Effects of jasmonic acid and of an elicitor from
Ceratocystis
fimbriata
f. sp.
platani
on the accumulation of phytoalexins in
leaves of susceptible and resistant plane trees
Alain Cle´rivet *, Ibtissam Alami
Laboratoire de Biotechnologie et Physiologie Ve´ge´tale Applique´e,EA728,cc002,Uni6ersite´ Montpellier2,place Euge`ne Bataillon,
34095Montpellier cedex5,France
Received 31 December 1998; received in revised form 15 June 1999; accepted 18 June 1999
Abstract
Jasmonic acid and a glycoprotein elicitor produced by Ceratocystis fimbriataf. sp. platani, the canker stain agent, were tested for induction of coumarin phytoalexin accumulation in detached leaves of resistant (Platanus occidentalis) and susceptible plane trees (Platanus acerifoliaandP.occidentalis). It was shown that leaves responded by different levels of phytoalexin accumulation after fungal elicitor treatment. The phytoalexins were excreted from elicited leaf tissues and accumulated in elicitor-containing droplets on the leaf surface. The highest level was found in leaves of resistant tree. Furthermore, pretreatment of leaves by jasmonic acid, sprayed before elicitor application, induced a further enhancement in phytoalexin accumulation which was higher in leaves of susceptible trees without any change in the ratio of the three phytoalexins, scopoletin, umbelliferone and xanthoarnol. However, jasmonic acid did not mimic biotic stress by itself. It only increased the response of leaf cells to fungal elicitor leading to an activation of coumarin metabolism. © 1999 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Ceratocystis fimbriataf. sp.platani; Elicitor; Jasmonic acid; Phytoalexins;Platanusspp
www.elsevier.com/locate/plantsci
1. Introduction
Plants respond to pathogen attack by an array of inducible defense responses that contribute to resistance against the parasitic microorganism. Phytoalexins are synthesized via the activation of the phenylpropanoid biosynthetic pathway and function as a chemical barrier to pathogen inva-sion [1 – 3]. In plane tree-Ceratocystis fimbriata f. sp. platani (Ell and Halst) Walter (Cfp) interac-tion, accumulation of two coumarin phytoalexins, scopoletin and umbelliferone, is part of the re-sponse against the canker stain agent [4]. We have previously shown that accumulation of these phe-nolics is associated with the restriction of fungal development both in infected young stems of
sus-ceptible Platanus acerifolia (Ait Willd) and in in-fected leaves of resistantPlatanus occidentalis[4,5]. In plant-pathogen interaction, it is well known that defense reactions are expressed when a patho-gen-derived elicitor interacts with a membrane re-ceptor resulting in the build-up of a complex signalling network and ultimately triggers the acti-vation of defense genes [6,7]. A 66 kD glyco-protein (GP66) has been identified from the culture medium of Cfp germlings as the major elicitor for phytoalexin accumulation in plane tree cell-suspension cultures [8]. In addition to scopo-letin and umbeliferone, a new dihydrofuranocou-marin phytoalexin, xanthoarnol also accumulates both in cell culture medium and in infected stems [4,9]. Accumulation of phenolic phytoalexins is generaly related to the initiation of a new phenyl-propanoid pathway and many data have previ-ously shown that jasmonic acid or methyl
* Corresponding author. Tel.:+33-4-67-14-46-10; fax: + 33-4-67-14-36-37.
E-mail address:[email protected] (A. Cle´rivet)
jasmonate can induce this secondary metabolic pathway [10 – 13], leading to protection against a fungal pathogen [14]. Jasmonates have been shown to derive from unsaturated octadecanoic acids via the lipoxygenase pathway [15]. These compounds are involved as intracellular signal transducers in stress responses and can change the pattern of gene expression in many plant species [16,17].
In the present work, we use a convenient exper-imental model consisting in the GP66 elicitor re-leased byCfp germlings and in detached leaves of plane trees susceptible or resistant to the canker stain agent. The objective is to study the effect of jasmonic acid and fungal elicitor on the accumula-tion of coumarin phytoalexins in relaaccumula-tion to host susceptibility or resistance to the parasitic fungus.
