle in a during Day 1 ED50 determinations until all mice were temperature-controlled environment with unrestricted food analgesic.
and water. All testing was conducted near mid-photophase
to minimize circadian fluctuations in morphine sensitivity. 2.5. Time–response studies Each mouse was used only once. Morphine sulfate was
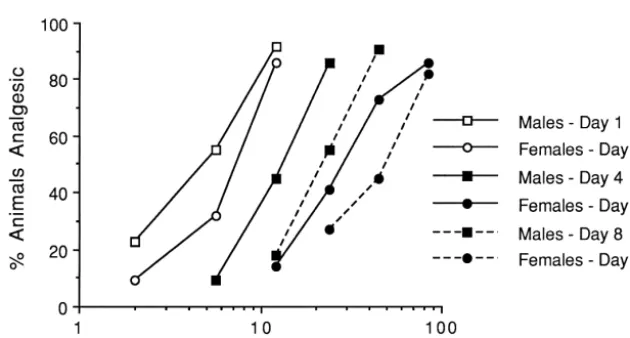
and all mice received a single 10 mg / kg morphine 4.160.63; females: 3.960.37), Day 4 (males 3.860.41; injection. Withdrawal latencies were re-assessed at 30-min females: 4.060.29) or Day 8 (males: 4.160.37; females: intervals for 2 h. Mice were then subject to 3 days of 4.260.40). In addition, male and female morphine ED50
morphine injections according to the dosing schedule values obtained on Day 1, prior to the start of the 3- or above. On Day 4, baseline latencies and morphine analge- 7-day tolerance induction protocol, were practically identi-sia were again determined at 30-min intervals for 2 h. cal and were thus pooled. As illustrated in Fig. 1, 3 days of Withdrawal latencies at peak analgesia (30 min) for each morphine administration produced a rightward shift in the sex on Day 1 and 4 were converted to %MPE scores morphine dose–response curve in both sexes on Day 4 [post-drug latency–baseline latency / cutoff latency– relative to Day 1. Table 1 shows that the resultant baseline latency)3100], and compared. The area under the morphine ED50 estimates was significantly increased on time3latency curve (AUC; min3s) for males and females Day 4 (males: 12.2 mg / kg; females: 28.6 mg / kg) relative was used to calculate %total analgesia (AUC / maximum to Day 1 (males: 4.1 mg / kg; females: 6.2 mg / kg) in both
possible AUC) and compared. males and females, indicative of tolerance. However, the
magnitude of tolerance was different between sexes.
2.6. Data analysis Whereas morphine potency was similar in males and
females on Day 1, equivalent dose–response shifts and Morphine dose–response data were analyzed using the ED50 estimate increases were not obtained on Day 4 (Fig. BLISS-21 computer program. This program maximizes the 1 and Table 1, respectively). In females, there was an log-likelihood function to fit a parallel set of Gaussian approximately 4.6-fold rightward shift in the morphine sigmoid curves to the dose–response data, and provides dose–response curve relative to an only three-fold shift in ED50 values, 95% confidence intervals (CI), and estimates males (Fig. 1), resulting in changes in morphine potencies of relative potency [42]. Baseline tail-withdrawal latencies, on Day 4 relative to Day 1 of 0.34 in males but 0.22 in and peak and total morphine analgesia between sexes on females (Table 1). ED50 estimates from morphine naive Day 1 was compared using an independent t-test. A two- mice (Day 1 in morphine-treated mice and Day 4 in saline way (one within, one between) repeated measures ANOVA control mice) did not differ within or between sex, was used to compare peak and %total morphine analgesia confirming that there were no significant initial differences on Days 1 and 4. An a level of 0.05 was used for all in morphine sensitivity between sexes, and indicating no
comparisons. effect of the repeated injection protocol.
Although morphine tolerance is increased in both sexes when morphine treatment and / or cumulative are increased 3. Results following 7 days of morphine treatment (Table 1 and Fig. 1), sex differences in tolerance were still observed.
