Cerium and lanthanum promote floral initiation and reproductive
growth of
Arabidopsis thaliana
Ya-Wen He, Chiang-Shiong Loh *
Department of Biological Sciences,National Uni6ersity of Singapore,14Science Dri6e4,Singapore117543,Singapore
Received 1 December 1999; received in revised form 2 June 2000; accepted 5 July 2000
Abstract
The effects of cerium and lanthanum on the vegetative growth, floral initiation and reproductive growth ofArabidopsis thaliana
were studied. Addition of cerium nitrate (0.5 – 10 mM) or lanthanum nitrate (0.5 – 50 mM) to the culture medium significantly
increased the lengths of primary roots, but had no significant effects on the number of rosette leaves produced per plant, plant heights and dry weights during the vegetative growth stage (17 days after seed germination). The percentage of plants bolted was significantly increased with the addition of 0.5 – 10.0mM cerium nitrate or lanthanum nitrate. The combination of 0.5mM cerium
nitrate and 0.5mM lanthanum nitrate was found to be most effective on the induction of floral initiation. The height, dry weight
and average number of flower numbers of 35-day-old plants growing in media containing cerium nitrate or/and lanthanum nitrate (0.5 – 10.0mM) were found to be significantly higher than those in the control medium. The endogenous levels of cytokinins (zeatin
riboside, dihydrozeatin riboside and isopentenyl adenosine) and carbohydrates (sucrose, glucose and fructose) in leaf and root tissues of plants growing in the medium supplemented with 0.5 mM cerium nitrate and 0.5 mM lanthanum nitrate were not
significantly different from those of plants in the control medium. Application of 0.5mM cerium nitrate and 0.5mM lanthanum
nitrate enhanced the effects of 10−6M IPA on root growth, plant height and flowering. The role of cerium and lanthanum in
promoting floral initiation and reproductive growth and the possibility of developing non-hormonal flowering promoting agents are discussed. © 2000 Elsevier Science Ireland Ltd. All rights reserved.
Keywords:Arabidopsis thaliana; Cerium; Lanthanum; Cytokinin; Flowering
www.elsevier.com/locate/plantsci
1. Introduction
The rare earth elements (REEs) comprise a group of 15 trivalent metallic elements with similar chemical properties. They normally occur as basic oxides and phosphate complexes in nature [1,2]. Since the introduction of ion exchange techniques, the separation of the rare earth elements from their ores and from one another has become prac-tical and many new uses of REEs have been developed [3]. The widespread industrial usage of
REEs makes it imperative that more detailed in-formation on the effects of REEs on biological systems be available.
The effects of REEs, especially lanthanum, in different animal tissues have been studied exten-sively [1]. Most of the work carried out on REEs in animal systems was based on the use of REE ion as a substitute or antagonist for Ca2+ to
monitor the movement of calcium and water, and to investigate the role of calcium in muscle and nerve activity [4].
The results from limited studies on the effect of REEs on plant growth are conflicting. Early re-ports indicated that the REE were inhibitory to plant growth. For example, La3+and Nd3+ were
found to inhibit elongation of oat coleoptile
sec-Abbre6iations: BA, benzyladenine; Ce, Cerium; DHZR,
dihy-drozeatin riboside; IPA, isopentenyl adenosine; La, Lanthanum; MS medium Murashige and Skoog (1962) medium; Nd, Neodymium; REEs, rare earth elements; ZR, zeatin riboside.
* Corresponding author. Tel.: +65-874-2916; fax:+65-779-5671.
E-mail address:[email protected] (C.-S. Loh).
tions [5]. Colloidal lanthanum caused an almost complete inhibition of cell division and root elon-gation in the root tips of barley plants [6]. La3+
had been shown to inhibit root elongation of wheat [7,8]. More recent reports, however, demonstrated some positive effects of REEs on plant growth. Diatloff et al. [9] reported that corn root growth increased significantly with the applications of cerium (0.63 mM) and lanthanum
(0.63 mM). Applications of lanthanum and
cerium were also reported to increase spike pro-duction in wheat [10]. In pot trials, applications of cerium sulphate (up to 100 mg/kg) enhanced root and shoot growth of Phaseolus radiatus and
Brassica pekinensis [11].