2. Materials and methods
2.1. Plant material
The 7-day-old leaves were cut off from 3 years old potted susceptible P. acerifolia (CV L11) and P. occidentalis (CV MO2S1) plants grown from seeds and 3 years old potted resistant P. occiden-talisplants grown from cuttings of resistant Amer-ican trees (CV M11) (McCraken, non published data) [18]. Plants were grown in a greenhouse at 2592°C, under natural day light and 70% humidity.
2.2. Elicitor and jasmonic acid treatment
The GP66 eliciting glycoprotein released from Cfp germlings in sterile distilled water was resus-pended in K-Pi buffer 10 mM, pH 7 according to Alami et al. [8]. Directly after sampling, plane tree leaves were sprayed or not on the lower surface with a solution of 20 mM (9)-jasmonic acid (Sigma) prepared in DMSO and then immediately placed in glass Petri dishes containing moist What-man filter paper. A total of 24 h later, 10 ml droplets of GP66 elicitor titrated at 0.5mg protein equivalent per ml K-Pi buffer (see above) were laid down on half (relative to the central vein) of the lower leaf surface. Protein content of GP66 solu-tion was determined by the Bradford assay [19]. The other half of the leaf was used as control with droplets (10 ml) of K-Pi buffer 10 mM, pH 7.
Droplets were regularly distributed (three droplets per cm2) along the leaf surface. Leaves were main-tained at 25°C in continuous light (40 mE s−1, Philips fluorescence tube, light day, 30 W) under 100% humidity (closed Petri dishes) to preserve the original volume of droplets over an experimenta-tion period of up to 72 h.
2.3. Phytoalexin determination
Extraction of coumarin phytoalexins was car-ried out from leaf areas exposed to GP66 droplets or control K-Pi buffer droplets as previously de-scribed [4,5]. Elicitor-containing droplets and con-trol droplets were recovered, filtered through a Millipore membrane (0.22 mm) and immediately analysed by HPLC. HPLC analysis (WATERS 990 with a photodiode bar receptor coupled with a WATERS 420 fluorescence detector) was carried out on a spherisorb C 18 column (particle size 5 mm, 250×5 mm) and samples were eluted in a solvent consisting in acetonitrile and water (ad-justed to pH 2.6 with orthophosphoric acid) with a gradient of 5 – 40% acetonitrile within 45 min with a 1 ml per min flow rate.
Phytoalexin content of droplets was expressed in mM of umbelliferone equivalent per ml of droplet covering 1 cm2of leaf surface (ml/cm2). From leaf tissues, phytoalexin content was expressed in mM of umbelliferone equivalent per mg fresh weight (FW) knowing that 1 cm2 of leaf surface weights 150 mg (FW). After 48 h of treatment with jas-monic acid and GP66 elicitor, each of the three phytoalexins (scopoletin, umbelliferone, and xan-thoarnol) was separately quantified in droplets and in leaf tissues and the contents expressed as described above. All data correspond to the means of three replicates9SE, each with three leaves from different trees.
3. Results
3.1. Effect of elicitor on phytoalexin accumulation
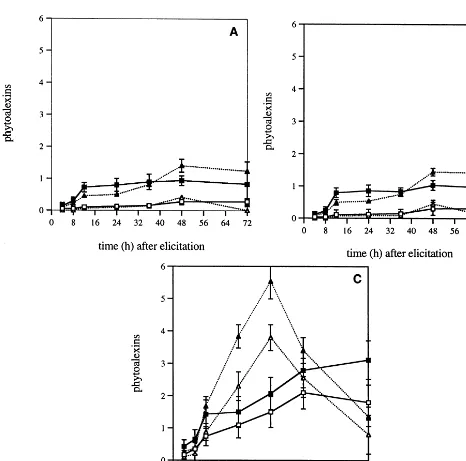
48 h. The strongest fluorescence was found in leaves of resistant P. occidentalis whereas no fluorescence was observed in control K-Pi buffer droplets.