3.1. Dose–response data Females displayed a significantly larger seven-fold
right-ward shift in the dose–response curve on Day 8 (ED :50
Mean baseline tail-withdrawal latencies did not differ 43.1) compared to only a 4.8-fold shift for males (ED :50
between males and females on either Day 1 (males: 19.5) (Fig. 1 and Table 1), resulting in relative potencies
Table 1
a
Morphine analgesic tolerance in male and female CD-1 mice
Treatment Day 1 Day 4 Day 8
[ED50(95% CI) / potency ratio] [ED50(95% CI) / potency ratio]
Male
Morphine 4.1 (2.9–5.8) 12.2 (9.3–15.8) / 0.34 19.5 (13.9–27.0) / 0.21
Saline – 5.0 (3.1–8.1) 6.5 (4.5–9.1)
Female
b b
Morphine 6.2 (4.8–8.1) 28.6 (22.5–36.2) / 0.22 43.1 (29.9–62.1) / 0.14
Saline 6.0 (3.7–9.4) 6.5 (4.6–9.1)
a
Separate groups of mice received subcutaneous saline or morphine injections for 3 and 7 days using an escalating dosing schedule. Morphine ED50and 95% confidence intervals (CI) estimates (mg / kg) were derived from cumulative dose–response curves obtained on Day 1, 4, and 8, respectively (see Fig. 1). Day 1 data from the two experiments were pooled for clarity. Potency ratio indicates proportion of analgesia retained after chronic treatment determined by ED50(Day 1) / ED50(Day 4 or 8).
b
Denotes significantly greater reduction in relative potency (i.e., tolerance) than males.
relative to Day 1 of 0.21 in males and 0.14 in females. morphine analgesia for Day 1 and 4 revealed decreased Again, a comparison of morphine naive mice from Day 1 peak morphine analgesia on Day 4 relative to Day 1 which and Day 8 indicate no effect from repeated injection did not differ between sex. Similar results were obtained following this longer 7-day injection paradigm in either for %total analgesia. Although there was a significant
sex. repeated measures effect (F( 1,14 )542.41; P,0.001),
in-dicating a decrease in morphine analgesia, between Days 1
3.2. Time–response data and 4, there was no effect of sex (F( 1,14 )50.84; n.s.) or
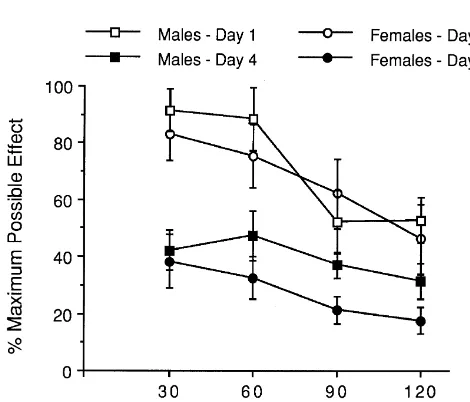
their interaction (F( 1,14 )50.14; n.s.). Similar to our ob-The analgesic time course for a single 10 mg / kg servations for dose–response studies, mean baseline tail-injection of morphine is shown in raw data form in Fig. 2. withdrawal latencies did not differ between males Peak morphine analgesia occurred by 30 min post-injection (4.360.66) and females (4.260.49).
for both sexes, and there was no difference in corre-sponding %MPE scores at that time. A two-way (one
between, one within) ANOVA for sex (F( 1,14 )50.44; n.s.), 4. Discussion repeated measures (time after injection) (F( 1,14 )545.5; P,
0.001), and their interaction (F( 1,14 )50.12; n.s.) on peak Three main findings emerge from the present study. First, female mice undergo greater analgesic tolerance to morphine than males. Significant rightward shifts in the morphine dose–response curve and increased ED50 esti-mates demonstrate tolerance for both male and female mice following 3 and 7days of repeated systemic morphine injections. Whereas they were not different on Day 1, the ED50 estimates on Days 4 and 8 were however sig-nificantly greater in females than males, indicating the development of greater analgesic tolerance in females regardless of the morphine treatment period. Our finding contrasts with previous rat studies in which greater or equal tolerance was observed in males [3,11,20], and highlights an important aim of this study. That is, subject species may influence the presence and / or the direction of sex differences in morphine tolerance. Although rats and mice differ in neural substrates that may contribute to potential differences in tolerance between these species [1,26,38,44], we are currently unaware of any sexual dimorphism exclusively present within one species that
Fig. 2. Time–response curves for morphine analgesia (10 mg / kg) on the could account for the contradictory findings between rats tail-withdrawal test in male and female mice before (Day 1) and after and mice on sex differences in tolerance. It is also worth (Day 4) 3 days of morphine injections. Significant (P,0.05) reductions in
noting that although greater tolerance was observed on
analgesia on Day 4 relative to Day 1 were observed for peak %MPE
Day 8 compared to Day 4, the relative sex difference in
(30-min post-injection latency) and %total analgesia (AUC / maximum