Results from field trials were also inconsistent. The increase in crop yield reported by workers from China ranged between 8 and 50%, with the common response being of the order of 8 – 15% [12,13]. However, no response was found by spraying and seed dressing of a summer fodder crop (Brassica sp.) with REEs in a field trial carried out in Australia (cited in [4]). In view of this, it is essential to study and elucidate the effects of REE on essential stages of growth and development of a model plant species such as
Arabidopsis thaliana. In this report, we investi-gated the effects of cerium (Ce) and lanthanum (La) on vegetative and reproductive growth of A.
thaliana and correlated some of the responses to increased sensitivity of cell to plant growth regu-lators.
2. Materials and methods
2.1. Plant materials, culture media and growth conditions
Seeds of A. thaliana L. Heynh cv. Columbia (LEHLE SEEDS, USA) were surface sterilized by soaking in 75% alcohol for 30 s and followed by 15% Clorox® for 15 min. The seeds were then
rinsed five times in sterilized water prior to cul-ture. The 1/4 strength Murashige and Skoog medium [14] was used for seed germination and as basal medium. The pH of the medium was adjusted to 5.8 before agar (Difco, 0.8%) was added. All media were autoclaved for 20 min at 121°C. Cerium nitrate hexahydrate (Ce(NO3)3
·6H2O, Sigma) and lanthanum nitrate
hexahy-drate (La(NO3)3·6H2O, Sigma) were dissolved in
Mili-Q water and sterilized by membrane filtra-tion (Millipore, 0.45 mm) and stored at room
temperature in the dark. Stock solutions of cerium nitrate and lanthanum nitrate were added to the autoclaved basal medium prior to dispens-ing into Magenta GA7® containers (Magenta
Corp., USA). Seeds were germinated in the dark and 2-day-old seedlings were placed under 16 h photoperiod (54 mmol−1m−2s−1 provided by
Cool White fluorescent lamps) at 2592°C.
2.2. Growth measurements
Lengths of primary roots were scored 10 days after seed germination. The number of leaves produced per plant was scored 17 days after seed germination. Plant heights and dry weights were scored 17 and 35 days after seed germination, respectively. Dry weights were taken by drying 100 plants in an oven (55°C) for 1 week. Floral initiation was recorded when the plant bolted with at least 1 cm long inflorescence stalk.
2.3. Extraction and determination of endogenous cytokinins and carbohydrates
Approximately 1 g fresh weight of tissues was homogenized in 4 ml of 80% ethanol followed by 1 h incubation at 4°C. After centrifugation at 1670×g for 3 min, the supernatant was trans-ferred to another centrifuge tube. The tissues were re-extracted with 2 ml of 80% ethanol, and the supernatant was pooled together after cen-trifugation. The extracts were vacuum evaporated at 4°C (Eppendorf Concentrator 5301) to 1/4 volume and then stored at −20°C after filter-sterilization (Milipore, 0.2 mm). The analysis of
zeatin riboside (ZR), dihydrozeatin riboside (DHZR), and isopentenyl adenosine (IPA) were performed by immunoassay detection kits (Sigma Chemical Company) according to the protocols provided by the manufacturer. All hormonal lev-els were expressed in terms of pmol per gram fresh weight (pmol/g.f.wt). The analysis of su-crose, glucose and fructose were performed by assay kits (Sigma Chemical Company) according to the protocols provided by the manufacturer. The levels of sucrose were expressed in term of mg per gram fresh weight (mg/g.f.wt). The levels of glucose and fructose were expressed in term of
3. Results
3.1. Effects on 6egetati6e growth
The vegetative and reproductive growth of A.
thaliana plant was separated. Prior to bolting, an
A. thaliana plant consisted of a rosette of small leaves with a main hypocotyl. In the present study, results showed that additions of 0.5 – 50mM
cerium or lanthanum had no significant effects on the vegetative growth in terms of height and dry weight. Cerium and lanthanum also had no sig-nificant effect on the average number of rosette leaves 17 days after seed germination (data not shown). Additions of higher concentrations of cerium or lanthanum inhibited the vegetative growth (data not shown).