In GP66 droplets of leaves of susceptible P. acerifolia (CV L11) and P. occidentalis (CV MO2S1), phytoalexin accumulation slightly in-creased from 4 to 48 h, reaching 0.27 and 0.30mM
equivalent umbelliferone in L11 and MO2S1, re-spectively. In leaf tissues of the two cultivars, phytoalexins also increased during elicitation, reaching 0.4 mM equivalent umbelliferone 48 h after elicitation (Fig. 1A, B).
In elicitor-containing droplets of leaves of resis-tant P. occidentalis (CV M11), the amount in phytoalexins increased progressively up to 48 h
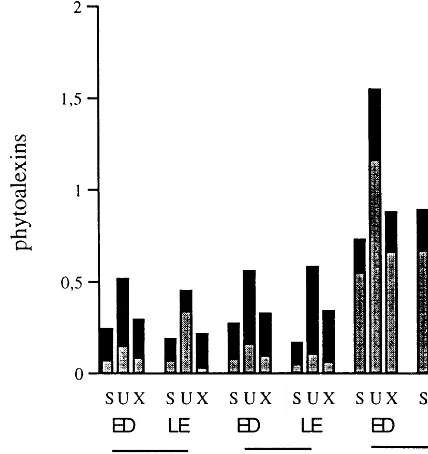
Fig. 2. Scopoletin, umbelliferone and xanthoarnol accumula-tion after elicitor applicaaccumula-tion (0.5 mg protein equivalent per ml) or after JA spraying (20mM, 24 h before elicitor applica-tion) on leaves of susceptible P. acerifolia (L11), and P.
occidentalis(MO2S1) and resistantP.occidentalis(M11). (S) scopoletin; (U) umbelliferone; and (X) xanthoarnol were ana-lyzed by HPLC as given in Section 2, 48 h after elicitor application. (ED) elicitor-containing droplets; (LE) leaf tis-sues; (a) amounts in coumarins under elicitation with GP66, or () additional amounts in coumarins under elicitation with GP66 after JA spraying. Phytoalexins were expressed in
mM per ml from elicitor-containing droplets and in mM per mg. FW from leaf extracts. Values are the mean of results from a triplicate set of experiments. For controls (elicitation with K-Pi buffer and spraying with DMSO solution) trace amounts of coumarins are not represented.
3.2. Effect of JA and elicitor on phytoalexin accumulation
When the leaf pretreatment by JA was not followed by elicitor application, only trace amounts of phytoalexins were detected both in susceptible and resistant leaves (data not shown). Pretreating susceptible and resistant leaves with JA before GP66 application induced a higher coumarin accumulation in susceptible leaves (3.5-fold increase) (Fig. 1A and B) than in resistant ones (1.3-fold increase) (Fig. 1C). However, de-spite the high increase in phytoalexin accumula-tion in eliciting droplets of susceptible leaves (0.93 mM in L11, 1.03 mM in MO2S1), this accu-mulation was lower than in resistant ones (2.79 mM in M11). Likewise, in leaf extracts, the cou-marin amounts were 1.39 mM in L11, 1.44 mM in MO2S1 and 5.53 mM in M11 (Fig. 1 A – C).
Furthermore, the ratio of umbelliferone, sco-poletin, and xanthoarnol accumulation was un-changed in susceptible and resistant leaves under JA pretreatment and further elicitor application (Fig. 2).
4. Discussion
In the present work, we have shown that a glycoprotein elicitor from Cfp germlings induced, in leaves of susceptible and resistant plane trees, the accumulation of the three coumarin phytoalexins, umbelliferone, scopoletin and xanthoarnol, previously identified both in inoculated stems and elicited cell cultures [4,9]. Furthermore, spraying of leaves with jasmonic acid before elicitor application induced an additionnal increase in phytoalexin accumulation. To date, phytoalexin accumulation in woody plants in response to fungal elicitation is only known for monoterpenes in some species of Pinus [20] and for sesquiterpenes in Datura stramonium [21] but not for coumarins. In these two plants, like in plane tree, high levels of phytoalexin accumulation were elicited by fungal preparations.