greater loss in potency in females). This suggests that the has invariably been shown to be exquisitely sensitive to duration and / or cumulative dose of morphine administra- NMDA receptor activation in rats and mice [14,27,33,34], tion may not impact on sex differences in tolerance in functional sex differences in NMDA receptor activity mice. Second, unlike previous rat studies where sex following chronic morphine may reasonably contribute to differences in tolerance were found, the differential mag- sex differences in morphine tolerance. Sex differences in nitude in tolerance between sexes could not be attributed to the anti-opioid effects of the endogenous peptides Tyr-sex differences in initial dose- or time-dependent measures MIF-1 and neuropeptide FF have also been documented of morphine sensitivity. In this regard, it would be of [23,24], and both peptides have been implicated in the interest to assess whether the greater morphine tolerance in development of morphine tolerance [16]. However, since female mice would be obtained in strains where initial these sexual dimorphisms have been demonstrated only morphine sensitivity differed in any way between sexes. with regard to morphine analgesia, their mechanistic
¨
Additionally, since ED50values in morphine naıve mice of relevance to sex differences in morphine tolerance war-both sexes obtained before (Day 1 morphine-treated group) rants further attention. Finally, we can not rule out the and after (Day 4 saline-treated group) 3 days of repeated possible contribution of sex differences in learning pro-injections did not significantly differ, sex differences in cesses on sex differences in morphine tolerance. Spe-response to the possible stress of repeated and chronic cifically, it has been repeatedly demonstrated in rats that an handling and injections also do not appear to contribute to association between environmental cues and the systemic the present sex differences in tolerance. Third and last, effects of morphine contributes to morphine tolerance there was no significant effect of sex in the magnitude of [4,40,41]. It is also well known that sex modulates the decreases in either peak %MPE or %total morphine classical conditioning, as well as several other types of analgesia after 3 days of morphine. This stands in contrast contextual learning paradigms, in rats (see reviews to the present demonstration of greater morphine tolerance, [39,43]). Thus, it is possible that males and females differ as indicated by significantly increased ED50 values, in in the rate at which environmental and contextual cues are females relative to males following the identical morphine associated with the effects of morphine, which could in dosing regimen. Thus, sex differences in morphine toler- turn lead to sex differences in the magnitude of tolerance. ance may depend on the method used to assess analgesia. We are currently clarifying the contribution of associative Alternatively, these apparently discrepant findings may be processes to sex differences in morphine tolerance in mice. attributable to the use of a single morphine dose on Day 1
and 4 to generate the AUC data as compared to multiple
doses used in generating dose–response curves. That is, Acknowledgements use of lower or higher morphine test doses on Days 1 and
4 may reveal sex differences in morphine tolerance in Supported in part by a SEED grant from the CSI / IBR AUC measures of analgesia that are dose–dependent. In Center for Developmental Neuroscience to BK. We would rats, sex differences in morphine tolerance are indeed like to thank Dr. Jeff Mogil for his assistance with data dependent on the test dose [11]. This possibility warrants analysis, and Anita Conte and Joanne Niekrash for their
further study. excellent care of the animals.
It is very difficult to postulate how sex differences in morphine tolerance may be affected given the lack of
studies on opioid tolerance in females in the literature, and References the myriad of biochemical and structural changes that
accompany opioid tolerance in males [10,14,16,34]. Refer- [1] E.E. Abdelhamid, M. Sultana, P.S. Portoghese, A.E. Takemori, Selective blockage of delta opioid receptors prevents the
develop-ence to the literature on sex differdevelop-ences in opioid analgesia
ment of morphine tolerance and dependence in mice, J. Pharmacol.
per se would also not be very likely revealing since current
Exp. Ther. 258 (1991) 299–303.
explanations of these differences are as varied as they are [2] A.I. Baamonde, A. Hidalgo, F. Andres-Trelles, Sex-related differ-´ equivocal (see review [28]). Nonetheless, some systems ences in the effects of morphine and stress on visceral pain,
with reported sexual dimorphisms have been shown else- Neuropharmacology 28 (1989) 967–970.
[3] D. Badillo-Martinez, A.L. Kirchgessner, P.D. Butler, R.J. Bodnar,
where to have great import with regard to morphine
Monosodium glutamate and analgesia induced by morphine,
test-tolerance, and require consideration. For example, male
specific effects, Neuropharmacology 23 (1984) 1141–1149.
and female rats differ in the expression pattern of the [4] M.A. Baptista, S. Siegel, G. MacQueen, L.T. Young, Pre-drug cues immediate early gene c-Fos following morphine, and the modulate morphine tolerance, striatal c-fos, and AP-1 DNA binding,
ability of antagonists of the N-methyl-D-aspartate (NMDA) Neuroreport 26 (1998) 3387–3390.