3.2. Effects on root growth
A. thaliana plants were observed to produce only primary roots 10 days after seed germina-tion. In REE-free medium, the length of primary roots was about 2.0 cm. Addition of 0.5 – 10.0mM
cerium nitrate to the culture medium significantly increased the lengths of primary roots with longest root (2.84 cm) observed in the medium containing 10.0 mM cerium nitrate (Table 1).
Sim-ilarly, addition of lanthanum (0.5 – 50.0 mM)
singly or in combination with cerium nitrate sig-nificantly increased the lengths of roots (Table 1).
3.3. Effects on floral initiation
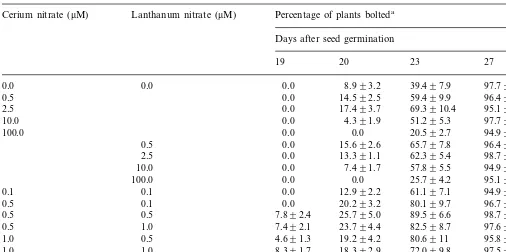
In basal medium, the first appearance of bolting (8.9%) was observed on day 20. About 39.4% of the plants bolted on day 23 and by day 27 more than 98% of the plants bolted (Table 2).
Addi-tions of cerium nitrate or lanthanum nitrate (0.5 – 2.5 mM) significantly increased the percentages of
plants bolted on days 20 and 23. For example, 69.3% of the plants growing in the medium with 2.5 mM cerium nitrate bolted on day 23 whereas
only 39.4% bolted in basal medium. About 65.7% of the plants in the medium containing 0.5 mM
lanthanum nitrate bolted on day 23 (Table 2). The percentages of plants bolted on day 27 were not significantly different between media with or without cerium nitrate or lanthanum nitrate (Table 2).
None of the plants growing in basal medium bolted on day 19. Additions of certain combina-tions of cerium nitrate and lanthanum nitrate (e.g. 0.5+0.5 mM, 0.5+1.0 mM, 1.0+0.5 mM, 1.0+
1.0 mM) resulted in early flowering; 4.6 – 8.3% of
the plants bolted as early as day 19. The combina-tion of 0.5 mM cerium nitrate and 0.5 mM
lan-thanum nitrate was most effective in induction of flowering. Nearly 90% of the plants bolted on day 23 in the medium containing 0.5 mM cerium
ni-trate and 0.5 mM lanthanum nitrate compared to
only 39.4% bolted in the basal medium. On day 27, no significant difference in the percentage of plants bolted was observed in the media with or without REE (Table 2).