The leaf response to Cfpelicitor is more intense in resistant leaves than in susceptible ones. This different level of leaf response has also been observed when leaves were directly infected with the fungus [5] but under these conditions phytoalexin accumulation was much lower than in GP66 elic-reaching 2.1 mM followed by a slow decrease
(Fig. 1C). In leaf extracts, phytoalexin accumula-tion increased rapidly up to 36 h reaching 3.8 mM, then strongly declined. In resistant leaves, the phytoalexin content in coumarins was ap-proximatively 7-fold higher in droplets and 9.5-fold higher in leaf extracts at the highest level of accumulation as compared to susceptible ones.
In droplets and in leaf extracts of susceptible and resistant leaves, the ratio of umbelliferone, scopoletin, and xanthoarnol accumulation was approximatively 2:1:1 excepted for leaf extract of L11 (2.5:1:0.5) showing that umbelliferone was the predominant accumulating compound (Fig. 2).
ited leaves. This fact may be related to cell death associated with fungal ingress [5]. By contrast, the GP66 elicitor induces a high accumulation of an-timicrobial coumarins, without causing the cell death. On the other hand, a dose effect of elicitor is not correlated with the level of reaction of leaves since elicitor concentration of droplets (0.5 mg per ml) was much lower than that measured in a suspension ofCfpgermlings (12mg per ml) applied on leaf surface [5,8]. As in inoculated leaves [5], a strong decline in phytoalexin content is observed in tissues of resistant leaves between 48 and 72 h. This decrease is in part related to phenolic excre-tion, but also to phenolic oxidaexcre-tion, as suggested by the browning of leaf tissues exposed to elicitor. Phytoalexins accumulated in leaf tissues exposed to elicitor are excreted in elicitor-containing droplets. Accumulation and excretion of umbellif-erone were higher than that of scopoletin and xanthoarnol. Excretion of phenolic compounds is largely reported in cell-suspension cultures after elicitation [22 – 24] but not in plant organs treated with biotic elicitors. Cell death caused by elicitor activity has been suggested in some systems to explain the mechanism of secondary product ex-cretion [25] but it is not likely to be the case for plane tree leaves since the addition of GP66 to cell-suspension culture did not cause any decline in cell viability [8]. Exocytosis or diffusion demon-strated for others systems [26] were probably in-volved here in the excretion of coumarins.
Our results show that pretreatment of resistant and susceptible leaves with JA before elicitor ap-plication induced an additional increase in phy-toalexin accumulation. Such effect of JA has already been reported for herbaceous plant cell cultures [27,28] but not yet for woody plant or-gans. The enhancement was stronger in the com-patible interaction than in the incomcom-patible one while the final level of coumarin accumulation was still lower in susceptible than in resistant leaves. The saturation level of phytoalexin accumulation with fungal elicitor is probably reached in resistant leaves and, as a consequence, the JA effect is restricted. When JA spray was not followed by elicitor application, phytoalexins were only de-tected at trace amounts like in control leaves treated with phosphate buffer. Similar results were also reported for potato and tomato [14] and for Hyoscyamus muticus root cultures [29], showing that jasmonate application by itself did not induce
the synthesis of secondary products. In susceptible leaves treated with JA before elicitor application, phytoalexin concentration was higher than the level required to inhibit the development of the pathogenic fungus in vitro. Indeed, with a mixture of scopoletin, umbelliferone and xanthoarnol (1:1:1), the ID50 for in vitro germination of Cfp conidia is around 0.85 mmol per ml [30]. Since the penetration of the fungus occurs in vivo through roots, trunk and branches [31] it would be interest-ing to study the response of woody organs of susceptible plane tree to experimental infections with Cfp after JA spraying. Moreover, JA spray-ing has been reported to protect tomato, potato, barley and Arabidopsis against pathogenic mi-croorganisms [14,32,33]. So, we can speculate that the protection of susceptible plane trees against Cfp would be triggered by a JA pretreatment before experimental infection. New experiments will be necessary to clarify the eventual role of JA spray in plane tree protection against fungal infec-tion and to understand its physiological role in th enhancement of elicitor activity leading to expres-sion of genes involved in defense responses toward the canker stain agent.