[5] K.J. Berkley, Sex differences in pain, Behav. Brain Sci. 20 (1997)
excitatory amino acid receptor to block this response is
371–380.
sexually dimorphic [13]. In the same study, a greater
[6] J.S. Boyer, M.M. Morgan, R.M. Craft, Microinjection of morphine
sensitivity to the behavioral effects of the NMDA antago- into the rostral ventromedial medulla produces greater antinocicep-nist dizocilpine maleate (MK-801) given without morphine tion in male compared to female rats, Brain Res. 796 (1998)
[7] J. Candido, K. Lutfy, B. Billings, V. Sierra, A. Duttaroy, C.E. [26] B. Kest, C.E. Lee, G.L. McLemore, C.E. Inturrisi, An antisense Inturrisi et al., Effect of adrenal and sex hormones on opioid oligodeoxynucleotide for the delta opioid receptor (DOR-1) inhibits analgesia and opioid receptor regulation, Pharmacol. Biochem. morphine tolerance and acute dependence, Brain Res. Bull. 39
Behav. 42 (1992) 685–692. (1996) 185–188.
[8] T.J. Cicero, B. Nock, E.R. Meyer, Gender-related differences in the [27] B. Kest, J.S. Mogil, S. Ben-Eliyahu, B. Kao, J.C. Liebeskind, P. antinociceptive properties of morphine, J. Pharmacol. Exp. Ther. Marek, The NMDA receptor antagonist MK-801 protects against the 279 (1996) 767–773. development of morphine tolerance after intrathecal administration, [9] T.J. Cicero, B. Nock, E.R. Meyer, Sex-related differences in Proc. West. Pharmacol. Soc. 36 (1993) 307–310.
morphine’s antinociceptive activity: relationship to serum and brain [28] B. Kest, E. Sarton, A. Dahan, Gender Differences in Opioid-morphine concentrations, J. Pharmacol. Exp. Ther. 282 (1997) Mediated analgesia: animal and human studies, Anesthestiology 93
939–944. (2000) 539–547.
[10] E. Collin, F. Cesselin, Neurobiological mechanisms of opioid [29] B. Kest, S.G. Wilson, J.S. Mogil, Sex differences in supraspinal tolerance and dependence, Clin. Neuropharmacol. 14 (1991) 465– morphine analgesia is dependent on genotype, J. Pharmacol. Exp.
488. Ther. 289 (1999) 1370–1375.
[11] R.M. Craft, J.A. Stratmann, R.E. Bartok, T.I. Walpole, S.J. King, [30] Y.A. Kolesnikov, S. Jain, R. Wilson, G.W. Pasternak, Lack of Sex differences in development of morphine tolerance and depen- morphine and enkephalin tolerance in 129 / SvEv mice: evidence for dence in the rat, Psychopharmacology 143 (1999) 1–7. a NMDA receptor defect, J. Pharmacol. Exp. Ther. 284 (1998) [12] F.E. D’Amour, D.L. Smith, A method for determining loss of pain 455–459.
sensation, J. Pharmacol. Exp. Ther. 72 (1941) 74–79. [31] E.K. Krzanowska, R.J. Bodnar, Morphine antinociception elicited [13] D.N. D’Souza, R.E. Harlan, M.M. Garcia, Sexual dimorphism in the from the ventrolateral periaqueductal gray is sensitive to sex and response to N-methyl-D-aspartate receptor antagonists and morphine gonadectomy differences in rats, Brain Res. 821 (1999) 224–230. on behavior and c-FOS induction in the rat brain, Neurosci. 93 [32] K. Lutfy, B. Sadowski, I.S. Kwon, E. Weber, Morphine analgesia (1999) 1539–1547. and tolerance in mice selectively bred for divergent swim stress-[14] K. Elliott, B. Kest, A. Man, B. Kao, C.E. Inturrisi, N-methyl-D- induced analgesia, Eur. J. Pharmacol. 265 (1994) 171–174.