In order to assess the effects of additional ni-trates from lanthanum nitrate and cerium nitrate on floral initiation, we replaced these two chemi-cals with potassium nitrate. The effects of addi-tional nitrates (0.3 or 1.0% of the total nitrate level in the 1/4 strength MS medium) on floral initiation were investigated. The percentages of plants bolted in the media supplemented with the additional nitrate were not significantly different from those in the basal medium (data not shown). Thus any effects of cerium nitrate and lanthanum
Table 1
Effects of cerium nitrate and lanthanum nitrate on the growth of primary root of A.thaliana 10 days after seed germination
Average root length (cm)a
Concentration (mM) Cerium nitrate Lanthanum nitrate Cerium nitrate and lanthanum nitrate
1.9690.04a 2.0490.06a
2.0490.06a 0.0
0.5 2.6290.05b 2.5890.04c 2.6590.03b 2.6590.04c 2.5890.04b 2.6390.04b
1.0
10.0 2.8490.04c 2.4790.05b 2.5490.05b 1.8190.03c 2.4090.04b
1.9290.04a 50.0
aValues were expressed as mean9SE. There were 81–95 plants per treatment. Means within the same column followed by the
Table 2
Effects of cerium or/and lanthanum on floral initiation ofA.thaliana
Lanthanum nitrate (mM)
Cerium nitrate (mM) Percentage of plants bolteda
Days after seed germination
19 20 23 27
0.0 8.993.2
0.0 0.0 39.497.9 97.791.4
0.0 14.592.5 59.499.9
0.5 96.491.0
2.5 0.0 17.493.7 69.3910.4 95.191.1 0.0 4.391.9 51.295.3
10.0 97.790.7
0.0 0.0
100.0 20.592.7 94.991.5
0.0 15.692.6
0.5 65.797.8 96.490.9
2.5 0.0 13.391.1 62.395.4 98.791.9 0.0 7.491.7
10.0 57.895.5 94.992.0
100.0 0.0 0.0 25.794.2 95.192.2 0.0
0.1 0.1 12.992.2 61.197.1 94.992.7
0.0 20.293.2
0.1 80.199.7
0.5 96.792.1
7.892.4 25.795.0
0.5 0.5 89.596.6 98.790.9
7.492.1 23.794.4
1.0 82.598.7
0.5 97.690.5
4.691.3 19.294.2
1.0 0.5 80.6911 95.891.6
8.391.7 18.392.9
1.0 72.099.8
1.0 97.591.3
0.0 17.893.4
2.5 2.5 60.397.5 98.490.9
aValues were expressed as mean9SE. There were 94–97 plants per treatment. Plants were grown under 16 h photoperiod and
at 2592°C.
nitrate on flowering were due to cerium or lanthanum.
3.4. Effects on reproducti6e growth
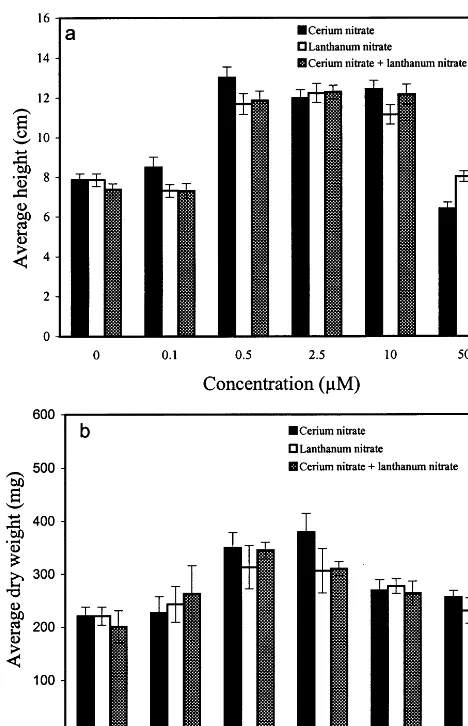
Thirty-five day-old plants growing in media with 0.5 – 10.0mM cerium nitrate were significantly
taller than those growing in basal medium (Fig. 1a). With 0.5 mM cerium nitrate, plant heights
reached 13.0 cm compared to 7.9 cm in basal medium. Combinations of cerium nitrate and lan-thanum nitrate did not show cumulative effects on plant heights (Fig. 1a).
The average dry weight of 35 day-old plants growing in media with 0.5 or 2.5 mM cerium
nitrate (350 and 379 mg respectively) were signifi-cantly higher than those growing in basal medium (221 mg) (Fig. 1b). Dry weights of plants were also significantly higher with the addition of lanthanum nitrate or both (0.5 – 2.5 mM) (Fig. 1b and Fig. 2).