Acknowledgements
We thank Dr A. Vigouroux (INRA, Plant Pathology Department, Montpellier, France) for supplying plant and fungal materials.
References
[1] R.A. Dixon, N.L. Paı¨va, Stress-induced phenyl-propanoid metabolism, Plant Cell 7 (1995) 1085 – 1097. [2] C.J. Smith, Accumulation of phytoalexins: defence
mech-anism and stimulus response system, New Phytol. 132 (1996) 1 – 45.
[3] J. Kuc, Molecular aspects of plant responses to patho-gens, Acta Physiol. Plantarum 19 (1997) 551 – 559. [4] C. El Modafar, A. Cle´rivet, A. Fleuriet, J.J. Macheix,
Inoculation of Platanus acerifolia with Ceratocystis fimbriataf. sp.plataniinduces scopoletin and umbellifer-one accumulation, Phytochemistry 34 (1993) 1271 – 1276. [5] C. El Modafar, A. Cle´rivet, A. Vigouroux, J.J. Macheix, Accumulation of phytoalexins in leaves of plane tree (Platanus spp.) expressing susceptibility or resistance to
Ceratocystis fimbriataf. sp.platani, Eur. J. Plant Pathol. 101 (1995) 503 – 509.
[7] J. Ebel, A. Mitho¨fer, Early events in the elicitation of plant defence, Planta 206 (1998) 335 – 348.
[8] I. Alami, S. Mari, A. Cle´rivet, A glycoprotein from
Ceratocystis fimbriata f. sp. platani triggers phytoalexin synthesis inPlatanus×acerifoliacell-suspension cultures, Phytochemistry 48 (1998) 771 – 776.
[9] I. Alami, A. Cle´rivet, M. Naji, M. VanMunster, J.J. Macheix, Elicitation of Platanus×acerifolia cell-suspen-sion cultures induces the synthesis of xanthoarnol, a new dihydrofuranocoumarin phytoalexin, Phytochemistry 51 (1999) 733 – 736.
[10] V.R. Franceschi, H.D. Grimes, Induction of vegetative strorage proteins and anthocyanins by low-level atmo-spheric methyl jasmonate, Proc. Natl. Acad. Sci. USA 83 (1991) 6745 – 6749.
[11] H. Gundlach, M.J. Muller, T.M. Kutchan, M.H. Zenk, Jasmonic acid is a signal transducer in elicitor-induced plant cell cultures, Proc. Natl. Acad. Sci. USA 89 (1992) 2389 – 2393.
[12] H. Kauss, K. Krause, W. Jeblick, Methyl jasmonate conditions parsley suspension cells for increased elicita-tion of phenylpropanoid defense responses, Biochem. Biophys. Res. Commun. 189 (1992) 304 – 308.
[13] H. Mizukami, Y. Tabira, B.E. Ellis, Methyl jasmonate-induced rosmarinic acid biosynthesis in Lithospermum eryrorhizon cell suspension cultures, Plant Cell Rep. 12 (1993) 706 – 709.
[14] Y. Cohen, U. Gisi, T. Niderman, Local and systemic protection against Phytophthora infestans induced in potato and tomato plants by jasmonic acid and jasmonic methyl ester, Phytopathology 83 (1993) 1054 – 1062. [15] R.A. Creelman, J.E. Mullet, Biosynthesis and action of
jasmonates in plants, Annu. Rev. Plant Physiol. Plant Mol. Biol. 48 (1997) 355 – 381.
[16] C. Wasternack, B. Parthier, Jasmonate-signalled plant gene expression, Trends Plant Sci. 2 (1997) 302 – 307. [17] G. Sembdner, B. Parthier, The biochemistry and the
physiological and molecular actions of jasmonates, Annu. Rev. Plant. Physiol. Plant Mol. Biol. 44 (1993) 569 – 589.