aspartate (NMDA) receptors, mu and kappa opioid tolerance, and [33] P. Marek, S. Ben-Eliyahu, M. Gold, J.C. Liebeskind, Excitatory perspectives on new drug development, Neuropsychopharmacology amino acid antagonists (kynurenic acid and MK-801) attenuate the 13 (1995) 347–356. development of morphine tolerance in the rat, Brain Res. 547 (1991) [15] M. Fernandes, S. Kluwe, H. Coper, The development of tolerance to 77–81.
morphine in the rat, Psychopharmacology 54 (1977) 197–201. [34] D.J. Mayer, J. Mao, Mechanisms of opioid tolerance: a current view [16] L.A. Harrison, A.J. Kastin, J.E. Zadina, Opiate tolerance and of cellular mechanisms, Pain Forum 8 (1999) 3–13.
dependence: receptors, G-proteins, and antiopiates, Peptides 19 [35] R.F. Mucha, H. Kalant, M.A. Linseman, Quantitative relationships (1998) 1603–1630. among measures of morphine tolerance and physical dependence in [17] I.K. Ho, H.H. Loh, E.L. Way, Morphine analgesia, tolerance, and the rat, Pharmacol. Biochem. Behav. 10 (1979) 397–405.
dependence in mice from different strains and vendors, J. Pharm. [36] M. Navarro, J.-C. Leza, I. Lizasoain, P. Lorenzo, Influence of Pharmac. 29 (1977) 583–584. psychogenetics in opiate tolerance and abstinence in mice, Gen. [18] O. Hoffmann, A. Plesan, Z. Wiesenfeld-Hallin, Genetic differences Pharmacol. 22 (1991) 713–716.
in morphine sensitivity, tolerance, and withdrawal in rats, Brain Res. [37] A. Oliverio, C. Castellano, Genotype-dependent sensitivity and 806 (1998) 232–237. tolerance to morphine and heroin: Dissociation between opiate-[19] A.K. Islam, M.L. Cooper, R.J. Bodnar, Interactions among aging, induced running and analgesia in the mouse, Psychopharmacologia
gender and gonadectomy effects upon morphine antinociception in 39 (1974) 13–22.
rats, Physiol. Behav. 54 (1993) 43–54. [38] L.E. Robson, M.G. Gillan, H.W. Kosterlitz, Species differences in [20] B.G. Kasson, R. George, Endocrine influences on the actions of the concentrations and distributions of opioid binding sites, Eur. J.
morphine. IV. Effects of sex and strain, Life Sci. 34 (1984) 1627– Pharmacol. 112 (1985) 65–71.
1634. [39] T.J. Shors, A.V. Beylin, G.E. Wood, E. Gould, The modulation of [21] M. Kavaliers, D.G.L. Innes, Sex and day-night differences in opiate- pavlovian memory, Behav. Brain Res. 110 (2000) 39–52.
induced responses of insular wild deer mice, Peromyscus man- [40] S. Siegel, Morphine analgesic tolerance: its situation specificity
iculatus triangularis, Pharmacol. Biochem. Behav. 27 (1987) 477– supports a Pavlovian conditioning model, Science 193 (1976) 323–
482. 325.
[22] M. Kavaliers, D.G.L. Innes, Developmental changes in opiate- [41] S. Siegel, Drug anticipation and drug addiction: The 1998 H. David induced analgesia in deer mice: sex and population differences, Archibald Lecture, Addiction 94 (1999) 1113–1124.
Brain Res. 516 (1990) 326–331. [42] J.G. Umans, C.E. Inturrisi, Pharmacodynamics of subcutaneously [23] M. Kavaliers, D.G.L. Innes, Sex differences in the effects of Tyr- administered diacetylmorphine, 6-acetylmorphine, and morphine in
MIF-1 on morphine- and stress-induced analgesia, Peptides 13 mice, J. Pharmacol. Exp. Ther. 218 (1981) 409–415.
(1992) 1295–1297. [43] F. Van Haaren, A. van Hest, R.P. Heinsbroek, Behavioral differences [24] M. Kavaliers, D.G.L. Innes, Sex differences in the effects of between male and female rats: effects of gonadal hormones on neuropeptide FF and IgG from neuropeptide FF on morphine- and learning and memory, Neurosci. Biobehav. Rev. 14 (1990) 23–33. stress-induced analgesia, Peptides 13 (1992) 603–607. [44] B.C. Yoburn, K. Lutfy, J. Candido, Species differences inm- and [25] K.L. Kepler, B. Kest, J.M. Kiefel, M.L. Cooper, R.J. Bodnar, Roles d-opioid receptors, Eur. J. Pharmacol. 193 (1991) 105–108.