Plants growing in basal medium produced an average of 7.4 flowers (Fig. 3). The average num-ber of flowers per plant in media containing cerium nitrate (0.5 – 10.0mM) ranged from 10.4 to
12.3. In media containing 0.5 – 10.0mM lanthanum
nitrate, the average number of flowers ranged
from 10.3 to 11.9 per plant (Fig. 3). Plants grow-ing in media with cerium nitrate and lanthanum nitrate (0.5 – 10.0mM) produced about 10.7 to 14.7
flowers. Medium containing combination of 2.5
mM cerium nitrate and 2.5 mM lanthanum nitrate
was observed most effective in flower production (Fig. 2 and Fig. 3).
3.5. Effects on the endogenous le6els of ZR, DHZR
and IPA
Changes in endogenous levels of ZR, DHZR and IPA in leaf and root tissues of plants growing in the basal medium and in the medium containing 0.5 mM cerium nitrate and 0.5 mM lanthanum
nitrate from day 12 to 18 were investigated. There was no significant difference in endogenous level of ZR, DHZR and IPA between the tissues in the basal medium and in REE-supplemented medium (data not shown).
increased from 969 to 1351 pmol/g.f.wt. on day 12 to 4057 – 4419 pmol/g.f.wt. on day 16. Subse-quently, a significant decrease in the level of IPA to 1120 – 1437 pmol/g.f.wt on day 18 was observed.
3.6. Effects on the endogenous le6els of
carbohydrate in leaf tissues
There was no significant difference in endoge-nous levels of sucrose, glucose and fructose be-tween the leaf tissues (12 – 18 days after seed germination) in the basal medium and in REE-supplemented medium (data not shown). The lev-els of sucrose (0.7 – 1.1 mg/g.f.wt) remained stable 12 – 16 days after germination but decreased to about 0.1 mg/g.f.wt. on day 18. In the control and REE-treated leaf tissues, the levels of glucose and fructose on days 16 and 18 were significantly higher than that on days 12 and 14 (data not shown).
3.7. Effects of 10−6 M IPA on root growth, plant
height and flowering in the presence of 0.5 mM cerium nitrate and 0.5 mM lanthanum nitrate
IPA was found to be most effective on root growth and flowering among six naturally occur-ring cytokinins tested (data not shown). In order to further study the mechanism of action of REEs on flowering, the effects of exogenous IPA with or without REEs on root growth and floral initiation were investigated (Table 3). Nine days after plants at 2-leaf stage (12 days after seed germination) were transferred to medium supplemented with 10−6 M IPA, root growth was observed
signifi-cantly increased. On day 20 after seed germina-tion, 32% of the plants in the medium containing 10−6 M IPA were observed to produce flower
buds compared to none in the control medium. However, addition of 10−6 M IPA significantly
reduced plant height (Table 3). Nine days after plants at 2-leaf stage were transplanted to the medium with 10−6M IPA, 0.5
mM cerium nitrate
and 0.5 mM lanthanum nitrate, no beneficial
ef-fects on root growth were observed. Plant height and the percentage of plants with flower buds on day 20 were further reduced (Fig. 4, Table 3). The effects of 10−6 M IPA in the presence of 0.5
mM
cerium nitrate and 0.5 mM lanthanum nitrate were
similar to that of 10−5 M IPA in terms of root
growth and plant height. Fig. 1. Effects of cerium nitrate and lanthanum nitrate on
height (a) and dry weight (b) ofA.thaliana35 days after seed germination. There were 100 plants per treatment. Bars indi-cate SE.
Fig. 2. Effects of 2.5 mM cerium nitrate and 2.5 mM
lan-thanum nitrate on height and flower production ofA.thaliana
Fig. 3. Effects of cerium nitrate and lanthanum nitrate on the number of flowers produced 35 days after seed germination. There were 100 plants per treatment. Bars indicate SE.