[18] A. Vigouroux, Preliminary results for obtaining a plane tree resistant to canker stain and adapted to European conditions, Acta Hort. 320 (1992) 91 – 96.
[19] M. Bradford, A rapid and sensitive method for quantifi-cation of microgram quantities of proteins utilizing the principle of protein dye-binding, Anal. Biochem. 72 (1976) 248 – 254.
[20] F. Lieutier, A.A. Berryman, J.A. Millstein, Preliminary study of the monoterpene response of three pines to
Ophiostoma cla6igerum(Ascomycetes: Ophiostomatales)
and two chemical elicitors, Ann. Sci. For. 48 (1991) 377 – 388.
[21] I.M. Whitehead, A.L. Atkinson, D.R. Threfall, Studies on the biosynthesis and metabolism of the phytoalexin lubimin and related compounds inDatura stramoniumL, Planta 182 (1990) 81 – 88.
[22] U. Matern, Coumarins and other phenylpropanoid com-pounds in the defense response of plants, Planta Med. 57 (1991) 15 – 19.
[23] F. Marinelli, S.D. Gregorio, V.N. Ronchi, Phytoalexin production and cell death in elicited carrot cell suspen-sion cultures, Plant Sci. 77 (1991) 261 – 266.
[24] Z.J. Guo, Y. Ohta, A synergistic effect of gluthatione-de-pletion and elicitation on the production of 6-methoxymellein in carrot cells, Plant Cell Rep. 12 (1993) 617 – 620.
[25] W. Barz, B. Beimen, B. Drger, U. Jacques, C. Otto, E. Sper, B. Upmeier, Turnover and storage of secondary products in cell cultures, in: B.V. Charlwood, M. Rhodes (Eds.), Secondary Products from Plant Tissue Culture, Clarendon Press, Oxford, 1990, pp. 79 – 102.
[26] J. Guern, J.P. Renaudin, S.C. Brown, The compartmen-tation of secondary metabolites in plant cell culture, in: Cell Culture in Phytochemistry, vol. 4, Academic Press, Inc, New York, 1987, pp. 49 – 90.
[27] H. Dittrich, T.M. Kutchan, M.H. Zenk, The jasmonate precursor, 12-oxo-phytodienoic acid, induces phytoalexin synthesis in Petroselinum crispum cell cultures, FEBS Lett. 309 (1992) 33 – 36.
[28] M. Sharan, G. Tagushi, K. Gonda, T. Jouke, M. Shi-mosaka, N. Hayashida, M. Okazaki, Effects of methyl jasmonate and elicitor on the activation of phenylalanine ammonia-lyase and the accumulation of scopoletin in tobacco cell cultures, Plant Sci. 132 (1998) 13 – 19. [29] G. Singh, J. Gavrieli, J.S. Oakey, W.R. Curtis,
Interac-tion of methyl jasmonate, wounding and fungal elicita-tion during sesquiterpene inducelicita-tion in Hyoscyamus muticusin root cultures, Plant Cell Rep. 17 (1998) 391 – 395.
[30] I. Alami, Elicitation de suspensions cellulaires de Pla
-tanus acerifolia par une glycoprote´ine isole´e de Cerato
-cystis fimbriata f. sp. platani. Modification du Me´tabolisme Phe´nolique et Implication de L’acide Jas-monique dans la Re´ponse Cellulaire. The`se Doctorat, Universite´ Montpellier 2, 1998.
[31] J.M. Walter, E.G. Rex, R. Schreiber, The rate of pro-gress and destructiveness of canker stain of plane trees, Phytopathology 42 (1952) 236 – 239.
[32] P. Schweizer, R. Gees, E. Mo¨singer, Effect of jasmonic acid on the interaction of barley (Hordeum 6ulgare L.)
with the powdery mildewErysiphe graminisf. sp.hordei, Plant Physiol. 102 (1993) 503 – 511.
[33] P. Vijayan, J. Shockey, C.A. Levesque, R.J. Cook, J. Browse, A role for jasmonate in pathogen defense of
Arabidopsis, Proc. Natl. Acad. Sci. USA 95 (1998) 7209 – 7214.