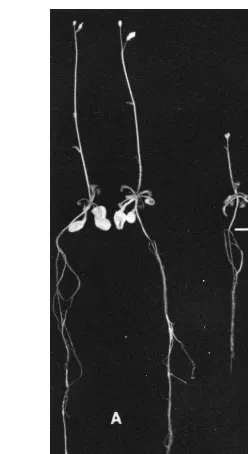
Fig. 4. Effects of 10−6M IPA on root growth and flowering
in the presence of 0.5 mM cerium nitrate and 0.5 mM
lan-thanum nitrate. Plants were grown under 16 h photoperiod and at 2592°C. Scale bar=1.5cm. (A) 10−6M IPA-treated
plant. (B) 10−6M IPA-treated plant in the presence of 0.5
mM cerium nitrate and 0.5mM lanthanum nitrate. 4. Discussion
Cerium and lanthanum are two important ele-ments of the REEs. The effects of REE on biolog-ical systems had attracted much attention since the 1970s. Regrettably, results from field trials, pot trials and laboratory study on the effects of REEs on plant growth and development are inconsistent [4]. This is partly attributed to the fact that ab-sorption of REEs could be influenced by factors such as soil pH, soil chelates and the available levels of fertilizers [4,15]. In view of this, the present investigation was conducted using a defined medium and under aseptic conditions. In this system, the composition of medium and the
amount of REEs can both be accurately con-trolled. The growth conditions of all plants are the same. In addition, among the limited reports re-garding REEs, investigations were only conducted on one or two stages of plant growth and develop-ment, such as root elongation [5,9,15], leaf and stem growth [11], spike production [10], and crop yield [12]. In the present study, the effects of REEs on root growth, vegetative and reproductive growth were all studied.
Table 3
Effects of IPA on root growth, plant height and flowering in the presence of 0.5mM cerium nitrate (Ce) and 0.5mM lanthanum
nitrate (La)
Average plant Average number of Average fresh weight of
Treatment % of plants with flower buds 20 days after seed
height (cm)a flowers per planta
roots from 50 plants (mg)a
germinationa
71.399.4a 9.190.4a 0a 8.890.5a Control
4.190.2b 10−6M IPA 104.5914.1b 4.690.2b 32.295.5b
21.493.7c 3.690.6b 10−6M IPA+0.5
mM 70.3917.2a 3.290.5c
Ce+0.5mM La
3.890.5b 52.497.9c
10−5M IPA 1.690.2d 0a
aValues were expressed as mean9SE. There were 50 plants per treatment. The average root fresh weight was scored 21 days
Experiments with corn and mungbean revealed that applications of lanthanum had no beneficial effect on the total dry matter yield [9]. In a field trial carried out in Australia, no response was found by spraying and seed dressing of a summer fodder crop (Brassicasp.) with REEs (cited in [4]). In the present study, addition of cerium and lan-thanum was found to have no beneficial effects on the vegetative growth of Arabidopsis (Fig. 1a and Fig. 3a). However, REEs were found to have beneficial effects on reproductive growth. Applica-tion of low concentraApplica-tions of cerium and lan-thanum significantly promoted reproductive growth in Arabidopsis in terms of height of bolt-ing, earliness of flowering and the number of flowers produced per plant (Tables 2 and 3, Fig. 1). The beneficial effects of REEs on reproductive growth were also reported in Day lily and narcis-sus (cited in [4]). Meehan et al. [10] reported that addition of REE promoted spike production in wheat.
In the present study, addition of 0.5 – 10.0 mM
cerium nitrate or lanthanum nitrate significantly increased root growth. For example, in the pres-ence of 1.0mM lanthanum nitrate the average root
length was 2.65 cm, about 32% increase over the control (Table 1). Similar findings were reported in corn and mungbean [9,15]. For example, addition of lanthanum at 0.63 mM increased the root
growth of corn by 36% and 0.19 mM lanthanum
increased mungbean root by 21% relative to the controls [9]. How cerium and/or lanthanum pro-moted root growth remains unclear. Roots of corns and mungbean were found to be able to accumulate high levels of lanthanum in solutions containing lanthanum [9]. Diatloff et al. [9], how-ever, suspected that the enhanced root growth of corn in solutions containing cerium or lanthanum might be due to amelioration of H+ toxicity. It
was also suggested that, at low levels, the rare earths acted as micronutrients, with toxicity occur-ring at high concentrations [11]. In the present study, the basal medium contained micronutrients and all media were prepared with the same pH. Thus it was possible that the increased root growth after REE application was not due to the effects of micronutrients or amelioration of H+
toxicity.
There was very little information available on the mechanism of action of REEs on flowering. According to Bernier et al. [16], cytokinins and
carbohydrates were two of the most important physiological signals that induced flowering. Ap-plication of 0.5mM cerium and 0.5mM lanthanum
onArabidopsiscould induce early flowering (Table 2), but had no effects on the endogenous levels of ZR, DHZR, IPA, sucrose, glucose and fructose in leaf and root tissues (data not shown). These results implied that the increased reproductive growth and flowering induced by REEs was not related to changes in the endogenous levels of cytokinins and carbohydrates.
On the other hand, addition of 0.5 mM cerium
nitrate and 0.5 mM lanthanum significantly
en-hanced the effects of 10−6M IPA on root growth
and floral initiation (Fig. 4, Table 3). Lanthanum had been reported to increase greatly the binding of auxin to A6ena coleoptile segments [17]. The
action of cytokinin in stimulating the adventitious bud initiation in Toreniastem segments was inhib-ited by 0.3 mM LaCl3[18]. These results suggested
that addition of REEs could increase the sensitiv-ity of cell to plant growth regulators. However, there was very little information available on how REEs increased the sensitivity of cell to plant growth regulators. We suggested that REEs might affect the sensitivity of cell through their effects on the membrane fluidity and membrane binding of hormones. Firstly, La3+, by virtue of an ionic
radius similar to Ca2+ and a valence higher than
Ca2+, could bind at superficially located Ca2+
absorption sites in a less reversible manner than does Ca2+ itself. Membrane stabilization takes
place when an agent, such as La3+ or Ca2+,
causes protein and protein/liquid complexes to become less fluid [19]. Some fluidity is essential to maintain optimum functions and destabilization may result in an improved membrane selectivity and overall function [4]. Secondly, it had been suggested that hormones acting through an attach-ment to site of action on a membrane might be subjected to the effects of solutes (especially La3+)
that alter membrane characteristics [20]. Low con-centrations of cerium nitrate and lanthanum ni-trate can be expected to be particularly significant in this respect because of its potency as membrane stabilizer [4].
or shoots. In certain breeding programs, earlier flowering of test materials allows earlier assess-ment of certain characteristics. Our study revealed that cerium and lanthanum had no significant effects on leaf production, dry weight and plant height of A. thaliana during the vegetative phase of growth. Instead, cerium and lanthanum, at low concentrations promoted early flowering and in-creased the number of flowers produced. This indicates that cerium and lanthanum (or other REE) may have the potential to be developed as non-hormonal flowering promoting agents for cer-tain crop species. However, we would like to point out that our studies were conducted using plants growing aseptically in enclosed Magenta culture vessels under low light intensity. Tissue culture media solidified with Difco-agar were used to grow the plants in this system. Compare with plants grown under natural conditions, plants growing in our system are relatively smaller al-though normal development is not affected. Nev-ertheless, this indicates that they were grown under sub-optimal conditions and might be under certain stress when compared to the field grown plants. Whether REEs have an effect on the flow-ering of Arabidopsis grow under natural condi-tions has yet to be investigated.
References
[1] T. Das, A. Sharma, G. Talukder, Effect of lanthanum in cellular systems, Biol. Trace Element Res. 18 (1988) 201 – 228.
[2] A. Kabata-Pendias, H. Pendias, Trace Elements in Soils and Plants, 2nd edn, CRC Press, Florida, 1992, pp. 166 – 178.
[3] T.J. Haley, Toxicity, in: K.A. Gschneidner, L. Eyring (Eds.), Handbook on the Physics and Chemistry of Rare Earths, vol. 15, North-Holland, 1979, pp. 553 – 585. [4] P.H. Brown, A.H. Rathjen, R.D. Graham, D.E. Tribe,
Rare earth elements in biological systems, in: K.A. Gschneidner Jr, L. Eyring (Eds.), Handbook on the Physics and Chemistry of Rare Earths, vol. 13, Elsevier, Amsterdam, 1990, pp. 423 – 452.
[5] B.G. Pickard, Comparison of calcium and lanthanum ions in the A6ena coleoptile growth tests, Planta 91
(1970) 314 – 320.
[6] R.F.M. Van Steveninck, M.E. Van Steveninck, D. Ches-coe, Intracellular binding of lanthanum in root tips of barley (Hordeum6ulgare), Protoplasma 90 (1976) 89 – 97.
[7] T.B. Kinraide, P.R. Ryan, L.V. Kochian, Interactive effects of Al3+, H+and other cations on root
elonga-tion considered in term of cell-surface electrical poten-tial, Plant Physiol. 99 (1992) 1461 – 1468.
[8] D.R. Parker, T.B. Kinraide, L.W. Zelazny, Aluminium speciation and phytotoxicity in dilute hydroxy-alu-minium solutions, Soil Sci. Soc. Am. J. 52 (1988) 438 – 444.
[9] E. Diatloff, F.W. Smith, C.J Asher, Rare earth elements and plant growth: III. Responses of corn and mungbean to low concentrations of cerium in dilute, continuously flowing nutrient solutions, J. Plant Nutri. 18 (1995) 1987 – 2003.
[10] B. Meehan, K. Peverill, A. Skroce, The impact of bioavailable rare earth elements in Australia agricultural soils, in: First National Workshop on Soil and Plant Analysis, 1993, pp. 36 – 41.
[11] J.R. Velasco, L.E. Domingo, A.S. Lansangan, Z.N. Sierra, Cultural studies on coconut Cadang: reaction of plants to the rare earths, thalium and certain soil sam-ples, The Philippine J. Coconut Stud. 4 (1979) 1 – 13. [12] B. Guo, Present and future situation of rare earth in
China’s agronomy, in: G. Xu, J. Xia (Eds.), Proceedings of International Conference on Rare Earth Development and Applications, Science Press, 1985, pp. 1522 – 1602. [13] R. Tang, G. Xiao, Rare earths, in: K. Wang, et al.
(Eds.), Trace Elements in Life Sciences, 2nd edn, China Measuring Publishing Press, 1996, pp. 450 – 509 (in Chi-nese).
[14] T. Murashige, F. Skoog, A revised medium for rapid growth and bioassays with tobacco tissue cultures, Phys-iol. Plant 15 (1962) 473 – 497.
[15] E. Diatloff, F.W. Smith, C.J. Asher, Rare earth elements and plant growth: II. Responses of corn and mungbean to low concentrations of lanthanum in dilute, continu-ously flowing nutrient solutions, J. Plant Nutri. 18 (1995) 1977 – 1986.
[16] G. Bernier, A. Hveiange, C. Houssa, A. Petitjean, P. Lejeune, Physiological signals that induce flowering, Plant Cell 5 (1993) 1147 – 1155.
[17] B.W. Poovaiah, A.C. Leopold, Effects of inorganic so-lutes on the binding of auxin, Plant Physiol. 58 (1976) 783 – 788.
[18] S. Tanimoto, H. Harada, Involvement of calcium in adventitious bud initiation in Torenia stem segments, Plant Cell Physiol. 27 (1986) 1 – 10.
[19] K.H. Harmet, Rapid growth response of A6ena sati6a
cultivar Victory coleoptile segments to lanthanum and other cations, Plant Physiol. 64 (1979) 1094 – 1098. [20] B.W. Poovaiah, A.C. Leopold, Effects of inorganic salts
on tissue permeability, Plant Physiol. 58 (1976) 182 – 185.